Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome
Abstract
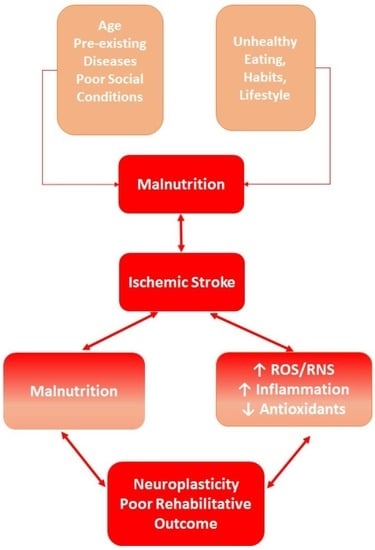
:1. Introduction
2. Nutritional Status and Healthy Brain
Oxidative Stress, Inflammation, and Healthy Nutrition
3. Ischemic Stroke and Nutritional Status
3.1. Malnutrition and Stroke-Modified Cross-Talking between Oxidative Stress and Inflammation
3.2. Stroke-Induced Modifications of Gut-Brain Axis: Involvement of Oxidative Stress and Inflammation
4. Neural Plasticity and Stroke-Induced Redox Imbalance and Inflammation
Neuronal Redox Status: A Dynamic Mechanism for Maintaining Adequate Neuronal Cell–Cell Signaling in Healthy Brains and after Ischemic Stroke
5. Impact of Malnutrition on Post-Stroke Neurorehabilitative Outcome
6. Nutritional Interventions in Post-Stroke Malnourished Patients Admitted to Rehabilitation
7. Limitations
8. Five-Year Perspective
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Stark, B.A.; Johnson, C.O.; Roth, G.A.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef] [PubMed]
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- GBD 2019 Risk Factors Collaborators. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2019, 396, 1223–1249. [Google Scholar] [CrossRef]
- Wafa, H.A.; Wolfe, C.D.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe. Stroke 2020, 51, 2418–2427. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef] [PubMed]
- Ciancarelli, I.; De Amicis, D.; Di Massimo, C.; Carolei, A.; Ciancarelli, M.G.T. Oxidative stress in post-acute ischemic stroke patients after intensive neurorehabilitation. Curr. Neurovasc. Res. 2012, 9, 266–273. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Morone, G.; Iosa, M.; Paolucci, S.; Pignolo, L.; Tonin, P.; Cerasa, A.; Ciancarelli, M.G.T. Adipokines as Potential Biomarkers in the Neurorehabilitation of Obese Stroke Patients. Curr. Neurovasc. Res. 2020, 17, 437–445. [Google Scholar] [CrossRef]
- Khan, F.; Amatya, B.; Galea, M.P.; Gonzenbach, R.; Kesselring, J. Neurorehabilitation: Applied neuroplasticity. J. Neurol. 2016, 264, 603–615. [Google Scholar] [CrossRef]
- Uchino, K.; Billheimer, D.; Cramer, S.C. Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke 2001, 32, 909–916. [Google Scholar] [CrossRef] [Green Version]
- Norman, K.; Haß, U.; Pirlich, M. Malnutrition in Older Adults—Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef]
- Nascimento, C.; Ingles, M.; Salvador-Pascual, A.; Cominetti, M.R.; Gomez-Cabrera, M.; Viña, J. Sarcopenia, frailty and their prevention by exercise. Free Radic. Biol. Med. 2018, 132, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Alaverdashvili, M.; Caine, S.; Li, X.; Hackett, M.J.; Bradley, M.P.; Nichol, H.; Paterson, P.G. Protein-Energy Malnutrition Exacerbates Stroke-Induced Forelimb Abnormalities and Dampens Neuroinflammation. Transl. Stroke Res. 2018, 9, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.; Qi, X.; Xia, X.; Wang, L.; Li, X. Malnutrition on admission increases the in-hospital mortality and length of stay in elder adults with acute ischemic stroke. J. Clin. Lab. Anal. 2021, 36, e24132. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Germanotta, M.; Guerrini, A.; Pascali, S.; Cipollini, V.; Cortellini, L.; Ruco, E.; Khazrai, Y.M.; De Gara, L.; Aprile, I. Relationship between Nutritional Status, Food Consumption and Sarcopenia in Post-Stroke Rehabilitation: Preliminary Data. Nutrients 2022, 14, 4825. [Google Scholar] [CrossRef] [PubMed]
- Kokura, Y.; Maeda, K.; Wakabayashi, H.; Nishioka, S.; Higashi, S. High Nutritional-Related Risk on Admission Predicts Less Improvement of Functional Independence Measure in Geriatric Stroke Patients: A Retrospective Cohort Study. J. Stroke Cerebrovasc. Dis. 2016, 25, 1335–1341. [Google Scholar] [CrossRef]
- Ciancarelli, I.; Mariangeli, F.; Tonin, P.; Ciofani, E.; Garo, M.L.; Ciancarelli, M.G.T. Influence of neurorehabilitation on stroke-induced modifications of the quadriceps muscle in elderly subacute stroke patients with paresis. Funct. Neurol. 2019, 34, 99–105. [Google Scholar] [PubMed]
- Ciancarelli, I.; Tonin, P.; Garo, M.L.; Ciancarelli, M.G.T. Effectiveness of intensive neurorehabilitation in obese subacute stroke patients. Funct. Neurol. 2019, 34, 45–51. [Google Scholar]
- Zhao, N.-N.; Zeng, K.-X.; Wang, Y.-L.; Sheng, P.-J.; Tang, C.-Z.; Xiao, P.; Liu, X.-W. Research on the nutrition and cognition of high-risk stroke groups in community and the relevant factors. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5408–5414. [Google Scholar]
- Lee, E.C.; Jeong, Y.G.; Jung, J.H.; Moon, H.I. Validity of the Controlling Nutritional Status score as a Nutritional Assessment Tool early after stroke. Int. J. Rehabilit. Res. 2021, 45, 58–64. [Google Scholar] [CrossRef]
- Kokura, Y.; Kimoto, K.; Okada, Y.; Kawakita, S. The Controlling Nutritional Status score as a functional prognostic marker in patients with acute stroke: A multicenter retrospective cohort study. Nutrition 2020, 79–80, 110889. [Google Scholar] [CrossRef]
- Irisawa, H.; Mizushima, T. Correlation of Body Composition and Nutritional Status with Functional Recovery in Stroke Rehabilitation Patients. Nutrients 2020, 12, 1923. [Google Scholar] [CrossRef] [PubMed]
- Zielińska-Nowak, E.; Cichon, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. Nutritional Supplements and Neuroprotective Diets and Their Potential Clinical Significance in Post-Stroke Rehabilitation. Nutrients 2021, 13, 2704. [Google Scholar] [CrossRef] [PubMed]
- Engelheart, S.; Andrén, D.; Repsilber, D.; Forslund, H.B.; Brummer, R.J. Nutritional status in older people—An explorative analysis. Clin. Nutr. ESPEN 2021, 46, 424–433. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). WHO Expert Committee. Physical Status: The Use and Interpretation of Anthropometry; World Health Organization (WHO): Geneva, Switzerland, 1995.
- World Health Organization (WHO). Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003.
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Norman, K.; Matthews, D.E. Editorial. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 311–313. [Google Scholar] [CrossRef]
- Holmes, C.; Racette, S. The Utility of Body Composition Assessment in Nutrition and Clinical Practice: An Overview of Current Methodology. Nutrients 2021, 13, 2493. [Google Scholar] [CrossRef]
- Guigoz, Y. The Mini-Nutritional Assessment (MNA®) Review of the Literature—What does it tell us? J. Nutr. Health Aging 2006, 10, 466–487. [Google Scholar] [PubMed]
- Kaiser, M.J.; Bauer, J.M.; Ramsch, C.; Uter, W.; Guigoz, Y.; Cederholm, T.; Thomas, D.R.; Anthony, P.; Charlton, K.E.; Maggio, M.; et al. Validation of the Mini Nutritional Assessment Short-Form (MNA®-SF): A practical tool for identification of nutritional status. J. Nutr. Health Aging 2009, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Ignacio de Ulíbarri, J.; González-Madroño, A.; de Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar]
- Han, X.; Cai, J.; Li, Y.; Rong, X.; Li, Y.; He, L.; Li, H.; Liang, Y.; Huang, H.; Xu, Y.; et al. Baseline Objective Malnutritional Indices as Immune-Nutritional Predictors of Long-Term Recurrence in Patients with Acute Ischemic Stroke. Nutrients 2022, 14, 1337. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Luo, X.; He, Q.; Cheng, Y.; Shen, W.; Xie, Z. Controlling nutritional status score and prognostic nutrition index predict the outcome after severe traumatic brain injury. Nutr. Neurosci. 2020, 25, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.-Y.; Cheng, S.; Lin, L.; Chen, M.-X. Effect of Controlling Nutritional Status Score (CONUT) and Prognostic Nutritional Index (PNI) on patients after spinal tuberculosis surgery. Sci. Rep. 2022, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Hata, N.; Kobayashi, N.; Okazaki, H.; Matsushita, M.; Shibata, Y.; Nishigoori, S.; Uchiyama, S.; Asai, K.; Shimizu, W. The prognostic impact of malnutrition in patients with severely decompensated acute heart failure, as assessed using the Prognostic Nutritional Index (PNI) and Controlling Nutritional Status (CONUT) score. Hear. Vessel. 2017, 33, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef] [PubMed]
- Colao, A.; Vetrani, C.; Muscogiuri, G.; Barrea, L.; Tricopoulou, A.; Soldati, L.; Piscitelli, P.; UNESCO Chair on Health Education and Sustainable Development. “Planeterranean” Diet: Extending worldwide the health benefits of Mediterranean Diet based on nutritional properties of locally available foods. J. Transl. Med. 2022, 20, 232. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef]
- Bourre, J.-M. Effets des nutriments sur les structures et les fonctions du cerveau: Le point sur la diététique du cerveau [The role of nutritional factors on the structure and function of the brain: An update on dietary requirements]. Rev. Neurol. 2004, 160, 767–792. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M.; Vitalini, S. Healthy Diets and Modifiable Risk Factors for Non-Communicable Diseases—The European Perspective. Foods 2020, 9, 940. [Google Scholar] [CrossRef]
- Caretto, A.; Lagattolla, V. Non-Communicable Diseases and Adherence to Mediterranean Diet. Endocrine Metab. Immune Disord.—Drug Targets 2015, 15, 10–17. [Google Scholar] [CrossRef]
- Carraro, E.; Schilirò, T.; Biorci, F.; Romanazzi, V.; Degan, R.; Buonocore, D.; Verri, M.; Dossena, M.; Bonetta, S.; Gilli, G. Physical Activity, Lifestyle Factors and Oxidative Stress in Middle Age Healthy Subjects. Int. J. Environ. Res. Public Health 2018, 15, 1152. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Biochemistry of Oxidative Stress. Angew. Chem. Int. Ed. 1986, 25, 1058–1071. [Google Scholar] [CrossRef]
- Sies, H.; Ursini, F. Homeostatic control of redox status and health. IUBMB Life 2021, 74, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ciancarelli, M.G.T.; Di Massimo, C.; De Amicis, D.; Ciancarelli, I. Mediterranean Diet and Health Promotion: Evidence and current concerns. Med. Res. Arch. 2017, 5, 7. [Google Scholar] [CrossRef]
- Gantenbein, K.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13, 1951. [Google Scholar] [CrossRef] [PubMed]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary Approaches to Stop Hypertension (DASH) Diet and Blood Pressure Reduction in Adults with and without Hypertension: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. Int. Rev. J. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Cherian, L.; Wang, Y.; Fakuda, K.; Leurgans, S.; Aggarwal, N.; Morris, M. Mediterranean-dash intervention for neurodegenerative delay (mind) diet slows cognitive decline after stroke. J. Prev. Alzheimer’s Dis. 2019, 6, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Springmann, M.; Spajic, L.; Clark, M.A.; Poore, J.; Herforth, A.; Webb, P.; Rayner, M.; Scarborough, P. The healthiness and sustainability of national and global food based dietary guidelines: Modelling study. BMJ 2020, 370, m2322. [Google Scholar] [CrossRef]
- Di Miceli, M.; Bosch-Bouju, C.; Layé, S. PUFA and their derivatives in neurotransmission and synapses: A new hallmark of synaptopathies. In Proceedings of the Nutrition Society; Cambridge University Press (CUP): Cambridge, UK, 2020; Volume 79, pp. 388–403. [Google Scholar]
- Djuricic, I.; Calder, P. Beneficial Outcomes of Omega-6 and Omega-3 Polyunsaturated Fatty Acids on Human Health: An Update for 2021. Nutrients 2021, 13, 2421. [Google Scholar] [CrossRef]
- Park, Y.; Watkins, B.A. Dietary PUFAs and Exercise Dynamic Actions on Endocannabinoids in Brain: Consequences for Neural Plasticity and Neuroinflammation. Adv. Nutr. Int. Rev. J. 2022, 13, 1989–2001. [Google Scholar] [CrossRef]
- Murphy, T.; Dias, G.P.; Thuret, S. Effects of Diet on Brain Plasticity in Animal and Human Studies: Mind the Gap. Neural Plast. 2014, 2014, 563160. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; He, Y.; Chen, S.; Qi, S.; Shen, J. Therapeutic targets of oxidative/nitrosative stress and neuroinflammation in ischemic stroke: Applications for natural product efficacy with omics and systemic biology. Pharmacol. Res. 2020, 158, 104877. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Wang, L.-P.; Wan, Q. Therapeutic targets of neuroprotection and neurorestoration in ischemic stroke: Applications for natural compounds from medicinal herbs. Biomed. Pharmacother. 2022, 148, 112719. [Google Scholar] [CrossRef]
- Jaeger, B.N.; Parylak, S.L.; Gage, F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Aspects Med. 2018, 61, 50–62. [Google Scholar] [CrossRef]
- Franco, M.N.; Galeano-Díaz, T.; López, Ó.; Fernández-Bolaños, J.G.; Sánchez, J.; De Miguel, C.; Gil, M.V.; Martín-Vertedor, D. Phenolic compounds and antioxidant capacity of virgin olive oil. Food Chem. 2014, 163, 289–298. [Google Scholar] [CrossRef]
- Del Rio, D.; Costa, L.G.; Lean, M.E.; Crozier, A. Polyphenols and health: What compounds are involved? Nutr. Metab. Cardiovasc. Dis. 2010, 20, 1–6. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Nguyen, T.T.J. Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutr. Neurosci. 2012, 15, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [Green Version]
- Sabbouh, T.; Torbey, M.T. Malnutrition in Stroke Patients: Risk Factors, Assessment, and Management. Neurocrit. Care 2017, 29, 374–384. [Google Scholar] [CrossRef]
- Ho, L.-C.; Wang, H.-K.; Chiu, L.-T.; Wang, H.-H.; Lee, Y.-C.; Hung, S.-Y.; Sun, Y.; Wei, C.-Y.; Hsu, K.-C.; Chen, Y.-W.; et al. Protein energy wasting–based nutritional assessment predicts outcomes of acute ischemic stroke and solves the epidemiologic paradox. Nutrition 2021, 93, 111431. [Google Scholar] [CrossRef]
- Su, Y.; Yuki, M.; Otsuki, M. Prevalence of stroke-related sarcopenia: A systematic review and meta-analysis. J. Stroke Cerebrovasc. Dis. 2020, 29. [Google Scholar] [CrossRef] [PubMed]
- Ciancarelli, I.; Di Massimo, C.; De Amicis, D.; Carolei, A.; Ciancarelli, M.G.T. Evidence of redox unbalance in post-acute ischemic stroke patients. Curr. Neurovascular Res. 2012, 9, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Siotto, M.; Santoro, M.; Aprile, I. Vitamin D and Rehabilitation after Stroke: Status of Art. Appl. Sci. 2020, 10, 1973. [Google Scholar] [CrossRef]
- Siotto, M.; Germanotta, M.; Santoro, M.; Canali, R.; Pascali, S.; Insalaco, S.; Cipollini, V.; Papadopoulou, D.; Antonacci, E.; Aprile, I. Oxidative Stress Status in Post Stroke Patients: Sex Differences. Healthcare 2022, 10, 869. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, C.; Dashe, J.F.; Scott, T.; Thaler, D.; Folstein, M.F.; Martin, A. Decreased Levels of Plasma Vitamin C and Increased Concentrations of Inflammatory and Oxidative Stress Markers After Stroke. Stroke 2004, 35, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Ciancarelli, I.; Di Massimo, C.; De Amicis, D.; Pistarini, C.; Ciancarelli, M.G.T. Uric Acid and Cu/Zn Superoxide Dismutase: Potential Strategies and Biomarkers in Functional Recovery of Post-Acute Ischemic Stroke Patients after Intensive Neurorehabilitation. Curr. Neurovasc. Res. 2015, 12, 120–127. [Google Scholar] [CrossRef]
- Mirończuk, A.; Kapica-Topczewska, K.; Socha, K.; Soroczyńska, J.; Jamiołkowski, J.; Kułakowska, A.; Kochanowicz, J. Selenium, Copper, Zinc Concentrations and Cu/Zn, Cu/Se Molar Ratios in the Serum of Patients with Acute Ischemic Stroke in Northeastern Poland-A New Insight into Stroke Pathophysiology. Nutrients 2021, 13, 2139. [Google Scholar] [CrossRef]
- Veronese, N.; Barbagallo, M. Magnesium and Micro-Elements in Older Persons. Nutrients 2021, 13, 847. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R. Leptin and zinc relation: In regulation of food intake and immunity. Indian J. Endocrinol. Metab. 2012, 16, S611–S616. [Google Scholar] [CrossRef]
- Elkind, M.S. Inflammation, atherosclerosis, and stroke. Neurologist 2006, 12, 140–148. [Google Scholar]
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, B.; Arya, A. Brain–gut axis after stroke. Brain Circ. 2018, 4, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; Bullich-Vilarrubias, C.; López-Almela, I.; Liébana-García, R.; Olivares, M.; Sanz, Y. The Microbiota and the Gut–Brain Axis in Controlling Food Intake and Energy Homeostasis. Int. J. Mol. Sci. 2021, 22, 5830. [Google Scholar] [CrossRef] [PubMed]
- Bauer, P.V.; Hamr, S.C.; Duca, F.A. Regulation of energy balance by a gut–brain axis and involvement of the gut microbiota. Cell. Mol. Life Sci. 2016, 73, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Chidambaram, S.B.; Rathipriya, A.G.; Mahalakshmi, A.M.; Sharma, S.; Hediyal, T.A.; Ray, B.; Sunanda, T.; Rungratanawanich, W.; Kashyap, R.S.; Qoronfleh, M.W.; et al. The Influence of Gut Dysbiosis in the Pathogenesis and Management of Ischemic Stroke. Cells 2022, 11, 1239. [Google Scholar] [CrossRef]
- Dumitrescu, L.; Popescu-Olaru, I.; Cozma, L.; Tulbă, D.; Hinescu, M.E.; Ceafalan, L.C.; Gherghiceanu, M.; Popescu, B.O. Oxidative Stress and the Microbiota-Gut-Brain Axis. Oxidative Med. Cell. Longev. 2018, 2018, 2406594. [Google Scholar] [CrossRef] [Green Version]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
- Chandra, R.K. Nutrition and the immune system from birth to old age. Eur. J. Clin. Nutr. 2002, 56, S73–S76. [Google Scholar] [CrossRef] [Green Version]
- Pourhassan, M.; Babel, N.; Sieske, L.; Westhoff, T.; Wirth, R. Longitudinal Changes of Cytokines and Appetite in Older Hospitalized Patients. Nutrients 2021, 13, 2508. [Google Scholar] [CrossRef]
- Takele, Y.; Adem, E.; Getahun, M.; Tajebe, F.; Kiflie, A.; Hailu, A.; Raynes, J.; Mengesha, B.; Ayele, T.A.; Shkedy, Z.; et al. Malnutrition in Healthy Individuals Results in Increased Mixed Cytokine Profiles, Altered Neutrophil Subsets and Function. PLoS ONE 2016, 11, e0157919. [Google Scholar] [CrossRef] [Green Version]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef] [Green Version]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Colombo, J.A.; Reisin, H.D.; Miguel-Hidalgo, J.J.; Rajkowska, G. Cerebral cortex astroglia and the brain of a genius: A propos of A. Einstein’s. Brain Res. Rev. 2006, 52, 257–263. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc. Trans. 2007, 35, 1147–1150. [Google Scholar] [CrossRef]
- Kugler, E.C.; Greenwood, J.; MacDonald, R.B. The “Neuro-Glial-Vascular” Unit: The Role of Glia in Neurovascular Unit Formation and Dysfunction. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef]
- Boveris, A.; Chance, B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973, 134, 707–716. [Google Scholar] [CrossRef]
- Kukreja, R.C.; Kontos, H.A.; Hess, M.L.; Ellis, E.F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ. Res. 1986, 59, 612–619. [Google Scholar] [CrossRef] [Green Version]
- McNally, J.S.; Davis, M.E.; Giddens, D.P.; Saha, A.; Hwang, J.; Dikalov, S.; Jo, H.; Harrison, D.G. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am. J. Physiol. Circ. Physiol. 2003, 285, H2290–H2297. [Google Scholar] [CrossRef] [Green Version]
- Radi, R. Oxygen radicals, nitric oxide, and peroxynitrite: Redox pathways in molecular medicine. Proc. Natl. Acad. Sci. USA 2018, 115, 5839–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [Green Version]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Baxter, P.S.; Hardingham, G.E. Adaptive regulation of the brain’s antioxidant defences by neurons and astrocytes. Free Radic. Biol. Med. 2016, 100, 147–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, L.T.; Klann, E. Potentiation of Hippocampal Synaptic Transmission by Superoxide Requires the Oxidative Activation of Protein Kinase C. J. Neurosci. 2002, 22, 674–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.Y.; Chung, K.; Chung, J.M. Involvement of Reactive Oxygen Species in Long-Term Potentiation in the Spinal Cord Dorsal Horn. J. Neurophysiol. 2010, 103, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Kessas, K.; Chouari, Z.; Ghzaiel, I.; Zarrouk, A.; Ksila, M.; Ghrairi, T.; El Midaoui, A.; Lizard, G.; Kharoubi, O. Role of Bioactive Compounds in the Regulation of Mitochondrial Dysfunctions in Brain and Age-Related Neurodegenerative Diseases. Cells 2022, 11, 257. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, R.; Azam, S.; Cho, D.-Y.; Su-Kim, I.; Choi, D.-K. Natural Phytochemicals as Novel Therapeutic Strategies to Prevent and Treat Parkinson’s Disease: Current Knowledge and Future Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 6680935. [Google Scholar] [CrossRef]
- Wu, A.-G.; Yong, Y.-Y.; Pan, Y.-R.; Zhang, L.; Wu, J.-M.; Zhang, Y.; Tang, Y.; Wei, J.; Yu, L.; Law, B.Y.-K.; et al. Targeting Nrf2-Mediated Oxidative Stress Response in Traumatic Brain Injury: Therapeutic Perspectives of Phytochemicals. Oxidative Med. Cell. Longev. 2022, 2022, 1015791. [Google Scholar] [CrossRef]
- Cichon, N.; Saluk-Bijak, J.; Gorniak, L.; Przyslo, L.; Bijak, M. Flavonoids as a Natural Enhancer of Neuroplasticity—An Overview of the Mechanism of Neurorestorative Action. Antioxidants 2020, 9, 1035. [Google Scholar] [CrossRef]
- Ye, R.; Shi, M.; Liu, Q.; Chen, J. Redox Imbalance and Stroke. Oxidat. Med. Cell. Longev. 2016, 2016, 3065263. [Google Scholar] [CrossRef]
- Shah, Z.; Li, R.-C.; Thimmulappa, R.; Kensler, T.; Yamamoto, M.; Biswal, S.; Doré, S. Role of reactive oxygen species in modulation of Nrf2 following ischemic reperfusion injury. Neuroscience 2007, 147, 53–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol Treatment in Different Time-Attenuated Neuronal Apoptosis After Oxygen and Glucose Deprivation/Reoxygenation via Enhancing the Activation of Nrf-2 Signaling Pathway In Vitro. Cell Transplant. 2018, 27, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Y.; Zhang, L.; Shi, D.-L.; Song, X.-H.; Shen, Y.-L.; Zheng, M.-Z.; Wang, L.-L. Resveratrol Attenuates Subacute Systemic Inflammation-Induced Spatial Memory Impairment via Inhibition of Astrocyte Activation and Enhancement of Synaptophysin Expression in the Hippocampus. Ann. Clin. Lab. Sci. 2017, 47, 17–24. [Google Scholar] [PubMed]
- Li, N.; Wang, X.; Sun, C.; Wu, X.; Lu, M.; Si, Y.; Ye, X.; Wang, T.; Yu, X.; Zhao, X.; et al. Change of intestinal microbiota in cerebral ischemic stroke patients. BMC Microbiol. 2019, 19, 191. [Google Scholar] [CrossRef] [Green Version]
- Yoo, S.-H.; Kim, J.S.; Kwon, S.U.; Yun, S.-C.; Koh, J.-Y.; Kang, D.-W. Undernutrition as a Predictor of Poor Clinical Outcomes in Acute Ischemic Stroke Patients. Arch. Neurol. 2008, 65, 39–43. [Google Scholar] [CrossRef]
- Scognamiglio, U.; Salvia, A.; Paolucci, S.; Garbagnati, F.; Caltagirone, C.; Musicco, M. Validity of a questionnaire for the semi-quantitative evaluation of dietary intake of hospitalized patients compared to weighed records. J. Human Nutr. Dietet. Off. J. British Dietet. Assoc. 2012, 25, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Gomes, F.; Emery, P.W.; Weekes, C.E. Risk of malnutrition is an independent predictor of mortality, length of hospital stay, and hospitalization costs in stroke patients. J. Stroke Cerebrovasc. Dis. 2016, 25, 799–806. [Google Scholar] [CrossRef] [Green Version]
- Bouziana, S.D.; Tziomalos, K. Malnutrition in Patients with Acute Stroke. J. Nutr. Metab. 2011, 2011, 167898. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, H.; Yozu, A.; Khono, Y.; Oose, H. Nutritional improvement is associated with better functional outcome in stroke rehabilitation: A cross-sectional study using controlling in nutritional status. J. Rehabil. Med. 2020, 52, jrm00028. [Google Scholar] [CrossRef] [Green Version]
- Morone, G.; Iosa, M.; Paolucci, T.; Muzzioli, L.; Paolucci, S. Relationship Between Body Mass Index and Rehabilitation Outcomes in Subacute Stroke With Dysphagia. Am. J. Phys. Med. Rehabilitat. 2019, 98, 608–612. [Google Scholar] [CrossRef]
- Korivi, M.; Wu, C.-Y.; Lin, K.-C. Potential predictive values of inflammatory biomarkers for stroke rehabilitation outcomes. J. Formos. Med. Assoc. 2013, 112, 735–737. [Google Scholar] [CrossRef]
- Aquilani, R.; Scocchi, M.; Boschi, F.; Viglio, S.; Iadarola, P.; Pastoris, O.; Verri, M. Effect of calorie-protein supplementation on the cognitive recovery of patients with subacute stroke. Nutr. Neurosci. 2008, 11, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Ricart, W.; Gonzalez-Huix, F.; Soler, S.; Marrugat, J.; Molins, A.; Suñer, R.; Genís, D. Effect of Malnutrition After Acute Stroke on Clinical Outcome. Stroke 1996, 27, 1028–1032. [Google Scholar] [CrossRef] [PubMed]
- Scherbakov, N.; Knops, M.; Ebner, N.; Valentova, M.; Sandek, A.; Grittner, U.; Dahinden, P.; Hettwer, S.; Schefold, J.C.; von Haehling, S.; et al. Evaluation of C-terminal Agrin Fragment as a marker of muscle wasting in patients after acute stroke during early rehabilitation. J. Cachex- Sarcopenia Muscle 2015, 7, 60–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimura, Y.; Yamada, M.; Kakehi, T.; Itagaki, A.; Tanaka, N.; Muroh, Y. Combination of Low Body Mass Index and Low Serum Albumin Level Leads to Poor Functional Recovery in Stroke Patients. J. Stroke Cerebrovasc. Dis. 2016, 26, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Leszczak, J.; Czenczek-Lewandowska, E.; Przysada, G.; Baran, J.; Weres, A.; Wyszyńska, J.; Mazur, A.; Kwolek, A. Association Between Body Mass Index and Results of Rehabilitation in Patients After Stroke: A 3-Month Observational Follow-Up Study. Med. Sci. Monit. 2019, 25, 4869–4876. [Google Scholar] [CrossRef]
- Andersen, K.K.; Olsen, T.S. The Obesity Paradox in Stroke: Lower Mortality and Lower Risk of Readmission for Recurrent Stroke in Obese Stroke Patients. Int. J. Stroke 2013, 10, 99–104. [Google Scholar] [CrossRef]
- Oesch, L.; Tatlisumak, T.; Arnold, M.; Sarikaya, H. Obesity paradox in stroke—Myth or reality? A systematic review. PLoS ONE 2017, 12, e0171334. [Google Scholar] [CrossRef] [Green Version]
- Trial Collaboration FOOD. Poor nutritional status on admission predicts poor outcomes after stroke: Observational data from the FOOD trial. Stroke 2003, 34, 1450–1456. [Google Scholar] [CrossRef] [Green Version]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a leucine-enriched amino acid supplement on muscle mass, muscle strength, and physical function in post-stroke patients with sarcopenia: A randomized controlled trial. Nutrition 2018, 58, 1–6. [Google Scholar] [CrossRef]
- Aquilani, R.; Scocchi, M.; Iadarola, P.; Franciscone, P.; Verri, M.; Boschi, F.; Pasini, E.; Viglio, S. Protein supplementation may enhance the spontaneous recovery of neurological alterations in patients with ischaemic stroke. Clin. Rehabilitat. 2008, 22, 1042–1050. [Google Scholar] [CrossRef]
- Kawakami, M.; Liu, M.; Wada, A.; Otsuka, T.; Nishimura, A. Resting Energy Expenditure in Patients with Stroke during the Subacute Phases—Relationships with Stroke Types, Location, Severity of Paresis, and Activities of Daily Living. Cerebrovasc. Dis. 2015, 39, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lieber, A.C.; Hong, E.; Putrino, D.; Nistal, D.A.; Pan, J.S.; Kellner, C.P. Nutrition, Energy Expenditure, Dysphagia, and Self-Efficacy in Stroke Rehabilitation: A Review of the Literature. Brain Sci. 2018, 8, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, A.; Wakabayashi, H.; Nishioka, S.; Nagano, A.; Momosaki, R. Energy Intake at Admission for Improving Activities of Daily Living and Nutritional Status among Convalescent Stroke Patients. Neurol. Medico-Chirurgica 2019, 59, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Dong, J.; Guo, J. The effects of nutrition supplement on rehabilitation for patients with stroke: Analysis based on 16 randomized controlled trials. Medicine 2022, 101, e29651. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Sessarego, P.; Iadarola, P.; Barbieri, A.; Boschi, F. Nutrition for brain recovery after ischemic stroke: An added value to rehabilitation. Nutr. Clin. Pract. 2011, 26, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Marek, K.; Cichoń, N.; Saluk-Bijak, J.; Bijak, M.; Miller, E. The Role of Vitamin D in Stroke Prevention and the Effects of Its Supplementation for Post-Stroke Rehabilitation: A Narrative Review. Nutrients 2022, 14, 2761. [Google Scholar] [CrossRef]
- Gomez-Pinilla, F.; Yang, X. System biology approach intersecting diet and cell metabolism with pathogenesis of brain disorders. Prog. Neurobiol. 2018, 169, 76–90. [Google Scholar] [CrossRef]
- Chen, R.; Snyder, M. Systems biology: Personalized medicine for the future? Curr. Opin. Pharmacol. 2012, 12, 623–628. [Google Scholar] [CrossRef] [Green Version]
- Pastorino, R.; Loreti, C.; Giovannini, S.; Ricciardi, W.; Padua, L.; Boccia, S. Challenges of Prevention for a Sustainable Personalized Medicine. J. Pers. Med. 2021, 11, 311. [Google Scholar] [CrossRef]
- Wagner, A.K.; Zitelli, K.T. A Rehabilomics focused perspective on molecular mechanisms underlying neurological injury, complications, and recovery after severe TBI. Pathophysiology 2013, 20, 39–48. [Google Scholar] [CrossRef]
- Bellini, G.; Benvenuti, L.; Ippolito, C.; Frosini, D.; Segnani, C.; Rettura, F.; Pancetti, A.; Bertani, L.; D’Antongiovanni, V.; Palermo, G.; et al. Intestinal histomorphological and molecular alterations in patients with Parkinson’s disease. Eur. J. Neurol. 2022. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciancarelli, I.; Morone, G.; Iosa, M.; Cerasa, A.; Calabrò, R.S.; Iolascon, G.; Gimigliano, F.; Tonin, P.; Tozzi Ciancarelli, M.G. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients 2023, 15, 108. https://doi.org/10.3390/nu15010108
Ciancarelli I, Morone G, Iosa M, Cerasa A, Calabrò RS, Iolascon G, Gimigliano F, Tonin P, Tozzi Ciancarelli MG. Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients. 2023; 15(1):108. https://doi.org/10.3390/nu15010108
Chicago/Turabian StyleCiancarelli, Irene, Giovanni Morone, Marco Iosa, Antonio Cerasa, Rocco Salvatore Calabrò, Giovanni Iolascon, Francesca Gimigliano, Paolo Tonin, and Maria Giuliana Tozzi Ciancarelli. 2023. "Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome" Nutrients 15, no. 1: 108. https://doi.org/10.3390/nu15010108
APA StyleCiancarelli, I., Morone, G., Iosa, M., Cerasa, A., Calabrò, R. S., Iolascon, G., Gimigliano, F., Tonin, P., & Tozzi Ciancarelli, M. G. (2023). Influence of Oxidative Stress and Inflammation on Nutritional Status and Neural Plasticity: New Perspectives on Post-Stroke Neurorehabilitative Outcome. Nutrients, 15(1), 108. https://doi.org/10.3390/nu15010108