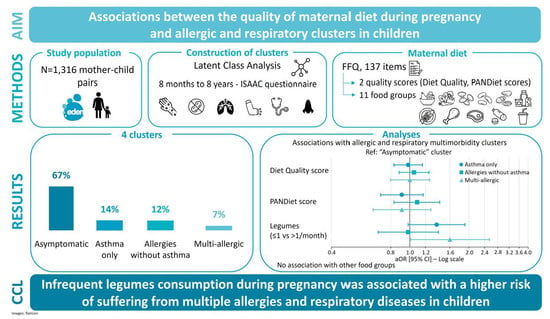
Maternal Diet Quality during Pregnancy and Allergic and Respiratory Multimorbidity Clusters in Children from the EDEN Mother–Child Cohort
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Ethics
2.3. Data Collection
2.3.1. Maternal Diet during Pregnancy
2.3.2. Allergic and Respiratory Variables
- Ever food allergy: at least one positive answer during the follow-up to the question “Has a doctor ever diagnosed your child with a food allergy?”.
- Ever eczema: at least one parental report of current eczema during the follow-up. Current eczema was characterized according to the criteria from the Mechanisms of Development of Allergy consortium (MeDALL) [2] as a positive answer to three items (“Has your child ever been diagnosed with eczema?”, “(Since last follow-up), has your child had an itchy rash (red patches, pimples, etc.) on the skin that appears and disappears intermittently?”, “Has this itchy rash affected any of the following areas: the folds of the elbows, behind the knees, in front of the ankles, under the buttocks, around the neck, around the eyes or ears?”).
- Ever wheezing: at least one positive answer during the follow-up to the question “Has your child had wheezing in the chest at any time (since last follow-up)?”.
- Ever medication for asthma attack: positive answers to two items (“(Since the last follow-up), has your child had an asthma attack?” and “Has this problem required treatment prescribed by a physician at least once?”).
- Ever asthma diagnosis: at least one positive answer during the follow-up to the question “Has your child ever been diagnosed with asthma by a doctor?”.
- Ever rhinitis: at least one parental report during the follow-up of current rhinitis. Current rhinitis was characterized according to the MeDALL criteria [2] as a positive answer to two items (“(Since last follow-up) has your child had sneezing, a runny nose or a stuffy nose without respiratory infection (no cold, no rhinopharyngitis, no flu...)?” and “Were these nose problems accompanied by watering (crying) or itching (scratching) of the eyes?”).
2.3.3. Other Variables
2.3.4. Study Sample
2.4. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Identification of Allergic and Respiratory Clusters
- “asymptomatic” cluster, (67%): This cluster corresponded to children with low prevalence of all allergic and respiratory outcomes considered in the LCA, in comparison with other clusters.
- “asthma only” cluster, (14%): A cluster characterised by a high prevalence of respiratory outcomes—wheezing (94.6%), asthma medication (90.1%), and medical diagnosis of asthma (76.7%)—and a low prevalence of food allergies (4.9%) and eczema (7.2%).
- “allergies without asthma” cluster, (12%): in this cluster children had a high prevalence of food allergy (40.2%), eczema (94.0%), and rhinitis (50.0%), but a low prevalence of asthma medication (1.1%), and medical diagnosis of asthma (6.5%).
- “multi-allergic” cluster, (7%): the prevalence of all allergic and respiratory outcomes considered in the LCA was high.
3.3. Maternal Diet Quality and Allergic and Respiratory Multimorbidity Clusters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. The Developmental Origins of Well-Being. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2004, 359, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Anto, J.M.; Bousquet, J.; Akdis, M.; Auffray, C.; Keil, T.; Momas, I.; Postma, D.S.; Valenta, R.; Wickman, M.; Cambon-Thomsen, A.; et al. Mechanisms of the Development of Allergy (MeDALL): Introducing Novel Concepts in Allergy Phenotypes. J. Allergy Clin. Immunol. 2017, 139, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asher, M.I.; Rutter, C.E.; Bissell, K.; Chiang, C.-Y.; El Sony, A.; Ellwood, E.; Ellwood, P.; García-Marcos, L.; Marks, G.B.; Morales, E.; et al. Worldwide Trends in the Burden of Asthma Symptoms in School-Aged Children: Global Asthma Network Phase I Cross-Sectional Study. Lancet 2021, 398, 1569–1580. [Google Scholar] [CrossRef] [PubMed]
- García-Marcos, L.; Asher, M.I.; Pearce, N.; Ellwood, E.; Bissell, K.; Chiang, C.-Y.; El Sony, A.; Ellwood, P.; Marks, G.B.; Mortimer, K.; et al. The Burden of Asthma, Hay Fever and Eczema in Children in 25 Countries: GAN Phase I Study. Eur. Respir. J. 2022, 60, 2102866. [Google Scholar] [CrossRef] [PubMed]
- Sicherer, S.H.; Sampson, H.A. Food Allergy: A Review and Update on Epidemiology, Pathogenesis, Diagnosis, Prevention, and Management. J. Allergy Clin. Immunol. 2018, 141, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Venter, C.; Agostoni, C.; Arshad, S.H.; Ben-Abdallah, M.; Du Toit, G.; Fleischer, D.M.; Greenhawt, M.; Glueck, D.H.; Groetch, M.; Lunjani, N.; et al. Dietary Factors during Pregnancy and Atopic Outcomes in Childhood: A Systematic Review from the European Academy of Allergy and Clinical Immunology. Pediatr. Allergy Immunol. 2020, 31, 889–912. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Zhu, X.; Wang, D.; Dai, J. Effects of Supplementation with Omega-3 Fatty Acids during Pregnancy on Asthma or Wheeze of Children: A Systematic Review and Meta-Analysis. J. Matern. Fetal. Neonatal. Med. 2020, 33, 1792–1801. [Google Scholar] [CrossRef]
- Jia, Y.; Huang, Y.; Wang, H.; Jiang, H. A Dose-Response Meta-Analysis of the Association between the Maternal Omega-3 Long-Chain Polyunsaturated Fatty Acids Supplement and Risk of Asthma/Wheeze in Offspring. BMC Pediatr. 2022, 22, 422. [Google Scholar] [CrossRef]
- Venter, C.; Smith, P.K.; Arshad, H. Dietary Strategies for the Prevention of Asthma in Children. Curr. Opin. Allergy. Clin. Immunol. 2022, 22, 123–131. [Google Scholar] [CrossRef]
- Hu, F.B. Dietary Pattern Analysis: A New Direction in Nutritional Epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Lange, N.E.; Rifas-Shiman, S.L.; Camargo, C.A.; Gold, D.R.; Gillman, M.W.; Litonjua, A.A. Maternal Dietary Pattern during Pregnancy Is Not Associated with Recurrent Wheeze in Children. J. Allergy Clin. Immunol. 2010, 126, 250–255.e1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moonesinghe, H.; Patil, V.K.; Dean, T.; Arshad, S.H.; Glasbey, G.; Grundy, J.; Venter, C. Association between Healthy Eating in Pregnancy and Allergic Status of the Offspring in Childhood. Ann. Allergy Asthma. Immunol. 2016, 116, 163–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianchi, C.M.; Mariotti, F.; Verger, E.O.; Huneau, J.-F. Pregnancy Requires Major Changes in the Quality of the Diet for Nutritional Adequacy: Simulations in the French and the United States Populations. PLoS ONE 2016, 11, e0149858. [Google Scholar] [CrossRef] [PubMed]
- Kadawathagedara, M.; Kersuzan, C.; Wagner, S.; Tichit, C.; Gojard, S.; Charles, M.A.; Lioret, S.; de Lauzon-Guillain, B. Adéquation des consommations alimentaires des femmes enceintes de l’étude ELFE aux recommandations du Programme national nutrition santé. Cah. De Nutr. Et De Diététique 2017, 52, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-W.; Lyons, B.; Navarro, P.; Shivappa, N.; Mehegan, J.; Murrin, C.M.; Hébert, J.R.; Kelleher, C.C.; Phillips, C.M. Maternal Dietary Inflammatory Potential and Quality Are Associated with Offspring Asthma Risk over 10-Year Follow-up: The Lifeways Cross-Generation Cohort Study. Am. J. Clin. Nutr. 2020, 111, 440–447. [Google Scholar] [CrossRef]
- Heude, B.; Forhan, A.; Slama, R.; Douhaud, L.; Bedel, S.; Saurel-Cubizolles, M.-J.; Hankard, R.; Thiebaugeorges, O.; De Agostini, M.; Annesi-Maesano, I.; et al. Cohort Profile: The EDEN Mother-Child Cohort on the Prenatal and Early Postnatal Determinants of Child Health and Development. Int. J. Epidemiol. 2016, 45, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, V.; de Lauzon-Guillain, B.; Lafay, L.; Borys, J.-M.; Charles, M.A.; Romon, M. Reproducibility and Relative Validity of a Food-Frequency Questionnaire among French Adults and Adolescents. Eur. J. Clin. Nutr. 2009, 63, 282–291. [Google Scholar] [CrossRef] [Green Version]
- Hercberg, S.; Deheeger, M.; Preziosi, P. Portions Alimentaires: Manuel Photos Pour L’estimation Des Quantités; Polytechnica Edn.: Paris, France, 2002. [Google Scholar]
- Etude NutriNet-Santé. Table de Composition des Aliments: Etude NutriNet-Santé; Economica: Bobigny, France, 2013; ISBN 978-2-7178-6537-0. [Google Scholar]
- Haut Conseil de la Santé Publique. Avis Relatif à la Révision des Repères Alimentaires Pour les Femmes Enceintes et Allaitantes; Polytechnica Edn.: Paris, France, 2022. [Google Scholar]
- Ministère des Solidarités et de la Santé. Programme National Nutrition Santé 2019-2023; Ministère des Solidarités et de la Santé: Paris, France, 2019. [Google Scholar]
- Asher, M.I.; Keil, U.; Anderson, H.R.; Beasley, R.; Crane, J.; Martinez, F.; Mitchell, E.A.; Pearce, N.; Sibbald, B.; Stewart, A.W.; et al. International Study of Asthma and Allergies in Childhood (ISAAC): Rationale and Methods. Eur. Respir. J. 1995, 8, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Pénard-Morand, C.; Raherison, C.; Kopferschmitt, C.; Caillaud, D.; Lavaud, F.; Charpin, D.; Bousquet, J.; Annesi-Maesano, I. Prevalence of Food Allergy and Its Relationship to Asthma and Allergic Rhinitis in Schoolchildren. Allergy 2005, 60, 1165–1171. [Google Scholar] [CrossRef]
- Garcia-Aymerich, J.; Benet, M.; Saeys, Y.; Pinart, M.; Basagaña, X.; Smit, H.A.; Siroux, V.; Just, J.; Momas, I.; Rancière, F.; et al. Phenotyping Asthma, Rhinitis and Eczema in MeDALL Population-Based Birth Cohorts: An Allergic Comorbidity Cluster. Allergy 2015, 70, 973–984. [Google Scholar] [CrossRef]
- Ahmad, K.; Kabir, E.; Ormsby, G.M.; Khanam, R. Clustering of Asthma and Related Comorbidities and Their Association with Maternal Health during Pregnancy: Evidence from an Australian Birth Cohort. BMC Public Health 2021, 21, 1952. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Okubo, H.; Sasaki, S.; Tanaka, K.; Hirota, Y. Maternal Dietary Patterns during Pregnancy and Risk of Wheeze and Eczema in Japanese Infants Aged 16–24 Months: The Osaka Maternal and Child Health Study. Pediatr. Allergy Immunol. 2011, 22, 734–741. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, S.O.; Northstone, K.; Newson, R.B.; Emmett, P.M.; Sherriff, A.; Henderson, A.J. Dietary Patterns in Pregnancy and Respiratory and Atopic Outcomes in Childhood. Thorax 2009, 64, 411–417. [Google Scholar] [CrossRef] [Green Version]
- Zulyniak, M.A.; de Souza, R.J.; Shaikh, M.; Ramasundarahettige, C.; Tam, K.; Williams, N.; Desai, D.; Lefebvre, D.L.; Gupta, M.; Subbarao, P.; et al. Ethnic Differences in Maternal Diet in Pregnancy and Infant Eczema. PLoS ONE 2020, 15, e0232170. [Google Scholar] [CrossRef] [PubMed]
- Brzozowska, A.; Podlecka, D.; Jankowska, A.; Król, A.; Kaleta, D.; Trafalska, E.; Nowakowska-Świrta, E.; Kałużny, P.; Hanke, W.; Bal-Gierańczyk, K.; et al. Maternal Diet during Pregnancy and Risk of Allergic Diseases in Children up to 7–9 Years Old from Polish Mother and Child Cohort Study. Env. Res. 2022, 208, 112682. [Google Scholar] [CrossRef] [PubMed]
- Nwaru, B.I.; Ahonen, S.; Kaila, M.; Erkkola, M.; Haapala, A.-M.; Kronberg-Kippilä, C.; Veijola, R.; Ilonen, J.; Simell, O.; Knip, M.; et al. Maternal Diet during Pregnancy and Allergic Sensitization in the Offspring by 5 Yrs of Age: A Prospective Cohort Study. Pediatr. Allergy. Immunol. 2010, 21, 29–37. [Google Scholar] [CrossRef]
- Gao, X.; Yan, Y.; Zeng, G.; Sha, T.; Liu, S.; He, Q.; Chen, C.; Li, L.; Xiang, S.; Li, H.; et al. Influence of Prenatal and Early-Life Exposures on Food Allergy and Eczema in Infancy: A Birth Cohort Study. BMC Pediatr. 2019, 19, 239. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, N.; Shimojo, N.; Suzuki, Y.; Ochiai, S.; Nakano, T.; Morita, Y.; Inoue, Y.; Arima, T.; Suzuki, S.; Kohno, Y. Maternal Intake of Natto, a Japan’s Traditional Fermented Soybean Food, during Pregnancy and the Risk of Eczema in Japanese Babies. Allergol. Int. 2014, 63, 261–266. [Google Scholar] [CrossRef] [Green Version]
- Chatzi, L.; Torrent, M.; Romieu, I.; Garcia-Esteban, R.; Ferrer, C.; Vioque, J.; Kogevinas, M.; Sunyer, J. Mediterranean Diet in Pregnancy Is Protective for Wheeze and Atopy in Childhood. Thorax 2008, 63, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Kalbermatter, C.; Fernandez Trigo, N.; Christensen, S.; Ganal-Vonarburg, S.C. Maternal Microbiota, Early Life Colonization and Breast Milk Drive Immune Development in the Newborn. Front Immunol 2021, 12, 683022. [Google Scholar] [CrossRef]
- Willers, S.M.; Devereux, G.; Craig, L.C.A.; McNeill, G.; Wijga, A.H.; Abou El-Magd, W.; Turner, S.W.; Helms, P.J.; Seaton, A. Maternal Food Consumption during Pregnancy and Asthma, Respiratory and Atopic Symptoms in 5-Year-Old Children. Thorax 2007, 62, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, Y.; Sasaki, S.; Tanaka, K.; Hirota, Y. Consumption of Vegetables, Fruit, and Antioxidants during Pregnancy and Wheeze and Eczema in Infants. Allergy 2010, 65, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Baïz, N.; Just, J.; Chastang, J.; Forhan, A.; de Lauzon-Guillain, B.; Magnier, A.-M.; Annesi-Maesano, I. EDEN Mother-Child Cohort Study Group Maternal Diet before and during Pregnancy and Risk of Asthma and Allergic Rhinitis in Children. Allergy Asthma Clin. Immunol. 2019, 15, 40. [Google Scholar] [CrossRef] [Green Version]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Vafa, M.R.; Sharma, S.; Kolahdooz, F. Fruit and Vegetable Intake and Risk of Wheezing and Asthma: A Systematic Review and Meta-Analysis. Nutr. Rev. 2014, 72, 411–428. [Google Scholar] [CrossRef]
- Milewska-Wróbel, D.; Lis-Święty, A. Does Maternal Diet during Pregnancy Influence Clinical and Laboratory Characteristics of Infantile-Onset Atopic Dermatitis? Eur. Ann. Allergy Clin. Immunol. 2020, 52, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Castro-Rodriguez, J.A.; Ramirez-Hernandez, M.; Padilla, O.; Pacheco-Gonzalez, R.M.; Pérez-Fernández, V.; Garcia-Marcos, L. Effect of Foods and Mediterranean Diet during Pregnancy and First Years of Life on Wheezing, Rhinitis and Dermatitis in Preschoolers. Allergol. Immunopathol. 2016, 44, 400–409. [Google Scholar] [CrossRef]
- Sausenthaler, S.; Koletzko, S.; Schaaf, B.; Lehmann, I.; Borte, M.; Herbarth, O.; von Berg, A.; Wichmann, H.-E.; Heinrich, J.; LISA Study Group. Maternal Diet during Pregnancy in Relation to Eczema and Allergic Sensitization in the Offspring at 2 y of Age. Am. J. Clin. Nutr. 2007, 85, 530–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grieger, J.A.; Pelecanos, A.M.; Hurst, C.; Tai, A.; Clifton, V.L. Pre-Conception Maternal Food Intake and the Association with Childhood Allergies. Nutrients 2019, 11, 1851. [Google Scholar] [CrossRef] [Green Version]
- Tuokkola, J.; Luukkainen, P.; Tapanainen, H.; Kaila, M.; Vaarala, O.; Kenward, M.G.; Virta, L.J.; Veijola, R.; Simell, O.; Ilonen, J.; et al. Maternal Diet during Pregnancy and Lactation and Cow’s Milk Allergy in Offspring. Eur. J. Clin. Nutr. 2016, 70, 554–559. [Google Scholar] [CrossRef]
- Alvarez Zallo, N.; Aguinaga-Ontoso, I.; Alvarez-Alvarez, I.; Marin-Fernandez, B.; Guillén-Grima, F.; Azcona-San Julián, C. Influence of the Mediterranean Diet during Pregnancy in the Development of Wheezing and Eczema in Infants in Pamplona, Spain. Allergol. Immunopathol. 2018, 46, 9–14. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Rifas-Shiman, S.L.; Platts-Mills, T.A.; Workman, L.; Sordillo, J.E.; Camargo, C.A.; Gillman, M.W.; Gold, D.R.; Litonjua, A.A. Peanut, Milk, and Wheat Intake during Pregnancy Is Associated with Reduced Allergy and Asthma in Children. J. Allergy Clin. Immunol. 2014, 133, 1373–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelé, F.; Bajeux, E.; Gendron, H.; Monfort, C.; Rouget, F.; Multigner, L.; Viel, J.-F.; Cordier, S. Maternal Fish and Shellfish Consumption and Wheeze, Eczema and Food Allergy at Age Two: A Prospective Cohort Study in Brittany, France. Env. Health. 2013, 12, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oien, T.; Storrø, O.; Johnsen, R. Do Early Intake of Fish and Fish Oil Protect against Eczema and Doctor-Diagnosed Asthma at 2 Years of Age? A Cohort Study. J. Epidemiol. Community Health 2010, 64, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Noakes, P.S.; Vlachava, M.; Kremmyda, L.-S.; Diaper, N.D.; Miles, E.A.; Erlewyn-Lajeunesse, M.; Williams, A.P.; Godfrey, K.M.; Calder, P.C. Increased Intake of Oily Fish in Pregnancy: Effects on Neonatal Immune Responses and on Clinical Outcomes in Infants at 6 Mo. Am. J. Clin. Nutr. 2012, 95, 395–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratakis, N.; Roumeliotaki, T.; Oken, E.; Ballester, F.; Barros, H.; Basterrechea, M.; Cordier, S.; de Groot, R.; den Dekker, H.T.; Duijts, L.; et al. Fish and Seafood Consumption during Pregnancy and the Risk of Asthma and Allergic Rhinitis in Childhood: A Pooled Analysis of 18 European and US Birth Cohorts. Int. J. Epidemiol. 2017, 46, 1465–1477. [Google Scholar] [CrossRef]
- Romieu, I.; Torrent, M.; Garcia-Esteban, R.; Ferrer, C.; Ribas-Fitó, N.; Antó, J.M.; Sunyer, J. Maternal Fish Intake during Pregnancy and Atopy and Asthma in Infancy. Clin. Exp. Allergy 2007, 37, 518–525. [Google Scholar] [CrossRef]
- Jedrychowski, W.; Perera, F.; Maugeri, U.; Mrozek-Budzyn, D.; Miller, R.L.; Flak, E.; Mroz, E.; Jacek, R.; Spengler, J.D. Effects of Prenatal and Perinatal Exposure to Fine Air Pollutants and Maternal Fish Consumption on the Occurrence of Infantile Eczema. Int. Arch. Allergy. Immunol. 2011, 155, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Leermakers, E.T.M.; Sonnenschein-van der Voort, A.M.M.; Heppe, D.H.M.; de Jongste, J.C.; Moll, H.A.; Franco, O.H.; Hofman, A.; Jaddoe, V.W.V.; Duijts, L. Maternal Fish Consumption during Pregnancy and Risks of Wheezing and Eczema in Childhood: The Generation R Study. Eur. J. Clin. Nutr. 2013, 67, 353–359. [Google Scholar] [CrossRef] [Green Version]
- Calvani, M.; Alessandri, C.; Sopo, S.M.; Panetta, V.; Pingitore, G.; Tripodi, S.; Zappalà, D.; Zicari, A.M. Lazio Association of Pediatric Allergology (APAL) Study Group Consumption of Fish, Butter and Margarine during Pregnancy and Development of Allergic Sensitizations in the Offspring: Role of Maternal Atopy. Pediatr. Allergy Immunol. 2006, 17, 94–102. [Google Scholar] [CrossRef]
- Gochfeld, M.; Burger, J. Good Fish/Bad Fish: A Composite Benefit-Risk by Dose Curve. Neurotoxicology 2005, 26, 511–520. [Google Scholar] [CrossRef]
- Drouillet-Pinard, P.; Huel, G.; Slama, R.; Forhan, A.; Sahuquillo, J.; Goua, V.; Thiébaugeorges, O.; Foliguet, B.; Magnin, G.; Kaminski, M.; et al. Prenatal Mercury Contamination: Relationship with Maternal Seafood Consumption during Pregnancy and Fetal Growth in the “EDEN Mother-Child” Cohort. Br. J. Nutr. 2010, 104, 1096–1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadawathagedara, M.; Ahluwalia, N.; Dufourg, M.-N.; Forhan, A.; Charles, M.A.; Lioret, S.; de Lauzon-Guillain, B. Diet during Pregnancy: Influence of Social Characteristics and Migration in the ELFE Cohort. Matern. Child. Nutr. 2021, 17, e13140. [Google Scholar] [CrossRef] [PubMed]

| % (n) or Mean ± sd | |
|---|---|
| Center | |
| Poitiers | 49.2% (647) |
| Nancy | 50.8% (669) |
| Maternal age at delivery (years) | 29.7 ± 4.8 |
| Maternal education level | |
| Up to lower secondary | 4.2% (55) |
| Upper secondary | 18.4% (242) |
| Intermediate | 17.9% (235) |
| 2-year university degree | 23.8% (313) |
| ≥3-year university degree | 35.8% (471) |
| Household income (euros/month) | |
| <800 | 2.9% (38) |
| 801–1500 | 9.9% (130) |
| 1501–2300 | 28.1% (370) |
| 2301–3000 | 28.8% (379) |
| >3000 | 30.3% (399) |
| Smoking during pregnancy | 22.9% (301) |
| Pre-pregnancy BMI (kg/m2) | 23.1 ± 4.3 |
| Primiparity | 46.2% (608) |
| Season of birth | |
| Autumn/winter | 45.5% (599) |
| Spring/summer | 54.5% (717) |
| Boys | 52.6% (692) |
| Family history of allergy | 53.0% (698) |
| Gestational age (weeks) | 39.3 ± 1.6 |
| Birth weight (g) | 3290 ± 494 |
| Total | Allergic and Respiratory Multimorbidity Clusters | ||||
|---|---|---|---|---|---|
| Asymptomatic | Asthma Only | Allergies without Asthma | Multi-Allergic | ||
| n = 1593 | n = 1075 | n = 223 | n = 184 | n = 111 | |
| Food allergy (0–8 years) | 11.4% (181) | 4.8% (52) | 4.9% (11) | 40.2% (74) | 39.6% (44) |
| Eczema (0–8 years) | 26.0% (414) | 11.2% (120) | 7.2% (16) | 94.0% (173) | 94.6% (105) |
| Wheezing (0–8 years) | 40.4% (643) | 21.4% (230) | 94.6% (211) | 51.1% (94) | 97.3% (108) |
| Asthma medication (0–8 years) | 21.0% (335) | 2.0% (21) | 90.1% (201) | 1.1% (2) | 100.0% (111) |
| Asthma diagnosis (0–8 years) | 18.5% (295) | 0.7% (8) | 76.7% (171) | 6.5% (12) | 93.7% (104) |
| Rhinitis (1–8 years) | 20.0% (319) | 10.8% (116) | 19.7% (44) | 50.0% (92) | 60.4% (67) |
| Total | Allergic and Respiratory Multimorbidity Clusters | |||||
|---|---|---|---|---|---|---|
| Asymptomatic | Asthma Only | Allergies without Asthma | Multi-allergic | p-Value † | ||
| n = 1316 | n = 880 | n = 190 | n = 154 | n = 92 | ||
| Diet Quality score (range 0–17) | 12.1 ± 1.2 | 12.1 ± 1.2 | 12.0 ± 1.2 | 12.1 ± 1.2 | 12.0 ± 1.2 | 0.74 |
| PANDiet score (range 0–100) | 64.3 ± 6.9 | 64.3 ± 6.9 | 64.3 ± 7.1 | 64.6 ± 7.0 | 64.3 ± 6.7 | 0.95 |
| Fruit (times/day) | 1.5 ± 1.6 | 1.5 ± 1.5 | 1.7 ± 2.0 | 1.4 ± 1.3 | 1.6 ± 1.8 | 0.55 |
| Vegetables (times/day) | 1.8 ± 1.2 | 1.7 ± 1.2 | 1.9 ± 1.2 | 1.8 ± 1.1 | 1.8 ± 1.2 | 0.78 |
| Legumes (>1/month) | 47.1% (620) | 48.1% (423) | 43.2% (82) | 50.0% (77) | 41.3% (38) | 0.35 |
| Starch and grains (times/day) | 2.8 ± 1.1 | 2.8 ± 1.2 | 2.8 ± 1.2 | 2.7 ± 1.2 | 2.8 ± 0.9 | 0.82 |
| Nuts (consumption) | 49.5% (652) | 49.1% (432) | 50.0% (95) | 48.7% (75) | 54.3% (50) | 0.81 |
| Milk and dairy products (≥3 times/day) | 72.3% (952) | 72.4% (637) | 73.7% (140) | 69.5% (107) | 73.9% (68) | 0.82 |
| Fish and shellfish (≥2 times/week) | 26.3% (346) | 25.9% (228) | 31.6% (60) | 24.7% (38) | 21.7% (20) | 0.26 |
| Red meat (<500 g/week) | 79.5% (1046) | 79.4% (699) | 81.1% (154) | 77.3% (119) | 80.4% (74) | 0.85 |
| Processed meat (<150 g/week) | 73.3% (965) | 72.7% (640) | 73.2% (139) | 76.6% (118) | 73.9% (68) | 0.79 |
| Poultry (g/week) | 136 ± 131 | 135 ± 132 | 142 ± 134 | 137 ± 131 | 138 ± 107 | 0.97 |
| Sugar-sweetened beverages (mL/day) | 294 ± 400 | 287 ± 398 | 341 ± 438 | 235 ± 306 | 346 ± 462 | 0.03 * |
| Total energy intake (kcal/J) | 2191 ± 729 | 2161 ± 707 | 2297 ± 812 | 2183 ± 735 | 2272 ± 734 | 0.08 |
| Allergic and Respiratory Multimorbidity Clusters (Ref = Asymptomatic) | |||
|---|---|---|---|
| Asthma Only | Allergies without Asthma | Multi-Allergic | |
| Diet Quality score (range 0–17) | 1.01 (0.86;1.18) | 1.05 (0.89;1.24) | 1.01 (0.82;1.25) |
| PANDiet score (range 0–100) | 0.91 (0.71;1.17) | 1.09 (0.83;1.42) | 0.91 (0.65;1.28) |
| Fruit (times/day) | 1.05 (0.95;1.17) | 0.93 (0.81;1.07) | 1.03 (0.89;1.20) |
| Vegetables (times/day) | 1.12 (0.97;1.30) | 1.00 (0.84;1.19) | 1.07 (0.87;1.32) |
| Legumes | |||
| ≤1/month | 1.37 (0.98;1.91) | 0.98 (0.69;1.40) | 1.60 (1.01;2.54) * |
| >1/month | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Starch and grains (times/day) | 0.98 (0.84;1.15) | 0.91 (0.76;1.08) | 1.05 (0.84;1.30) |
| Nuts | |||
| No consumption | 1.05 (0.75;1.47) | 1.02 (0.71;1.46) | 0.90 (0.57;1.43) |
| Consumption | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Milk and dairy products | |||
| <3 times/day | 1.01 (0.67;1.53) | 1.30 (0.85;2.00) | 0.98 (0.56;1.71) |
| ≥3 times/day | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Fish and shellfish | |||
| <2 times/week | 0.75 (0.51;1.10) | 1.12 (0.73;1.74) | 1.35 (0.76;2.39) |
| ≥2 times/week | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| Red meat | |||
| <500 g/week | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| ≥500 g/week | 0.78 (0.51;1.19) | 1.10 (0.71;1.70) | 0.83 (0.46;1.49) |
| Processed meat | |||
| <150 g/week | 1 (Ref) | 1 (Ref) | 1 (Ref) |
| ≥150 g/week | 0.76 (0.52;1.13) | 0.79 (0.51;1.21) | 0.74 (0.43;1.25) |
| Poultry (g/week) | 1.01 (0.95;1.07) | 1.00 (0.94;1.08) | 1.00 (0.92;1.09) |
| Sugar-sweetened beverages (mL/day) | 1.00 (0.96;1.04) | 0.96 (0.91;1.02) | 1.01 (0.95;1.07) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delvert, R.; Ghozal, M.; Adel-Patient, K.; Kadawathagedara, M.; Heude, B.; Charles, M.-A.; Annesi-Maesano, I.; Tafflet, M.; Leynaert, B.; Varraso, R.; et al. Maternal Diet Quality during Pregnancy and Allergic and Respiratory Multimorbidity Clusters in Children from the EDEN Mother–Child Cohort. Nutrients 2023, 15, 146. https://doi.org/10.3390/nu15010146
Delvert R, Ghozal M, Adel-Patient K, Kadawathagedara M, Heude B, Charles M-A, Annesi-Maesano I, Tafflet M, Leynaert B, Varraso R, et al. Maternal Diet Quality during Pregnancy and Allergic and Respiratory Multimorbidity Clusters in Children from the EDEN Mother–Child Cohort. Nutrients. 2023; 15(1):146. https://doi.org/10.3390/nu15010146
Chicago/Turabian StyleDelvert, Rosalie, Manel Ghozal, Karine Adel-Patient, Manik Kadawathagedara, Barbara Heude, Marie-Aline Charles, Isabella Annesi-Maesano, Muriel Tafflet, Bénédicte Leynaert, Raphaëlle Varraso, and et al. 2023. "Maternal Diet Quality during Pregnancy and Allergic and Respiratory Multimorbidity Clusters in Children from the EDEN Mother–Child Cohort" Nutrients 15, no. 1: 146. https://doi.org/10.3390/nu15010146
APA StyleDelvert, R., Ghozal, M., Adel-Patient, K., Kadawathagedara, M., Heude, B., Charles, M. -A., Annesi-Maesano, I., Tafflet, M., Leynaert, B., Varraso, R., de Lauzon-Guillain, B., & Bédard, A. (2023). Maternal Diet Quality during Pregnancy and Allergic and Respiratory Multimorbidity Clusters in Children from the EDEN Mother–Child Cohort. Nutrients, 15(1), 146. https://doi.org/10.3390/nu15010146