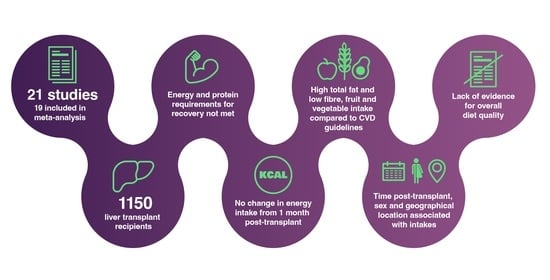
Nutritional Intake after Liver Transplant: Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Risk of Bias
2.6. Data Synthesis and Analysis
3. Results
3.1. Nutritional Intake
3.1.1. Narrative Synthesis
3.1.2. Meta-Analysis
3.2. Potential Determinants of Nutritional Intake
3.2.1. Narrative Synthesis
3.2.2. Meta-Regression
3.3. Risk of Bias (ROB)
3.4. Sensitivity Analysis
3.4.1. Risk of Bias
3.4.2. Converted Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef] [PubMed]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-Up Study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.A.; Garden, O.; Davidson, H. Reduction in energy expenditure after liver transplantation. Nutrition 2001, 17, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, H.S.; Anastácio, L.R.; Ferreira, L.; Lima, A.S.; Correia, M.I.T. Energy expenditure and balance among long term liver recipients. Clin. Nutr. 2014, 33, 1147–1152. [Google Scholar] [CrossRef]
- Richards, J.; Gunson, B.; Johnson, J.; Neuberger, J. Weight gain and obesity after liver transplantation. Transpl. Int. 2005, 18, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Akarsu, M.; Bakir, Y.; Karademir, S.; Unek, T.; Bacakoglu, A.; Astarcioglu, I. Prevalence and risk factors for obesity after liver transplantation: A single-center experience. Hepat. Mon. 2013, 13, e7569. [Google Scholar] [PubMed]
- Anastácio, L.R.; Ferreira, L.G.; Ribeiro, H.D.S.; Lima, A.S.; Vilela, E.G.; Correia, M.I.T.D. Body Composition and Overweight of Liver Transplant Recipients. Transplantation 2011, 92, 947–951. [Google Scholar] [CrossRef]
- Everhart, J.E.; Lombardero, M.; Lake, J.R.; Wiesner, R.H.; Zetterman, R.K.; Hoofnagle, J.H. Weight change and obesity after liver transplantation: Incidence and risk factors. Liver Transpl. Surg. 1998, 4, 285–296. [Google Scholar] [CrossRef]
- Ribeiro Hde, S.; Anastácio, L.R.; Ferreira, L.G.; Lagares, E.B.; Lima, A.S.; Correia, M.I.T.D. Prevalence and factors associated with dyslipidemia after liver transplantation. Rev. Assoc. Med. Bras. 2014, 60, 365–372. [Google Scholar] [CrossRef]
- Pinto, A.S.; Chedid, M.F.; Guerra, L.T.; Cabeleira, D.D.; Kruel, C.D.P. Dietary Management for dyslipidemia in liver transplant recipients. Braz. Arch. Dig. Surg. 2016, 29, 246–251. [Google Scholar] [CrossRef]
- Albeldawi, M.; Aggarwal, A.; Madhwal, S.; Cywinski, J.; Lopez, R.; Eghtesad, B.; Zein, N.N. Cumulative risk of cardiovascular events after orthotopic liver transplantation. Liver Transplant. 2012, 18, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, S.M.; Taghavi, S.A.R.; Eshraghian, A.; Gholami, S.; Imanieh, M.H.; Bordbar, M.R.; Malek-Hosseini, S.A. Hyperlipidemia in Iranian liver transplant recipients: Prevalence and risk factors. J. Gastroenterol. 2007, 42, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D.; Danaei, G.; Shibuya, K.; Adair-Rohani, H.; AlMazroa, M.A.; Amann, M.; Anderson, H.R.; Andrews, K.G.; et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2224–2260. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Hooper, L.; Martin, N.; Jimoh, O.F.; Kirk, C.; Foster, E.; Abdelhamid, A.S. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database Syst. Rev. 2020, 5, CD011737. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef]
- Morze, J.; Danielewicz, A.; Przybyłowicz, K.; Zeng, H.; Hoffmann, G.; Schwingshackl, L. An updated systematic review and meta-analysis on adherence to mediterranean diet and risk of cancer. Eur. J. Nutr. 2021, 60, 1561–1586. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: Systematic review and dose-response meta-analysis of prospective studies. BMJ 2016, 353, i2716. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Critical Appraisal Skills Programme. CASP Cohort Study Checklist. Available online: https://casp-uk.b-cdn.net/wp-content/uploads/2018/03/CASP-Cohort-Study-Checklist-2018_fillable_form.pdf (accessed on 3 July 2019).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; White, I.R.; Anzures-Cabrera, J. Meta-analysis of skewed data: Combining results reported on log-transformed or raw scales. Stat. Med. 2008, 27, 6072–6092. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.T.J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (Updated February 2022); John Wiley & Sons: Hoboken, NJ, USA, 2022; Available online: www.training.cochrane.org/handbook (accessed on 3 March 2022).
- Bender, D. Introduction to Nutrition and Metabolism, 4th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Hanley, J.A.; Negassa, A.; Edwardes, M.D.D.; Forrester, J.E. Statistical Analysis of Correlated Data Using Generalized Estimating Equations: An Orientation. Am. J. Epidemiol. 2003, 157, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, L.R.; Ribeiro Hde, S.; Ferreira, L.G.; Lima, A.S.; Vilela, E.G.; Correia, M.I.T.D. Incidence and risk factors for diabetes, hypertension and obesity after liver transplantation. Nutr. Hosp. 2013, 28, 643–648. [Google Scholar] [PubMed]
- Anastácio, L.R.; Diniz, K.G.; Ribeiro, H.S.; Ferreira, L.G.; Lima, A.S.; Correia, M.I.; Vilela, E.G. Prospective evaluation of metabolic syndrome and its components among long-term liver recipients. Liver Int. 2014, 34, 1094–1101. [Google Scholar] [CrossRef]
- Bakshi, N.; Singh, K. Nutrition management of acute postliver transplant recipients. Indian J. Crit. Care Med. 2018, 22, 773–780. [Google Scholar] [CrossRef]
- Alves, B.C.; Bruch-Bertani, J.P.; Galinatti, C.B.M.; Garbin, C.C.; Álvares-da-Silva, M.R.; Dall’Alba, V. Obesity, dynapenia and high cardiovascular risk co-exist in post-liver transplant setting: Results of a cross-sectional study. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 140–147. [Google Scholar] [CrossRef]
- Andrade, C.P.R.; Portugal, M.L.T.; Paz, E.S.; Freitas, P.A.; Daltro, A.F.C.S.; Almondes, K.G.S.; Maia, C.S.C.; Oliveira, A.C. Influence of liver transplantation in the nutritional profile of severe cirrhotic patients. Nutr. Hosp. 2018, 35, 104–109. [Google Scholar]
- Bajaj, J.S.; Kakiyama, G.; Cox, I.J.; Nittono, H.; Takei, H.; White, M.; Fagan, A.; Gavis, E.A.; Heuman, D.M.; Gilles, H.C.; et al. Alterations in gut microbial function following liver transplant. Liver Transplant. 2018, 24, 752–761. [Google Scholar] [CrossRef]
- Brito-Costa, A.; Pereira-da-Silva, L.; Papoila, A.L.; Alves, M.; Mateus, É.; Nolasco, F.; Barroso, E. Factors Associated with Changes in Body Composition Shortly After Orthotopic Liver Transplantation: The Potential Influence of Immunosuppressive Agents. Transplantation 2016, 100, 1714–1722. [Google Scholar] [CrossRef]
- Brito-Costa, A.; Pereira-Da-Silva, L.; Papoila, A.; Alves, M.; Mateus, É.; Nolasco, F.; Barroso, E. Preoperative metabolic status is associated with different evolution of resting energy expenditure after liver transplant in adults. Nutr. Hosp. 2017, 34, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Alves, V.; Hack Mendes, R.; Pinto Kruel, C.D. Nutritional status, lipid profile and HOMA-IR in post-liver transplant patients. Nutr. Hosp. 2014, 29, 1154–1162. [Google Scholar] [PubMed]
- De Carvalho, L.; Parise, E.R.; Samuel, D. Factors associated with nutritional status in liver transplant patients who survived the first year after transplantation. J. Gastroenterol. Hepatol. 2010, 25, 391–396. [Google Scholar] [CrossRef] [PubMed]
- De Luis, D.A.; Izaola, O.; Velicia, M.C.; Antolín, G.S.; Pajares, F.G.; Terroba, M.C.; Cuéllar, L. Impact of dietary intake and nutritional status on outcomes after liver transplantation. Rev. Esp. Enferm. Dig. 2006, 98, 6–13. [Google Scholar] [PubMed]
- Ferreira, L.G.; Santos, L.F.; Anastácio, L.R.; Lima, A.S.; Correia, M.I. Resting energy expenditure, body composition, and dietary intake: A longitudinal study before and after liver transplantation. Transplantation 2013, 96, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Hickman, I.J.; Hannigan, A.K.B.; Johnston, H.E.B.; Elvin-Walsh, L.B.; Mayr, H.L.; Staudacher, H.M.; Barnett, A.M.; Stoney, R.; Salisbury, C.B.; Jarrett, M.B.; et al. Telehealth-delivered, Cardioprotective Diet and Exercise Program for Liver Transplant Recipients: A Randomized Feasibility Study. Transplant. Direct 2021, 7, e667. [Google Scholar] [CrossRef]
- Jagielska, A.; Jankowski, K.; Okręglicka, K.; Skupin, P.; Kozłowska, A.; Durlik, M.; Pruszczyk, P.; Nitsch-Osuch, A.; Tronina, O. Lifestyle risk factors of non-communicable diseases among patients after liver transplantation—A pilot study. Fam. Med. Prim. Care Rev. 2017, 3, 214–220. [Google Scholar] [CrossRef]
- Krasnoff, J.B.; Vintro, A.Q.; Ascher, N.L.; Bass, N.M.; Paul, S.M.; Dodd, M.J.; Painter, P.L. A Randomized Trial of Exercise and Dietary Counseling After Liver Transplantation. Am. J. Transplant. 2006, 6, 1896–1905. [Google Scholar] [CrossRef]
- Lunati, M.E.; Grancini, V.; Agnelli, F.; Gatti, S.; Masserini, B.; Zimbalatti, D.; Pugliese, G.; Rossi, G.; Donato, M.F.; Colombo, M.; et al. Metabolic syndrome after liver transplantation: Short-term prevalence and pre- and post-operative risk factors. Dig. Liver Dis. 2013, 45, 833–883. [Google Scholar] [CrossRef]
- McCoy, S.M.; Campbell, K.L.; Lassemillante, A.-C.M.; Wallen, M.P.; Fawcett, J.; Jarrett, M.; Macdonald, G.A.; Hickman, I.J. Changes in dietary patterns and body composition within 12 months of liver transplantation. HepatoBiliary Surg. Nutr. 2017, 6, 317–326. [Google Scholar] [CrossRef]
- Merli, M.; Giusto, M.; Riggio, O.; Gentili, F.; Molinaro, A.; Attili, A.F.; Corradini, S.G.; Rossi, M. Improvement of nutritional status in malnourished cirrhotic patients one year after liver transplantation. e-SPEN Eur. e-J. Clin. Nutr. Metab. 2011, 6, e142–e147. [Google Scholar] [CrossRef]
- Ribeiro, H.S.; Coury, N.C.; de Vasconcelos Generoso, S.; Lima, A.S.; Correia, M. Energy Balance and Nutrition Status: A Prospective Assessment of Patients Undergoing Liver Transplantation. Nutr. Clin. Pract. 2020, 35, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.F.; Monteze, N.M.; Fagundes, G.B.P.; Monteiro, B.L.; Lima, A.S.; Correia, M.I.T.D.; Generoso, S.D.V.; Ferreira, A.V.M. Hypometabolism as a potential risk factor for overweight and obesity in liver recipients. Nutrition 2019, 61, 16–20. [Google Scholar] [CrossRef]
- World Health Organization; Food and Agriculture Organization; United Nations University. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization Technical Report Series; WHO: Geneva, Switzerland, 2007; 265p.
- World Health Organization. Fact Sheet No 394: Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 23 March 2022).
- U.S. Department of Agriculture; U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025, 9th ed.; U.S. Department of Agriculture: Washington, DC, USA, 2020.
- Scientific Advisory Committee on Nutrition. Carbohydrates and Health; The Stationary Office: London, UK, 2015. [Google Scholar]
- Lindqvist, C.; Nordstedt, P.; Vidgren, M.; Nowak, G. Protein intake early after liver transplantation and postoperative outcome: An observational study comparing two nutritional protocols. Nutrition 2022, 98, 111635. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Nikolic, N.; Denhaerynck, K.; Binet, I.; Koller, M.; Boely, E.; De Geest, S.; Psychosocial Interest Group; Swiss Transplant Cohort Study. Evolution of body weight parameters up to 3 years after solid organ transplantation: The prospective Swiss Transplant Cohort Study. Clin. Transplant. 2017, 31, e12896. [Google Scholar] [CrossRef] [PubMed]
- Rezende Anastácio, L.; García Ferreira, L.; Costa Liboredo, J.; de Sena Ribeiro, H.; Soares Lima, A.; García Vilela, E.; Correia, M.I. Overweight, obesity and weight gain up to three years after liver transplantation. Nutr. Hosp. 2012, 27, 1351–1356. [Google Scholar]
- Madhwal, S.; Atreja, A.; Albeldawi, M.; Lopez, R.; Post, A.; Costa, M.A. Is liver transplantation a risk factor for cardiovascular disease? A meta-analysis of observational studies. Liver Transpl. 2012, 18, 1140–1146. [Google Scholar] [CrossRef]
- Van den Berg-Emons, R.; Kazemier, G.; van Ginneken, B.; Nieuwenhuijsen, C.; Tilanus, H.; Stam, H. Fatigue, level of everyday physical activity and quality of life after liver transplantation. J. Rehabil. Med. 2006, 38, 124–129. [Google Scholar] [CrossRef]
- Jagielska, A.; Tronina, O.; Jankowski, K.; Kozłowska, A.; Okręglicka, K.; Jagielski, P.; Durlik, M.; Pruszczyk, P.; Nitsch–Osuch, A. Factors Affecting Health-Related Quality of Life in Liver Transplant Patients. Adv. Exp. Med. Biol. 2018, 1133, 49–54. [Google Scholar]
- Krasnoff, J.B.; Vintro, A.Q.; Ascher, N.L.; Bass, N.M.; Dodd, M.J.; Painter, P.L. Objective measures of health-related quality of life over 24 months post-liver transplantation. Clin. Transplant. 2005, 19, 1–9. [Google Scholar] [CrossRef]
- Rana, A.; Witte, E.D.; Halazun, K.J.; Sood, G.K.; Mindikoglu, A.L.; Sussman, N.L.; Vierling, J.M.; Kueht, M.L.; Galvan, N.T.N.; Cotton, R.T.; et al. Liver transplant length of stay (LOS) index: A novel predictive score for hospital length of stay following liver transplantation. Clin. Transplant. 2017, 31, e13141. [Google Scholar] [CrossRef] [PubMed]
- Francke, A.L.; Smit, M.C.; de Veer, A.J.; Mistiaen, P. Factors influencing the implementation of clinical guidelines for health care professionals: A systematic meta-review. BMC Med. Inform. Decis. Mak. 2008, 8, 38. [Google Scholar] [CrossRef] [PubMed]
- Tapsell, L.C.; Neale, E.P.; Satija, A.; Hu, F.B. Foods, Nutrients, and Dietary Patterns: Interconnections and Implications for Dietary Guidelines. Adv. Nutr. Int. Rev. J. 2016, 7, 445–454. [Google Scholar] [CrossRef]
- Petersen, K.S.; Kris-Etherton, P.M. Diet Quality Assessment and the Relationship between Diet Quality and Cardiovascular Disease Risk. Nutrients 2021, 13, 4305. [Google Scholar] [CrossRef]
- Stok, F.M.; Hoffmann, S.; Volkert, D.; Boeing, H.; Ensenauer, R.; Stelmach-Mardas, M.; Kiesswetter, E.; Weber, A.; Rohm, H.; Lien, N.; et al. The DONE framework: Creation, evaluation, and updating of an interdisciplinary, dynamic framework 2.0 of determinants of nutrition and eating. PLoS ONE 2017, 12, e0171077. [Google Scholar] [CrossRef]
- Plank, L.D.; Russell, K. Nutrition in liver transplantation: Too little or too much? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Micha, R.; Khatibzadeh, S.; Fahimi, S.; Shi, P.; Powles, J.; Mozaffarian, D. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group. Dietary quality among men and women in 187 countries in 1990 and 2010: A systematic assessment. Lancet Glob. Health 2015, 3, e132–e142. [Google Scholar] [CrossRef]
- Hwang, S.; Choi-Kwon, S. Nutritional Status of Liver Transplantation Recipients and Factors Influencing Nutritional Status. J. Korean Acad. Nurs. 2019, 49, 340–348. [Google Scholar] [CrossRef]
- Salyer, J.; An, K.; Siddiqui, S. Abstract P046: Cardiometabolic Risk Liver Transplant Survivors. Circulation 2017, 135, AP046. [Google Scholar] [CrossRef]
- De Simone, P.; Ducci, J.; Elisei, M.; Petruccelli, S.; Giannessi, E.; Magni, B.; Masetti, M.; Meocci, F.; Baldoni, L.; Filipponi, F. The dietary habits of liver transplant recipients: Results of a single-center, cross-sectional analysis. Liver Transplant. 2011, 7, S169. [Google Scholar]
- Bahari, H.; Aliakbarian, M.; Norouzy, A.; Mansourian, M.; Akhavan-Rezayat, K.; Khadem-Rezaiyan, M.; Esfehani, A.J.; Nematy, M. Assessment of the nutritional status of patients before, one, and three months after liver transplantation: A multi-center longitudinal study. Clin. Nutr. ESPEN 2022, 53, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Cade, J.E.; Consortium, O.B.O.T.D.; Warthon-Medina, M.; Albar, S.; Alwan, N.A.; Ness, A.; Roe, M.; Wark, P.A.; Greathead, K.; Burley, V.J.; et al. DIET@NET: Best Practice Guidelines for dietary assessment in health research. BMC Med. 2017, 15, 202. [Google Scholar] [CrossRef] [PubMed]




| Author, Year * | Country | Study Design | Participant Details | Time Post-Transplant | Dietary Assessment Method |
|---|---|---|---|---|---|
| Alves et al., 2019 [30] | Brazil | Cross-sectional | n: 69 Median age (IQR): 51.6 (51–64) Males (n): 42 Females (n): 27 | Median (IQR) Normal CIMT: 1 (4–9) year Abnormal CIMT: 2 (1–4) years | 3-day food diary |
| Anastacio et al., 2013 [27]; 2014 [28] | Brazil | Prospective cohort | n: 148 Median age (range): 51.5 (21–75) Males (n): 90 Females (n): 58 | Median (range) Baseline: 3 (0–13) years Follow-up: 7 (3–17) years | Diet history +/− 3-day food diary |
| Andrade et al., 2018 [31] | Brazil | Prospective cohort | n: 23 Age range: 18–65 Males (n): 11 Females (n): 12 | 3 months | 3 24-h recalls |
| Bajaj et al., 2018 [32] | USA | Prospective cohort | n: 40 Mean (SD) age: 56 (7) Males (n): 35 Females (n): 5 | Mean (SD): 7 (3) months | 1 24-h recall |
| Bakshi and Singh, 2018 [29] | India | Prospective cohort | n: 54 Mean (SD) age: 48.6 (10.2) Males (n): 39 Females (n): 15 | Range: 1–12 days | Hospital records + 2 24-h recalls |
| Brito-Costa et al., 2016 [33]; 2017 [34] | Portugal | Prospective cohort | n: 56 Mean age (SD): 53.7 (8.5) Males (n): 49 Females (n): 7 | Median (IQR): Time 1: 9 (7–12) days, Time 2: 36 (31–43) days | 1 24-h recall |
| Da Silva Alves et al., 2014 [35] | Brazil | Cross-sectional | n: 36 Mean (SD) age: 53.3 (10.6) Males (n): 22 Females (n): 14 | Range: 1–24 months | 1 24-h recall |
| De Carvalho et al., 2010 [36] | France | Prospective cohort | n: 70 Mean (range) age: 47.5 (23–69) Males (n): 41 Females (n): 15 | 45, 90, 180 and 365 days | 3-day food diary |
| De Luis et al., 2006 [37] | Spain | Prospective cohort | n: 31 Mean (SD) age: 56.2 (8.1) Males (n): 25 Females (n): 6 | 6 months | 3-day food diary |
| Ferreira et al., 2013 [38] | Brazil | Prospective cohort | n: 17 Mean (range) age: 52 (29–65) Males (n): 12 Females (n): 5 | Mean (SD): Time 1: 41 (20) days Time 2: 110 (26) days Time 3: 192 (14) days Time 4: 287 (24) days Time 5: 379 (96) days. | 3-day food diary |
| Hickman et al., 2021 [39] | Australia | Randomised (feasibility) controlled trial | n: 35 Mean (SD) age, intervention: 50 (15), control: 51 (15) Males (n): 25 Females (n): 10 | Median (IQR): 4 (2–6) years | Mediterranean diet adherence screener |
| Jagielska et al., 2017 [40] | Poland | Cross-sectional | n: 44 Mean (SD) age: 51.5 (11) Males (n): 31 Females (n): 24 | Not reported | 7-day food diary |
| Krasnoff et al., 2006 [41] | USA | Randomised control trial | n: 151 Mean (SD) age, intervention: 49.5 (11.3), usual care: 50.6 (11.3) Males (n): 47 Females (n): 72 | 2, 6 and 12 months | The Block 95 full-length dietary questionnaire |
| Lunati et al., 2013 [42] | Italy | Prospective cohort | n: 84 Mean (SD) age: 53.9 (9.3) Males (n): 63 Females (n): 21 | 3, 6 and 12 months | 3-day food diary |
| McCoy et al., 2017 [43] | Australia | Prospective cohort | n: 17 Median (IQR) age: 54 (16) Males (n): 14 Females (n): 3 | 6 and 12 months | Diet history |
| Merli et al., 2011 [44] | Italy | Prospective cohort | n: 25 Median (range) age: 55 (21–64) Males (n): 19 Females (n): 6 | 3, 6 and 12 months | Diet interviews |
| Ribeiro et al., 2014 [4] | Brazil | Cross-sectional | n: 42 Mean (SD) age: 50.1 (13.1) Males (n): 22 Females (n): 20 | Mean (range): 6.5 (1.1–15) years | 3-day food diary |
| Ribeiro et al., 2014 [9] | Brazil | Cross-sectional | n: 136 Mean (SD) age: 52.2 (13) Males (n): 83 Females (n): 53 | Mean (SD): 4 (3) years | Diet history |
| Ribeiro et al., 2020 [45] | Brazil | Prospective cohort | n: 29 Mean (SD) age: 54.1 (11.5) Males (n): 23 Females (n): 6 | Mean (SD): Time 1: 2.4 (1.2) days Time 2: 8.1 (2.8) days | 3-day food diary |
| Richardson et al., 2001 [3] | UK | Prospective cohort | n: 23 Mean (SD) age: 53.9 (1.9) Males (n): 10 Females (n): 13 | 3, 6 and 12 months | 3-day food diary |
| Rodrigues et al., 2019 [46] | Brazil | Cross-sectional | n: 20 Mean (SD) age: 50 (3) Males (n): 14 Females (n): 6 | Mean (SD): 26 (2) months | 3-day food diary |
| Nutrient Reference Period * | Comparand * | Coefficient | 95% CI | p-Values | I2 | |
|---|---|---|---|---|---|---|
| Energy (kcal) | ||||||
| <1 month | 1 to <6 months | 608.9 | 292.0 | 925.8 | 0.002 | 63.0 |
| 6 to <12 months | 251.3 | 100.3 | 402.4 | 0.003 | 74.2 | |
| ≥12 months | 135.1 | 23.8 | 246.5 | 0.021 | 95.1 | |
| 1 to <6 months | 6 to <12 months | −110.1 | −330.8 | 110.5 | 0.307 | 62.1 |
| ≥12 months | −103.7 | −224.8 | 17.4 | 0.089 | 93.4 | |
| 6 to <12 months | ≥12 months | −101.5 | −311.8 | 108.9 | 0.328 | 92.9 |
| Protein (% energy) | ||||||
| <1 month | 1 to <6 months | −1.9 | −5.8 | 1.9 | 0.283 | 87.2 |
| 6 to <12 months | −1.1 | −2.8 | 0.5 | 0.160 | 86.3 | |
| ≥12 months | −0.7 | −1.7 | 0.3 | 0.163 | 86.2 | |
| 1 to <6 months | 6 to <12 months | −0.3 | −3.1 | 2.4 | 0.813 | 88.9 |
| ≥12 months | −0.1 | −1.3 | 1.2 | 0.940 | 88.7 | |
| 6 to <12 months | ≥12 months | 0.2 | −2.0 | 2.4 | 0.843 | 88.1 |
| Carbohydrate (% energy) | ||||||
| <1 month | 1 to <6 months † | |||||
| 6 to <12 months | 0.7 | −4.5 | 6.0 | 0.748 | 77.7 | |
| ≥12 months | 1.0 | −1.2 | 3.2 | 0.338 | 79.9 | |
| 1 to <6 months | 6 to <12 months | 1.0 | −5.6 | 6.7 | 0.659 | 73.2 |
| ≥12 months | 0.4 | −1.3 | 2.0 | 0.630 | 76.1 | |
| 6 to <12 months | ≥12 months | 1.5 | −2.1 | 5.0 | 0.399 | 79.0 |
| Fat (% energy) | ||||||
| <1 month | 1 to <6 months | 0.1 | −1.3 | 1.6 | 0.748 | 79.3 |
| 6 to <12 months † | ||||||
| ≥12 months | 0.2 | −4.1 | 4.6 | 0.659 | 75.4 | |
| 1 to <6 months | 6 to <12 months | 1.8 | −3.0 | 6.6 | 0.630 | 81.9 |
| ≥12 months | 0.4 | −1.4 | 2.1 | 0.399 | 81.6 | |
| 6 to <12 months | ≥12 months | −1.1 | −4.2 | 2.0 | 0.748 | 76.4 |
| Saturated fat (% energy) | ||||||
| <1 month | 1 to <6 months † | |||||
| 6 to <12 months † | ||||||
| ≥12 months † | ||||||
| 1 to <6 months | 6 to <12 months † | |||||
| ≥12 months | 1.9 | −0.3 | 4.0 | 0.087 | 96.6 | |
| 6 to <12 months | ≥12 months | −0.3 | −3.1 | 2.4 | 0.781 | 86.5 |
| Study-Level Variable * | Coef | 95% CI | p-Values | I2 | |
|---|---|---|---|---|---|
| Aetiology—proportion with ARLD (15 studies) | |||||
| Energy (kcal) | −1.0 | −7.5 | 5.4 | 0.746 | 89.9 |
| Protein (% energy) | 0.0 | 0.0 | 0.1 | 0.184 | 85.4 |
| Carbohydrate (% energy) | 0.0 | −0.1 | 0.1 | 0.512 | 67.8 |
| Fat (% energy) | 0.0 | −0.1 | 0.1 | 0.736 | 77.5 |
| Saturated fat (% energy) | |||||
| Average age (18 studies) | |||||
| Energy (kcal) | 49.7 | 9.0 | 90.4 | 0.018 | 88.1 |
| Protein (% energy) | 0.0 | −0.4 | 0.4 | 0.901 | 87.5 |
| Carbohydrate (% energy) | −0.5 | −1.2 | −0.2 | 0.148 | 78.7 |
| Fat (% energy) | 0.5 | −0.1 | 1.1 | 0.083 | 74.2 |
| Saturated fat (% energy) | 0.0 | −0.4 | 0.4 | 0.988 | 85.0 |
| Sex—proportion male (18 studies) | |||||
| Energy (kcal) | −0.6 | −7.2 | 6.0 | 0.848 | 92.2 |
| Protein (% energy) | 0.0 | −0.0 | 0.1 | 0.237 | 88.2 |
| Carbohydrate (% energy) | −0.1 | −0.1 | 0.0 | 0.083 | 73.0 |
| Fat (% energy) | 0.0 | −0.1 | 0.1 | 0.948 | 77.2 |
| Saturated fat (% energy) | 0.2 | 0.1 | 0.3 | 0.001 | 86.8 |
| Year of publication (19 studies) | |||||
| Energy (kcal) | −20.9 | −40.2 | −1.7 | 0.034 | 89.9 |
| Protein (% energy) | 0.3 | 0.1 | 0.4 | <0.001 | 87.4 |
| Carbohydrate (% energy) | 0.0 | −0.3 | 0.3 | 0.923 | 79.1 |
| Fat (% energy) | −0.3 | −0.5 | −0.1 | 0.014 | 75.0 |
| Saturated fat (% energy) | −0.1 | −0.9 | 0.7 | 0.798 | 98.8 |
| Continent—Europe vs. other (19 studies) | |||||
| Energy (kcal) | 200.6 | 7.6 | 393.6 | 0.042 | 88.7 |
| Protein (% energy) | −2.5 | −4.1 | −0.9 | 0.004 | 88.2 |
| Carbohydrate (% energy) | 2.3 | −0.6 | 5.2 | 0.110 | 73.9 |
| Fat (% energy) | 3.7 | 1.2 | 6.2 | 0.005 | 73.3 |
| Saturated fat (% energy) | 1.1 | −2.6 | 4.7 | 0.528 | 98.9 |
| Dietary assessment method—food diary vs. other (19 studies) | |||||
| Energy (kcal) | −41.4 | −144.0 | 61.3 | 0.418 | 92.4 |
| Protein (% energy) | −0.4 | −1.3 | 0.5 | 0.395 | 88.5 |
| Carbohydrate (% energy) | −0.3 | −1.8 | 1.3 | 0.746 | 79.0 |
| Fat (% energy) | 0.5 | −1.0 | 1.9 | 0.520 | 78.8 |
| Saturated fat (% energy) | 2.3 | −0.2 | 4.7 | 0.065 | 97.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spillman, L.N.; Madden, A.M.; Richardson, H.; Imamura, F.; Jones, D.; Nash, M.; Lim, H.K.; Hellawell, H.N.; Rennie, K.L.; Oude Griep, L.M.; et al. Nutritional Intake after Liver Transplant: Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2487. https://doi.org/10.3390/nu15112487
Spillman LN, Madden AM, Richardson H, Imamura F, Jones D, Nash M, Lim HK, Hellawell HN, Rennie KL, Oude Griep LM, et al. Nutritional Intake after Liver Transplant: Systematic Review and Meta-Analysis. Nutrients. 2023; 15(11):2487. https://doi.org/10.3390/nu15112487
Chicago/Turabian StyleSpillman, Lynsey N., Angela M. Madden, Holly Richardson, Fumiaki Imamura, Danielle Jones, Marilyn Nash, Hong Kai Lim, Holly N. Hellawell, Kirsten L. Rennie, Linda M. Oude Griep, and et al. 2023. "Nutritional Intake after Liver Transplant: Systematic Review and Meta-Analysis" Nutrients 15, no. 11: 2487. https://doi.org/10.3390/nu15112487
APA StyleSpillman, L. N., Madden, A. M., Richardson, H., Imamura, F., Jones, D., Nash, M., Lim, H. K., Hellawell, H. N., Rennie, K. L., Oude Griep, L. M., Allison, M., & Griffin, S. J. (2023). Nutritional Intake after Liver Transplant: Systematic Review and Meta-Analysis. Nutrients, 15(11), 2487. https://doi.org/10.3390/nu15112487