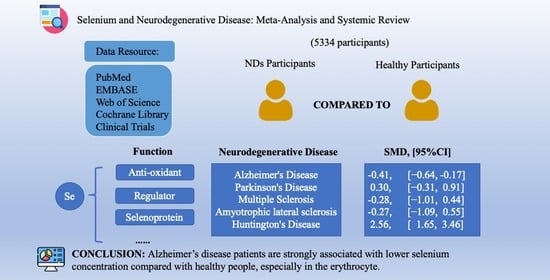
Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis
Abstract
:1. Introduction
2. Methods and Materials
2.1. Protocol and Registration
2.2. Literature Searching Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection
3.2. Baseline Characteristics of Included Studies
3.3. Analysis of Selenium and AD
3.4. The Relationship between Selenium and PD, MS, ALS and HD
3.5. Assessment of Publication Bias
4. Discussion
4.1. AD
4.2. PD
4.3. MS
4.4. ALS
4.5. HD
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. Met. Ions. Life Sci. 2013, 13, 359–387. [Google Scholar]
- Ward, R.J.; Zucca, F.A.; Duyn, J.H.; Crichton, R.R.; Zecca, L. The role of iron in brain ageing and neurodegenerative disorders. Lancet Neurol. 2014, 13, 1045–1060. [Google Scholar]
- Mezzaroba, L.; Alfieri, D.F.; Colado Simão, A.N.; Vissoci Reiche, E.M. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Stuss, M.; Michalska-Kasiczak, M.; Sewerynek, E. The role of selenium in thyroid gland pathophysiology. Endokrynol. Pol. 2017, 68, 440–465. [Google Scholar] [CrossRef]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Roberts, B.R.; Bush, A.I.; Hare, D.J. Selenium, selenoproteins and neurodegenerative diseases. Metallomics 2015, 7, 1213–1228. [Google Scholar]
- Dexter, D.; Carter, C.; Wells, F.; Javoy-Agid, F.; Agid, Y.; Lees, A.; Jenner, P.; Marsden, C.D. Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J. Neurochem. 1989, 52, 381–389. [Google Scholar] [CrossRef]
- Aaseth, J.; Skalny, A.V.; Roos, P.M.; Alexander, J.; Aschner, M.; Tinkov, A.A. Copper, Iron, Selenium and Lipo-Glycemic Dysmetabolism in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 9461. [Google Scholar] [CrossRef]
- Whanger, P. Selenium and the brain: A review. Nutr. Neurosci. 2001, 4, 81–97. [Google Scholar] [CrossRef] [PubMed]
- Öz, A.; Çelik, Ö. Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: Involvement of transfection procedure. Mol. Membr. Biol. 2016, 33, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Rita Cardoso, B.; Apolinário, D.; da Silva Bandeira, V.; Busse, A.L.; Magaldi, R.M.; Jacob-Filho, W.; Cozzolino, S.M. Effects of Brazil nut consumption on selenium status and cognitive performance in older adults with mild cognitive impairment: A randomized controlled pilot trial. Eur. J. Nutr. 2016, 55, 107–116. [Google Scholar] [PubMed]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of plasma magnesium, copper, zinc, iron and selenium concentrations and some related erythrocyte antioxidant enzyme activities in patients with Alzheimer’s disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Cornett, C.R.; Ehmann, W.D.; Wekstein, D.R.; Markesbery, W.R. Trace elements in Alzheimer’s disease pituitary glands. Biol. Trace Elem. Res. 1998, 62, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Meseguer, I.; Molina, J.A.; Jiménez-Jiménez, F.J.; Aguilar, M.V.; Mateos-Vega, C.J.; González-Muñoz, M.J.; de Bustos, F.; Ortí-Pareja, M.; Zurdo, M.; Berbel, A.; et al. Cerebrospinal fluid levels of selenium in patients with Alzheimer’s disease. J. Neural Transm. 1999, 106, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Rani, P. Evaluation of selenium, redox status and their association with plasma amyloid/tau in Alzheimer’s disease. Biol. Trace Elem. Res. 2014, 158, 158–165. [Google Scholar] [CrossRef]
- Salaramoli, S.; Joshaghani, H.; Hashemy, S.I. Selenium Effects on Oxidative Stress-Induced Calcium Signaling Pathways in Parkinson’s Disease. Indian J. Clin. Biochem. 2022, 37, 257–266. [Google Scholar]
- Zhang, X.; Liu, R.P.; Cheng, W.H.; Zhu, J.H. Prioritized brain selenium retention and selenoprotein expression: Nutritional insights into Parkinson’s disease. Mech. Ageing Dev. 2019, 180, 89–96. [Google Scholar] [CrossRef]
- Mischley, L.K.; Allen, J.; Bradley, R. Coenzyme Q10 deficiency in patients with Parkinson’s disease. J. Neurol. Sci. 2012, 318, 72–75. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Azzolin, V.F.; Rodrigues Dos Passos, G.; Turra, B.O.; Alves, A.O.; Bressanim, A.C.M.; Canton, L.E.L.; Vieira Dos Santos, A.C.; Mastella, M.H.; Barbisan, F.; et al. A coffee enriched with guarana, selenium, and l-carnitine (GSC) has nutrigenomic effects on oxi-inflammatory markers of relapsing-remitting multiple sclerosis patients: A pilot study. Mult. Scler. Relat. Disord. 2023, 71, 104515. [Google Scholar]
- Vinceti, M.; Solovyev, N.; Mandrioli, J.; Crespi, C.M.; Bonvicini, F.; Arcolin, E.; Georgoulopoulou, E.; Michalke, B. Cerebrospinal fluid of newly diagnosed amyotrophic lateral sclerosis patients exhibits abnormal levels of selenium species including elevated selenite. Neurotoxicology 2013, 38, 25–32. [Google Scholar] [PubMed]
- Vinceti, M.; Guidetti, D.; Pinotti, M.; Rovesti, S.; Merlin, M.; Vescovi, L.; Bergomi, M.; Vivoli, G. Amyotrophic lateral sclerosis after long-term exposure to drinking water with high selenium content. Epidemiology 1996, 7, 529–532. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Marks, E.; Chen, J.; Moline, J.; Barrows, L.; Raisbeck, M.; Volitakis, I.; Cherny, R.A.; Chopra, V.; Bush, A.I.; et al. Altered selenium status in Huntington’s disease: Neuroprotection by selenite in the N171-82Q mouse model. Neurobiol. Dis. 2014, 71, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, J.; Xiao, H.; Wu, L.; Jiang, H.; Zhou, Y. Application of artificial neural network model in diagnosis of Alzheimer’s disease. BMC Neurol. 2019, 19, 154. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, G.L. Characterization of metal profiles in serum during the progression of Alzheimer’s disease. Met. Integr. Biometal Sci. 2014, 6, 292–300. [Google Scholar] [CrossRef]
- Koç, E.R.; Ilhan, A.; Zübeyde, A.; Acar, B.; Gürler, M.; Altuntaş, A.; Karapirli, M.; Bodur, A.S. A comparison of hair and serum trace elements in patients with Alzheimer disease and healthy participants. Turk. J. Med. Sci. 2015, 45, 1034–1039. [Google Scholar] [CrossRef]
- Socha, K.; Klimiuk, K.; Naliwajko, S.K.; Soroczyńska, J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Kochanowicz, J. Dietary Habits, Selenium, Copper, Zinc and Total Antioxidant Status in Serum in Relation to Cognitive Functions of Patients with Alzheimer’s Disease. Nutrients 2021, 13, 287. [Google Scholar] [CrossRef]
- Paglia, G.; Miedico, O.; Cristofano, A.; Vitale, M.; Angiolillo, A.; Chiaravalle, A.E.; Corso, G.; Di Costanzo, A. Distinctive Pattern of Serum Elements During the Progression of Alzheimer’s Disease. Sci. Rep. 2016, 6, 22769. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Ong, T.P.; Jacob-Filho, W.; Jaluul, O.; Freitas, M.I.; Cominetti, C.; Cozzolino, S.M. Glutathione peroxidase 1 Pro198Leu polymorphism in Brazilian Alzheimer’s disease patients: Relations to the enzyme activity and to selenium status. J. Nutr. Nutr. 2012, 5, 72–80. [Google Scholar] [CrossRef]
- Giacoppo, S.; Galuppo, M.; Calabrò, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potortì, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy metals and neurodegenerative diseases: An observational study. Biol. Trace Elem. Res. 2014, 161, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Stosnach, H.; Parkes, H.G.; Hye, A.; Powell, J.; So, P.W. Publisher Correction: Pattern of Altered Plasma Elemental Phosphorus, Calcium, Zinc, and Iron in Alzheimer’s Disease. Sci. Rep. 2019, 9, 6343. [Google Scholar] [CrossRef] [PubMed]
- Ceballos-Picot, I.; Merad-Boudia, M.; Nicole, A.; Thevenin, M.; Hellier, G.; Legrain, S.; Berr, C. Peripheral antioxidant enzyme activities and selenium in elderly subjects and in dementia of Alzheimer’s type--place of the extracellular glutathione peroxidase. Free Radic. Biol. Med. 1996, 20, 579–587. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Li, Q.X.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Ganio, K.; Lothian, A.; Mukherjee, S.; et al. Selenium Levels in Serum, Red Blood Cells, and Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Report from the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). J. Alzheimers Dis. 2017, 57, 183–193. [Google Scholar] [PubMed]
- Baum, L.; Chan, I.H.; Cheung, S.K.; Goggins, W.B.; Mok, V.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; Woo, J.; et al. Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 2010, 23, 173–179. [Google Scholar] [CrossRef]
- Wenstrup, D.; Ehmann, W.D.; Markesbery, W.R. Trace element imbalances in isolated subcellular fractions of Alzheimer’s disease brains. Brain Res. 1990, 533, 125–131. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Ong, T.P.; Jacob-Filho, W.; Jaluul, O.; Freitas, M.I.; Cozzolino, S.M. Nutritional status of selenium in Alzheimer’s disease patients. Br. J. Nutr. 2010, 103, 803–806. [Google Scholar] [CrossRef]
- Chmatalova, Z.; Vyhnalek, M.; Laczo, J.; Hort, J.; Pospisilova, R.; Pechova, M.; Skoumalova, A. Relation of plasma selenium and lipid peroxidation end products in patients with Alzheimer’s disease. Physiol. Res. 2017, 66, 1049–1056. [Google Scholar] [CrossRef]
- González-Domínguez, R.; García-Barrera, T.; Gómez-Ariza, J.L. Homeostasis of metals in the progression of Alzheimer’s disease. Biometals 2014, 27, 539–549. [Google Scholar]
- Part, P. Differences in trace element concentrations between Alzheimer and “normal” human brain tissue using instrumental neutron activation analysis (INAA). J. Radioanal. Nucl. Chem. 2001, 249, 437–441. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Lind, M.; McLean, C.A.; Volitakis, I.; Laws, S.M.; Masters, C.L.; Bush, A.I.; Roberts, B.R. The APOE ε4 Allele Is Associated with Lower Selenium Levels in the Brain: Implications for Alzheimer’s Disease. ACS Chem. Neurosci. 2017, 8, 1459–1464. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, R.D.; Reddy, B.S.; Sattar, S.A.; Rao, A.D.P. Trace element imbalances in blood serum of Alzheimer’s disease patients. Environ. Pollut. 2023, 318, 120782. [Google Scholar] [CrossRef]
- Strumylaite, L.; Kregzdyte, R.; Kucikiene, O.; Baranauskiene, D.; Simakauskiene, V.; Naginiene, R.; Damuleviciene, G.; Lesauskaite, V.; Zemaitiene, R. Alzheimer’s Disease Association with Metals and Metalloids Concentration in Blood and Urine. Int. J. Environ. Res. Public Health 2022, 19, 7309. [Google Scholar] [CrossRef] [PubMed]
- Maass, F.; Michalke, B.; Willkommen, D.; Schulte, C.; Tönges, L.; Boerger, M.; Zerr, I.; Bähr, M.; Lingor, P. Selenium speciation analysis in the cerebrospinal fluid of patients with Parkinson’s disease. J. Trace Elem. Med. Biol. 2020, 57, 126412. [Google Scholar] [CrossRef]
- Hemmati-Dinarvand, M.; Taher-Aghdam, A.A.; Mota, A.; Zununi Vahed, S.; Samadi, N. Dysregulation of serum NADPH oxidase1 and ferritin levels provides insights into diagnosis of Parkinson’s disease. Clin. Biochem. 2017, 50, 1087–1092. [Google Scholar] [CrossRef]
- Zhao, H.W.; Lin, J.; Wang, X.B.; Cheng, X.; Wang, J.Y.; Hu, B.L.; Zhang, Y.; Zhang, X.; Zhu, J.H. Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PLoS ONE 2013, 8, e83060. [Google Scholar] [CrossRef]
- Aguilar, M.V.; Jiménez-Jiménez, F.J.; Molina, J.A.; Meseguer, I.; Mateos-Vega, C.J.; González-Muñoz, M.J.; de Bustos, F.; Gómez-Escalonilla, C.; Ort-Pareja, M.; Zurdo, M.; et al. Cerebrospinal fluid selenium and chromium levels in patients with Parkinson’s disease. J. Neural Transm. 1998, 105, 1245–1251. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Molina, J.A.; Arrieta, F.J.; Aguilar, M.V.; Cabrera-Valdivia, F.; Vázquez, A.; Jorge-Santamaría, A.; Seijas, V.; Fernández-Calle, P.; Martínez-Para, M.C. Decreased serum selenium concentrations in patients with Parkinson’s disease. Eur. J. Neurol. 1995, 2, 111–114. [Google Scholar] [CrossRef]
- Maass, F.; Michalke, B.; Leha, A.; Boerger, M.; Zerr, I.; Koch, J.C.; Tönges, L.; Bähr, M.; Lingor, P. Elemental fingerprint as a cerebrospinal fluid biomarker for the diagnosis of Parkinson’s disease. J. Neurochem. 2018, 145, 342–351. [Google Scholar] [CrossRef]
- Nikam, S.; Nikam, P.; Ahaley, S.K. Role of free radical and antioxidant imbalance in pathogenesis of Parkinson’s disease. Biomed. Res. 2009, 20, 55–58. [Google Scholar]
- Younes-Mhenni, S.; Aissi, M.; Mokni, N.; Boughammoura-Bouatay, A.; Chebel, S.; Frih-Ayed, M.; Kerkeni, A.; Bost, M.; Chazot, G.; Sfar, M.T.; et al. Serum copper, zinc and selenium levels in Tunisian patients with Parkinson’s disease. Tunis. Med. 2013, 91, 402–405. [Google Scholar] [PubMed]
- Qureshi, G.A.; Qureshi, A.A.; Memon, S.A.; Parvez, S.H. Impact of selenium, iron, copper and zinc in on/off Parkinson’s patients on L-dopa therapy. J. Neural Transm. Suppl. 2006, 71, 229–236. [Google Scholar]
- Mousavi, S.S.S.; Sadatborhani, M. Support vectors machine-based model for diagnosis of multiple sclerosis using the plasma levels of selenium, vitamin B12, and vitamin D3. Inform. Med. Unlocked 2020, 20, 100382. [Google Scholar]
- Socha, K.; Kochanowicz, J.; Karpińska, E.; Soroczyńska, J.; Jakoniuk, M.; Mariak, Z.; Borawska, M.H. Dietary habits and selenium, glutathione peroxidase and total antioxidant status in the serum of patients with relapsing-remitting multiple sclerosis. Nutr. J. 2014, 13, 62. [Google Scholar] [CrossRef]
- Wikström, J.; Westermarck, T.; Palo, J. Selenium, vitamin E and copper in multiple sclerosis. Acta Neurol. Scand. 1976, 54, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Tamburo, E.; Varrica, D.; Dongarrà, G.; Grimaldi, L.M. Trace elements in scalp hair samples from patients with relapsing-remitting multiple sclerosis. PLoS ONE 2015, 10, e0122142. [Google Scholar] [CrossRef]
- Mehrpour, M.; Kyani, A.; Tafazzoli, M.; Fathi, F.; Joghataie, M.T. A metabonomics investigation of multiple sclerosis by nuclear magnetic resonance. Magn. Reson. Chem. 2013, 51, 102–109. [Google Scholar] [CrossRef]
- Korpela, H.; Kinnunen, E.; Juntunen, J.; Kumpulainen, J.; Koskenvuo, M. Serum selenium concentration, glutathione peroxidase activity and lipid peroxides in a co-twin control study on multiple sclerosis. J. Neurol. Sci. 1989, 91, 79–84. [Google Scholar] [CrossRef]
- De Benedetti, S.; Lucchini, G.; Del Bò, C.; Deon, V.; Marocchi, A.; Penco, S.; Lunetta, C.; Gianazza, E.; Bonomi, F.; Iametti, S. Blood trace metals in a sporadic amyotrophic lateral sclerosis geographical cluster. Biometals 2017, 30, 355–365. [Google Scholar] [CrossRef]
- Vinceti, M.; Guidetti, D.; Bergomi, M.; Caselgrandi, E.; Vivoli, R.; Olmi, M.; Rinaldi, L.; Rovesti, S.; Solimè, F. Lead, cadmium, and selenium in the blood of patients with sporadic amyotrophic lateral sclerosis. Ital. J. Neurol. Sci. 1997, 18, 87–92. [Google Scholar] [CrossRef]
- Bonnefont-Rousselot, D.; Lacomblez, L.; Jaudon, M.; Lepage, S.; Salachas, F.; Bensimon, G.; Bizard, C.; Doppler, V.; Delattre, J.; Meininger, V. Blood oxidative stress in amyotrophic lateral sclerosis. J. Neurol. Sci. 2000, 178, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Peters, S.; Broberg, K.; Gallo, V.; Levi, M.; Kippler, M.; Vineis, P.; Veldink, J.; van den Berg, L.; Middleton, L.; Travis, R.C.; et al. Blood Metal Levels and Amyotrophic Lateral Sclerosis Risk: A Prospective Cohort. Ann. Neurol. 2021, 89, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Blasco, H.; Garcon, G.; Patin, F.; Veyrat-Durebex, C.; Boyer, J.; Devos, D.; Vourc’h, P.; Andres, C.R.; Corcia, P. Panel of Oxidative Stress and Inflammatory Biomarkers in ALS: A Pilot Study. Can. J. Neurol. Sci. 2017, 44, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Moriwaka, F.; Satoh, H.; Ejima, A.; Watanabe, C.; Tashiro, K.; Hamada, T.; Matsumoto, A.; Shima, K.; Yanagihara, T.; Fukazawa, T.; et al. Mercury and selenium contents in amyotrophic lateral sclerosis in Hokkaido, the northernmost island of Japan. J. Neurol. Sci. 1993, 118, 38–42. [Google Scholar] [CrossRef]
- Nagata, H.; Miyata, S.; Nakamura, S.; Kameyama, M.; Katsui, Y. Heavy metal concentrations in blood cells in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 1985, 67, 173–178. [Google Scholar] [CrossRef]
- Barros, A.; Felipe, M.; Barbosa, I.R.; Leite-Lais, L.; Pedrosa, L.F.C. Dietary Intake of Micronutrients and Disease Severity in Patients with Amyotrophic Lateral Sclerosis. Metabolites 2023, 13, 696. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Abete, M.C.; Brusco, A. Trace elements profile in the blood of Huntington’ disease patients. J. Trace Elem. Med. Biol. 2020, 57, 18–20. [Google Scholar] [CrossRef]
- Hill, V.M.; O’Connor, R.M.; Sissoko, G.B.; Irobunda, I.S.; Leong, S.; Canman, J.C.; Stavropoulos, N.; Shirasu-Hiza, M. A bidirectional relationship between sleep and oxidative stress in Drosophila. PLoS Biol. 2018, 16, e2005206. [Google Scholar] [CrossRef]
- Crack, P.J.; Taylor, J.M.; Flentjar, N.J.; De Haan, J.; Hertzog, P.; Iannello, R.C.; Kola, I. Increased infarct size and exacerbated apoptosis in the glutathione peroxidase-1 (Gpx-1) knockout mouse brain in response to ischemia/reperfusion injury. J. Neurochem. 2001, 78, 1389–1399. [Google Scholar] [CrossRef]
- Yoneda, S.; Suzuki, K.T. Equimolar Hg-Se complex binds to selenoprotein P. Biochem. Biophys. Res. Commun. 1997, 231, 7–11. [Google Scholar] [CrossRef]
- Yan, J.; Barrett, J.N. Purification from bovine serum of a survival-promoting factor for cultured central neurons and its identification as selenoprotein-P. J. Neurosci. 1998, 18, 8682–8691. [Google Scholar] [CrossRef] [PubMed]
- Mostert, V.; Lombeck, I.; Abel, J. A novel method for the purification of selenoprotein P from human plasma. Arch. Biochem. Biophys. 1998, 357, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Sasakura, C.; Suzuki, K.T. Biological interaction between transition metals (Ag, Cd and Hg), selenide/sulfide and selenoprotein P. J. Inorg. Biochem. 1998, 71, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Zhou, J.; McMahan, W.J.; Motley, A.K.; Atkins, J.F.; Gesteland, R.F.; Burk, R.F. Deletion of selenoprotein P alters distribution of selenium in the mouse. J. Biol. Chem. 2003, 278, 13640–13646. [Google Scholar] [CrossRef]
- Arteel, G.; Mostert, V.; Oubrahim, H.; Briviba, K.; Abel, J.; Sies, H. Protection by selenoprotein P in human plasma against peroxynitrite-mediated oxidation and nitration. Biol. Chem. 1998, 379, 1201–1205. [Google Scholar] [PubMed]
- Saito, Y.; Takahashi, K. Characterization of selenoprotein P as a selenium supply protein. Eur. J. Biochem. 2002, 269, 5746–5751. [Google Scholar] [CrossRef]
- Gray, S.C.; Kinghorn, K.J.; Woodling, N.S. Shifting equilibriums in Alzheimer’s disease: The complex roles of microglia in neuroinflammation, neuronal survival and neurogenesis. Neural Regen. Res. 2020, 15, 1208–1219. [Google Scholar]
- Pascual, M.; Ibáñez, F.; Guerri, C. Exosomes as mediators of neuron-glia communication in neuroinflammation. Neural Regen. Res. 2020, 15, 796–801. [Google Scholar] [CrossRef]
- Li, L.X.; Chu, J.H.; Chen, X.W.; Gao, P.C.; Wang, Z.Y.; Liu, C.; Fan, R.F. Selenium ameliorates mercuric chloride-induced brain damage through activating BDNF/TrKB/PI3K/AKT and inhibiting NF-κB signaling pathways. J. Inorg. Biochem. 2022, 229, 111716. [Google Scholar] [CrossRef]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta 2014, 1842, 1219–1231. [Google Scholar]
- Picco, A.; Polidori, M.C.; Ferrara, M.; Cecchetti, R.; Arnaldi, D.; Baglioni, M.; Morbelli, S.; Bastiani, P.; Bossert, I.; Fiorucci, G.; et al. Plasma antioxidants and brain glucose metabolism in elderly subjects with cognitive complaints. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 764–775. [Google Scholar] [CrossRef]
- Haider, S.; Saleem, S.; Perveen, T.; Tabassum, S.; Batool, Z.; Sadir, S.; Liaquat, L.; Madiha, S. Age-related learning and memory deficits in rats: Role of altered brain neurotransmitters, acetylcholinesterase activity and changes in antioxidant defense system. Age Dordr. 2014, 36, 9653. [Google Scholar] [CrossRef]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Raichle, M.E.; Mintun, M.A. Brain work and brain imaging. Annu. Rev. Neurosci. 2006, 29, 449–476. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, H.; Huo, Y.; Wei, X.; Li, Y. Emerging roles of selenium on metabolism and type 2 diabetes. Front. Nutr. 2022, 9, 1027629. [Google Scholar] [CrossRef]
- Behne, D.; Wolters, W. Distribution of selenium and glutathione peroxidase in the rat. J. Nutr. 1983, 113, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Dauer, W.; Przedborski, S. Parkinson’s Disease: Mechanisms and Models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef] [PubMed]
- Kish, S.J.; Morito, C.; Hornykiewicz, O. Glutathione peroxidase activity in Parkinson’s disease brain. Neurosci. Lett. 1985, 58, 343–346. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.; Zhang, P.; Agid, Y.; Javoy-Agid, F. Glutathione peroxidase, glial cells and Parkinson’s disease. Neuroscience 1993, 52, 1–6. [Google Scholar] [CrossRef]
- Shahar, A.; Patel, K.V.; Semba, R.D.; Bandinelli, S.; Shahar, D.R.; Ferrucci, L.; Guralnik, J.M. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov. Disord. 2010, 25, 1909–1915. [Google Scholar] [CrossRef]
- Gandhi, R.; Laroni, A.; Weiner, H.L. Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2010, 221, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Sanjuan, I.; Bates, G.P. The importance of integrating basic and clinical research toward the development of new therapies for Huntington disease. J. Clin. Investig. 2011, 121, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef]
- Sorolla, M.A.; Reverter-Branchat, G.; Tamarit, J.; Ferrer, I.; Ros, J.; Cabiscol, E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008, 45, 667–678. [Google Scholar] [CrossRef] [PubMed]




| Study | Year | Country | Tissue Type | Patients | Healthy Controls | Unit | Study Type | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Selenium Level (Mean ± Sd.) | No. | Selenium Level (Mean ± Sd.) | |||||||
| Huseyin Vural [14] | 2010 | Turkey | Plasma | 23M | 58.15 ± 10.63 | 24M | 67.84 ± 9.69 | μg/L | Case–control | 8 |
| 27F | 58.43 ± 11.26 | 26F | 68.70 ± 12.58 | |||||||
| Naibo Wang [25] | 2019 | China | Urine and Blood | 89 | 2.26 ± 0.59 | 178 | 2.61 ± 1.07 | umol/mL | Nested case–control | 6 |
| I. Meseguer [16] | 1999 | Spain | CSF | 27 | 11.40 ± 7.80 | 34 | 13.30 ± 7.00 | ng/mL | Case–control | 5 |
| Serum | 27 | 28.50 ± 13.00 | 34 | 22.50 ± 17.50 | ||||||
| R.González-Domínguez [26] | 2014 | Spain | Serum | 30 | 112.80 ± 25.76 | 30 | 118.50 ± 26.84 | μg/L | Case–control | 8 |
| EMİNE RABİA KOÇ [27] | 2015 | Turkey | Hair | 37 | 0.50 ± 0.10 | 31 | 0.60 ± 0.10 | µg/g | Case–control | 7 |
| Serum | 40 | 0.75 ± 0.20 | 33 | 0.83 ± 0.40 | µg/mL | |||||
| Katarzyna Socha [28] | 2021 | Poland | Serum | 110 | 69.10 ± 19.30 | 60 | 79.80 ± 22.00 | μg/L | Case–control | 7 |
| Giuseppe paglia [29] | 2016 | Italy | Serum | 34 | 70.36 ± 19.28 | 40 | 82.62 ± 23.40 | μg/L | Case–control | 6 |
| Sreeram Krishnan [17] | 2014 | India | Blood | 30 | 173.63 ± 31.01 | 40 | 187.53 ± 48.73 | ppb | Case–control | 8 |
| Bárbara Rita Cardoso [30] | 2012 | Brazil | Plasma | 20 | 31.44 ± 21.42 | 21 | 54.87 ± 23.54 | μg/L | Case–control | 7 |
| Erythrocytes | 20 | 40.25 ± 17.74 | 20 | 87.75 ± 51.24 | ||||||
| Sabrina Giacoppo [31] | 2014 | Italy | Blood | 15 | 42.78 ± 9.772 | 10 | 73.27 ± 10.05 | μg/L | Case–control | 5 |
| Azhaar Ashraf [32] | 2019 | NK | plasma | 44 | 0.106 ± 0.067 | 44 | 0.112 ± 0.049 | μg/L | Case–control | 6 |
| IrèneCeballos-Picot [33] | 1996 | France | Plasma | 40 | 54.10 ± 12.90 | 34 | 49.70 ± 9.60 | ng/mL | Case–control | 6 |
| Erythrocytes | 40 | 0.286 ± 0.048 | 34 | 0.291 ± 0.0038 | ||||||
| Bárbara R. Cardoso [34] | 2017 | Australia | Erythrocytes | 36 | 68.36 ± 5.09 | 39 | 92.17 ± 6.59 | μg/L | Case–control | 6 |
| Serum | 29 | 114.13 ± 19.85 | 30 | 115.10 ± 23.72 | ||||||
| CSF | 10 | 1.09 ± 0.16 | 31 | 1.69 ± 1.00 | ||||||
| Larry Baum [35] | 2010 | China | Serum | 44 | 1420 ± 230 | 41 | 1390 ± 240 | nmol/L | Case–control | 5 |
| David Wenstrup [36] | 1990 | USA | Whole tissue | 10 | 865 ± 109 | 12 | 901 ± 162 | ng/g | Case–control | 5 |
| C. R. CORNET [15] | 1998 | USA | Pituitary tissues | 43 | 0.92 ± 0.11 | 15 | 0.86 ± 0.19 | μg/g | Case–control | 5 |
| Bárbara Rita Cardoso [37] | 2010 | Brazil | Plasma | 28 | 32.59 ± 21.99 | 29 | 50.99 ± 21.07 | μg/g | Case–control | 7 |
| Erythrocytes | 28 | 43.74 ± 23.02 | 29 | 79.16 ± 46.38 | ||||||
| Nails | 28 | 0.3 ± 0.14 | 29 | 0.4 ± 0.13 | ||||||
| Z. CHMATALOVA [38] | 2017 | Czech Republic | Plasma | 11 | 76.07 ± 18.45 | 12 | 90.72 ± 17.56 | μg/L | Case–control | 6 |
| Raúl González-Domínguez [39] | 2014 | NK | Serum | 25 | 120.5 ± 31.12 | 15 | 122.9 ± 24.14 | μg/L | Case–control | 5 |
| A. E. Panayi [40] | 2000 | Netherlands | Superior frontal gyrus (dry mass) | 16 | 0.133 ± 0.044 | 8 | 0.128 ± 0.029 | μg/L | Case–control | 6 |
| Superior parietal gyrus (dry mass) | 14 | 0.143 ± 0.037 | 9 | 0.128 ± 0.015 | ||||||
| Superior temporal gyrus (dry mass) | 12 | 0.129 ± 0.040 | 9 | 0.104 ± 0.036 | ||||||
| BáŕbaraR.Cardoso [41] | 2017 | America | Membrane fraction of wet tissue | 33 | 53.99 ± 17.71 | 38 | 64.67 ± 20.56 | ng/g | Case–control | 5 |
| R. D. Lavanya [42] | 2021 | India | Serum | 30 | 422.2 ± 41.0 | 18 | 314.4 ± 47.8 | ng/g | Case–control | 5 |
| Loreta Strumylaite [43] | 2022 | Italy | NK | 53 | 10.97 ± 8.09 | 217 | 13.53 ± 15.55 | kg × 10−9/m3 × 10−3 | Case–control | 6 |
| Study | Year | Country | Tissue Type | Patients | Healthy Controls | Unit | Study Type | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Selenium Level (Mean ± Sd.) | No. | Selenium Level (Mean ± Sd.) | |||||||
| Fabian Maass [44] | 2020 | Germany | CSF | 75 | 2695 ± 1358 | 68 | 2745 ± 1848 | ng/L | Case–control | 6 |
| Mohsen Hemmati-Dinarvand [45] | 2017 | Iran | Serum | 40 | 96.48 ± 9.11 | 40 | 83.67 ± 8.65 | μg/L | Case–control | 5 |
| Hai-Wen Zhao [46] | 2013 | China | Plasma | 238 | 115 ± 37 | 302 | 105 ± 33 | μg/L | Case–control | 7 |
| M. V. Aguilar [47] | 1998 | Spain | CSF | 28 | 17.9 ± 12.3 | 43 | 13.5 ± 8.2 | ng/mL | Case–control | 7 |
| Serum | 28 | 29.8 ± 16.9 | 43 | 22.5 ± 17.5 | ||||||
| F J Jiménez-Jiménez [48] | 1995 | Spain | Serum | 29 | 34.60 ± 2.35 | 30 | 45.20 ± 3.83 | μg/L | Case–control | 6 |
| Urine | 29 | 47.10 ± 6.25 | 30 | 45.50 ± 5.38 | μg/24 h | |||||
| Fabian Maass [49] | 2018 | Germany | CSF | 36 | 9.4 ± 7.6 | 42 | 5.9 ± 6.6 | μg/L | Case–control | 6 |
| Shashikant Nikam [50] | 2009 | India | Plasma | 22 | 14.58 ± 0.98 | 22 | 19.05 ± 1.42 | μgm/dL | Case–control | 7 |
| Younes-Mhenni Samia [51] | 2013 | Tunisia | Plasma | 48 | 98.5 ± 17.6 | 36 | 95.8 ± 14.4 | μg/L | Case–control | 6 |
| G. A. Qureshi [52] | 2016 | Sweden | CSF | 17 | 19.7 ± 1.9 | 21 | 14.2 ± 1.8 | μg/L | Case–control | 5 |
| Study | Year | Country | Tissue Type | Patients | Healthy Controls | Unit | Study Type | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Selenium Level (Mean ± Sd.) | No. | Selenium Level (Mean ± Sd.) | |||||||
| Seyed Sajjad Sharifmousavi [53] | 2020 | Iran | Plasma | 99 | 68.84 ± 23.17 | 81 | 53.33 ± 3.66 | μg/L | Case–control | 7 |
| Katarzyna Socha [54] | 2014 | Poland | Serum | 101 | 55.2 ± 16.2 | 63 | 79.2 ± 20.6 | μg/L | Case–control | 6 |
| J. Wikstrom [55] | 1976 | Finland | Whole blood | 15 | 56.0 ± 14.5 | 18 | 61.8 ± 15.5 | ng/mL | Case–control | 5 |
| Serum | 27 | 46.4 ± 12.9 | 18 | 43.6 ± 13.0 | ||||||
| Elisa Tamburo [56] | 2015 | Italy | Hair | 48 | 1.00 ± 0.69 | 51 | 0.8 ± 0.47 | μg/g | Case–control | 7 |
| Masoud Mehrpour [57] | 2012 | Iran | Serum | 23 | 61 ± 13 | 28 | 89 ± 13 | μg/L | Case–control | 5 |
| Sabrina Giacoppo [31] | 2014 | Italy | Blood | 41 | 68.60 ± 19.02 | 23 | 71.10 ± 18.09 | μg/L | Case–control | 5 |
| Heikki Korpela [58] | 1989 | Finland | Serum | 12 | 123 ± 17 | 11 | 120 ± 18 | μg/L | Nested Case–control | 5 |
| Study | Year | Country | Tissue Type | Patients | Healthy Controls | Unit | Study Type | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Selenium Level (Mean ± Sd.) | No. | Selenium Level (Mean ± Sd.) | |||||||
| Stefano De Benedetti [59] | 2017 | Italy | Serum | 6 | 100 ± 12 | 5 | 90 ± 8 | μg/L | Case–control | 7 |
| whole blood | 6 | 110 ± 18 | 5 | 121 ± 17 | ||||||
| Vinceti M [60] | 1997 | Italy | Serum | 16 | 68.6 ± 16.9 | 38 | 76.8 ± 11.8 | μg/L | Case–control | 7 |
| Dominique Bonnefont-Rousselot [61] | 2000 | France | Plasma | 167 | 1.21 ± 0.21 | 62 | 1.18 ± 0.18 | μmol/L | Case–control | 6 |
| Susan Peters [62] | 2021 | NK | Erythrocytes | 107 | 115 ± 1.25 | 319 | 117.70 ± 1.31 | ng/g | Nested Case–control | 6 |
| Hélène Blasco [63] | 2021 | France | Blood | 9 | 108.75 ± 17.14 | 10 | 106.40 ± 13.82 | μg/L | Case–control | 6 |
| F. Moriwaka [64] | 1993 | Japan | Plasma | 21 | 81.20 ± 46.42 | 35 | 120.61 ± 20.55 | ng/g | Case–control | 6 |
| Blood cells | 20 | 134.63 ± 73.36 | 35 | 191.55 ± 35.88 | ||||||
| Hiroshi Nagata [65] | 1985 | Japan | Blood cells | 40 | 1.16 ± 0.24 | 25 | 0.84 ± 0.17 | ng/mg | Case–control | 5 |
| Acsa Nara [66] | 2023 | Brazil | Plasma | 33 | 64.00 ± 91.00 | 36 | 35 ± 35 | μg/L | Case–control | 5 |
| Study | Year | Country | Tissue Type | Patients | Healthy Controls | Unit | Study Type | NOS Score | ||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | Selenium Level (Mean ± Sd.) | No. | Selenium Level (Mean ± Sd.) | |||||||
| Stefania Squadrone [67] | 2019 | Italy | Blood | 18 | 138 ± 12 | 18 | 101 ± 16 | μg/L | Case–control | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, W.; Cao, Z.; Lian, S.; Li, J.; Nie, J.; Huang, Y.; Zhao, K.; He, J.; Liu, C. Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis. Nutrients 2023, 15, 3706. https://doi.org/10.3390/nu15173706
Zhou J, Zhang W, Cao Z, Lian S, Li J, Nie J, Huang Y, Zhao K, He J, Liu C. Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis. Nutrients. 2023; 15(17):3706. https://doi.org/10.3390/nu15173706
Chicago/Turabian StyleZhou, Jiaxin, Wenfen Zhang, Zhiwen Cao, Shaoyan Lian, Jieying Li, Jiaying Nie, Ying Huang, Ke Zhao, Jiang He, and Chaoqun Liu. 2023. "Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis" Nutrients 15, no. 17: 3706. https://doi.org/10.3390/nu15173706
APA StyleZhou, J., Zhang, W., Cao, Z., Lian, S., Li, J., Nie, J., Huang, Y., Zhao, K., He, J., & Liu, C. (2023). Association of Selenium Levels with Neurodegenerative Disease: A Systemic Review and Meta-Analysis. Nutrients, 15(17), 3706. https://doi.org/10.3390/nu15173706