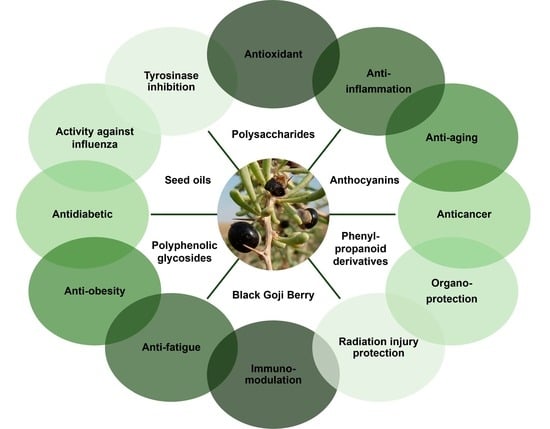
Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity
Abstract
:1. Introduction
2. Phytochemical Composition
3. Pharmacological Properties
3.1. Anti-Inflammatory Effects
3.2. Anti-Aging Effects
3.3. Anticancer Effects
3.4. Protective Effects
3.4.1. Hepatoprotective Effects
3.4.2. Neuroprotective Effects
3.4.3. Cardioprotective Effects
3.4.4. Protection against Radiation Injury
3.5. Immunomodulatory Effects
3.6. Other Effects
3.6.1. Anti-Fatigue Activity
3.6.2. Anti-Obesity and Antidiabetic Activity
3.6.3. Activity against Influenza
3.6.4. Tyrosinase Inhibitory Activity
3.6.5. Antioxidant Effects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 6-OHDA | 6-Hydroxydopamine hydrobromide |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline)-6-sulfonic acid |
| AGEs | Advanced glycation end products |
| ALT | Alanine transaminase |
| AST | Aspartate transaminase |
| BUN | Blood urea nitrogen |
| CC50 | 50% Cytotoxicity concentration |
| CEase | Cholesterol esterase |
| CK-MB | Creatine kinase-myocardial band |
| CPK | Creatine phosphokinase |
| Cy | Cyclophosphamide |
| d-gal | d-galactose |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| FDA | Fluorescein diacetate |
| GPx | Glutathione peroxidase |
| H&E | Hematoxylin and eosin |
| H3N2 | Influenza A/Novosibirsk/RII-27192S/2020 |
| IC50 | 50% Inhibitory concentration |
| ICD | International Classification of Diseases |
| ICR | Institute of Cancer Research |
| IL | Interleukin |
| LDH | Lactate dehydrogenase |
| LPS | Lipopolysaccharides |
| LRP3 | LRM polysaccharide 3 |
| LRPP5 | LRM polysaccharide pectin-5 |
| LRM | Lycium ruthenicum Murray |
| LRP3-S1 | LRM polysaccharide 3-S1 |
| LRP4&AC | LRM polysaccharide 4 and anthocyanin |
| MAO-B | Monoamine oxidase B |
| MDA | Malondialdehyde |
| MDCK | Madin-Darby canine kidney |
| MTT | 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| MTX | Methotrexate |
| NAFLD | Nonalcoholic fatty liver disease |
| NC | Normal control |
| NF-κB | Nuclear factor-κB |
| NO | Nitric oxide |
| PI | Propidium iodide |
| RA | Rheumatoid arthritis |
| RAGEs | AGEs and their receptors |
| ROS | Reactive oxygen species |
| SF | Synovial fibroblasts |
| SOD | Superoxide dismutase |
| TG | Triglyceride |
| TNF-α | Tumor necrosis factor-α |
| WD | Western diet |
References
- Salo, H.M.; Nguyen, N.; Alakärppä, E.; Klavins, L.; Hykkerud, A.L.; Karppinen, K.; Jaakola, L.; Klavins, M.; Häggman, H. Authentication of Berries and Berry-Based Food Products. Compr. Rev. Food Sci. Food Saf. 2021, 20, 5197–5225. [Google Scholar] [CrossRef]
- Golovinskaia, O.; Wang, C.-K. Review of Functional and Pharmacological Activities of Berries. Molecules 2021, 26, 3904. [Google Scholar] [CrossRef]
- Yao, R.; Heinrich, M.; Weckerle, C.S. The Genus Lycium as Food and Medicine: A Botanical, Ethnobotanical and Historical Review. J. Ethnopharmacol. 2018, 212, 50–66. [Google Scholar] [CrossRef] [PubMed]
- da Miguel, M.G. Chemical and Biological Properties of Three Poorly Studied Species of Lycium Genus—Short Review. Metabolites 2022, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Potterat, O. Goji (Lycium barbarum and L. Chinense): Phytochemistry, Pharmacology and Safety in the Perspective of Traditional Uses and Recent Popularity. Planta Med. 2010, 76, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Zhou, Z.-W.; Sheng, H.-P.; He, L.-J.; Fan, X.-W.; He, Z.-X.; Sun, T.; Zhang, X.; Zhao, R.J.; Gu, L.; et al. An Evidence-Based Update on the Pharmacological Activities and Possible Molecular Targets of Lycium Barbarum Polysaccharides. Drug Des. Devel. Ther. 2015, 9, 33–78. [Google Scholar]
- Ma, Z.F.; Zhang, H.; Teh, S.S.; Wang, C.W.; Zhang, Y.; Hayford, F.; Wang, L.; Ma, T.; Dong, Z.; Zhang, Y.; et al. Goji Berries as a Potential Natural Antioxidant Medicine: An Insight into Their Molecular Mechanisms of Action. Oxid. Med. Cell. Longev. 2019, 2019, e2437397. [Google Scholar] [CrossRef]
- Peng, Q.; Lv, X.; Xu, Q.; Li, Y.; Huang, L.; Du, Y. Isolation and Structural Characterization of the Polysaccharide LRGP1 from Lycium Ruthenicum. Carbohydr. Polym. 2012, 90, 95–101. [Google Scholar] [CrossRef]
- Islam, T.; Yu, X.; Badwal, T.S.; Xu, B. Comparative Studies on Phenolic Profiles, Antioxidant Capacities and Carotenoid Contents of Red Goji Berry (Lycium barbarum) and Black Goji Berry (Lycium ruthenicum). Chem. Cent. J. 2017, 11, 59. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Tao, W.; Zhang, X.; Gao, X.; Yong, J.; Zhao, J.; Zhang, L.; Li, Y.; Duan, J. Lycium Ruthenicum Studies: Molecular Biology, Phytochemistry and Pharmacology. Food Chem. 2018, 240, 759–766. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, B.; Wen, H.; Tao, Y.; Shao, Y. Phytochemical Profiles, Nutritional Constituents and Antioxidant Activity of Black Wolfberry (Lycium ruthenicum Murr.). Ind. Crops Prod. 2020, 154, 112692. [Google Scholar] [CrossRef]
- Sharma, R.; Raghuvanshi, R.; Kumar, R.; Thakur, M.S.; Kumar, S.; Patel, M.K.; Chaurasia, O.P.; Saxena, S. Current Findings and Future Prospective of High-Value Trans Himalayan Medicinal Plant Lycium Ruthenicum Murr: A Systematic Review. Clin. Phytoscience 2022, 8, 3. [Google Scholar] [CrossRef]
- Chen, S.; Wang, H.; Hu, N. Long-Term Dietary Lycium Ruthenicum Murr. Anthocyanins Intake Alleviated Oxidative Stress-Mediated Aging-Related Liver Injury and Abnormal Amino Acid Metabolism. Foods 2022, 11, 3377. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hu, N.; Wang, H.; Wu, Y.; Li, G. Bioactivity-Guided Isolation of the Major Anthocyanin from Lycium Ruthenicum Murr. Fruit and Its Antioxidant Activity and Neuroprotective Effects in Vitro and in Vivo. Food Funct. 2022, 13, 3247–3257. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, X.; Liu, Y.; Wang, M.; Shan, Y.; Yin, Y.; Meng, X.; Sun, F.; Li, H.; Li, Z. Molecular and Biochemical Investigations of the Anti-Fatigue Effects of Tea Polyphenols and Fruit Extracts of Lycium Ruthenicum Murr. on Mice with Exercise-Induced Fatigue. Front. Mol. Biosci. 2023, 10, 1223411. [Google Scholar] [CrossRef]
- Lu, K.; Wang, J.; Yu, Y.; Wu, Y.; He, Z. Lycium Ruthenicum Murr. Alleviates Nonalcoholic Fatty Liver in Mice. Food Sci. Nutr. 2020, 8, 2588–2597. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, H.; Zhang, G.; Meng, J.; Deng, K.; Zhou, W.; Wang, H.; Wang, Z.; Hu, N.; Suo, Y. Anthocyanins from Lycium Ruthenicum Murr. Ameliorated d-Galactose-Induced Memory Impairment, Oxidative Stress, and Neuroinflammation in Adult Rats. J. Agric. Food Chem. 2019, 67, 3140–3149. [Google Scholar] [CrossRef]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. HSF-1 and SIR-2.1 Linked Insulin-like Signaling Is Involved in Goji Berry (Lycium Spp.) Extracts Promoting Lifespan Extension of Caenorhabditis Elegans. Food Funct. 2021, 12, 7851–7866. [Google Scholar] [CrossRef]
- Xiong, L.; Deng, N.; Zheng, B.; Li, T.; Liu, R.H. Goji Berry (Lycium spp.) Extracts Exhibit Antiproliferative Activity via Modulating Cell Cycle Arrest, Cell Apoptosis, and the P53 Signaling Pathway. Food Funct. 2021, 12, 6513–6525. [Google Scholar] [CrossRef]
- He, F.; Zhang, S.; Li, Y.; Chen, X.; Du, Z.; Shao, C.; Ding, K. The Structure Elucidation of Novel Arabinogalactan LRP1-S2 against Pancreatic Cancer Cells Growth in Vitro and in Vivo. Carbohydr. Polym. 2021, 267, 118172. [Google Scholar] [CrossRef]
- Zhang, S.; He, F.; Chen, X.; Ding, K. Isolation and Structural Characterization of a Pectin from Lycium Ruthenicum Murr and Its Anti-Pancreatic Ductal Adenocarcinoma Cell Activity. Carbohydr. Polym. 2019, 223, 115104. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Wang, X.; Xu, K.; Yang, X.; Wang, Q.; Liu, C.; Wang, X.; Guo, X.; Sun, J.; Li, L.; et al. Synergistic Antitumor Effects of Polysaccharides and Anthocyanins from Lycium Ruthenicum Murr. on Human Colorectal Carcinoma LoVo Cells and the Molecular Mechanism. Food Sci. Nutr. 2022, 10, 2956–2968. [Google Scholar] [CrossRef] [PubMed]
- Deng, K.; Li, Y.; Xiao, M.; Wang, F.; Zhou, P.; Zhang, W.; Heep, A.; Li, X. Lycium Ruthenicum Murr Polysaccharide Protects Cortical Neurons against Oxygen-Glucose Deprivation/Reperfusion in Neonatal Hypoxic-Ischemic Encephalopathy. Int. J. Biol. Macromol. 2020, 158, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-K.; Bai, X.-L.; Yuan, H.; Zhang, Y.; Ayeni, E.A.; Liao, X. Polyphenolic Glycosides from the Fruits Extract of Lycium Ruthenicum Murr and Their Monoamine Oxidase B Inhibitory and Neuroprotective Activities. J. Agric. Food Chem. 2022, 70, 7968–7980. [Google Scholar] [CrossRef]
- Yossa Nzeuwa, I.B.; Xia, H.; Shi, Y.; Yang, C.; Shah, M.W.; Guo, B.; Wang, L.; Sun, G. Fatty Acid and Mineral Contents of Lycium Ruthenicum Murr. and Antioxidant Activity against Isoproterenol-Induced Acute Myocardial Ischemia in Mice. Food Sci. Nutr. 2020, 8, 1075–1081. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, F.; Yao, X.; Zhu, J.; Wang, C.; Zhang, J.; Li, X. Protective Effect of Lycium Ruthenicum Murr. Against Radiation Injury in Mice. Int. J. Environ. Res. Public Health 2015, 12, 8332–8347. [Google Scholar] [CrossRef]
- Peng, Q.; Xu, Q.; Yin, H.; Huang, L.; Du, Y. Characterization of an Immunologically Active Pectin from the Fruits of Lycium Ruthenicum. Int. J. Biol. Macromol. 2014, 64, 69–75. [Google Scholar] [CrossRef]
- Gong, Y.; Wu, J.; Li, S.-T. Immuno-Enhancement Effects of Lycium Ruthenicum Murr. Polysaccharide on Cyclophosphamide-Induced Immunosuppression in Mice. Int. J. Clin. Exp. Med. 2015, 8, 20631–20637. [Google Scholar]
- Xu, K.; Qin, X.; Zhang, Y.; Yang, M.; Zheng, H.; Li, Y.; Yang, X.; Xu, Q.; Li, Y.; Xu, P.; et al. Lycium Ruthenicum Murr. Anthocyanins Inhibit Hyperproliferation of Synovial Fibroblasts from Rheumatoid Patients and the Mechanism Study Powered by Network Pharmacology. Phytomedicine 2023, 118, 154949. [Google Scholar] [CrossRef]
- Ni, W.; Gao, T.; Wang, H.; Du, Y.; Li, J.; Li, C.; Wei, L.; Bi, H. Anti-Fatigue Activity of Polysaccharides from the Fruits of Four Tibetan Plateau Indigenous Medicinal Plants. J. Ethnopharmacol. 2013, 150, 529–535. [Google Scholar] [CrossRef]
- Zhao, X.; Dong, B.; Li, P.; Wei, W.; Dang, J.; Liu, Z.; Tao, Y.; Han, H.; Shao, Y.; Yue, H. Fatty Acid and Phytosterol Composition, and Biological Activities of Lycium Ruthenicum Murr. Seed Oil. J. Food Sci. 2018, 83, 2448–2456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-S.; Li, S.; Luo, Z.-H.; Zhou, Z.-Q.; Li, N.; Wang, Y.; Yao, X.-S.; Gao, H. Bioactive Phenylpropanoid Derivatives from the Fruits of Lycium Ruthenicum Murr. Bioorganic Chem. 2021, 116, 105307. [Google Scholar] [CrossRef] [PubMed]
- Kurskaya, O.; Prokopyeva, E.; Bi, H.; Sobolev, I.; Murashkina, T.; Shestopalov, A.; Wei, L.; Sharshov, K. Anti-Influenza Activity of Medicinal Material Extracts from Qinghai-Tibet Plateau. Viruses 2022, 14, 360. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, K.; Liang, Y.; Liu, G.; Sang, J.; Li, C. Extraction Optimization and Purification of Anthocyanins from Lycium Ruthenicum Murr. and Evaluation of Tyrosinase Inhibitory Activity of the Anthocyanins. J. Food Sci. 2020, 85, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Narumiya, S. Prostaglandin-Cytokine Crosstalk in Chronic Inflammation. Br. J. Pharmacol. 2019, 176, 337–354. [Google Scholar] [CrossRef]
- Lawrence, T. The Nuclear Factor NF-κB Pathway in Inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Moskalev, A.; Guvatova, Z.; Lopes, I.D.A.; Beckett, C.W.; Kennedy, B.K.; De Magalhaes, J.P.; Makarov, A.A. Targeting Aging Mechanisms: Pharmacological Perspectives. Trends Endocrinol. Metab. 2022, 33, 266–280. [Google Scholar] [CrossRef]
- Campisi, J.; Kapahi, P.; Lithgow, G.J.; Melov, S.; Newman, J.C.; Verdin, E. From Discoveries in Ageing Research to Therapeutics for Healthy Ageing. Nature 2019, 571, 183–192. [Google Scholar] [CrossRef]
- Lin, Y.; Qi, X.; Liu, H.; Xue, K.; Xu, S.; Tian, Z. The Anti-Cancer Effects of Fucoidan: A Review of Both in Vivo and in Vitro Investigations. Cancer Cell Int. 2020, 20, 154. [Google Scholar] [CrossRef]
- Gao, H.-M.; Hong, J.-S. Why Neurodegenerative Diseases Are Progressive: Uncontrolled Inflammation Drives Disease Progression. Trends Immunol. 2008, 29, 357–365. [Google Scholar] [CrossRef]
- Hajialyani, M.; Hosein Farzaei, M.; Echeverría, J.; Nabavi, S.M.; Uriarte, E.; Sobarzo-Sánchez, E. Hesperidin as a Neuroprotective Agent: A Review of Animal and Clinical Evidence. Molecules 2019, 24, 648. [Google Scholar] [CrossRef]
- Liu, L.; Liu, J.; Bao, J.; Bai, Q.; Wang, G. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Youdim, M.B.H.; Lavie, L. Selective MAO-A and B Inhibitors, Radical Scavengers and Nitric Oxide Synthase Inhibitors in Parkinson’s Desease. Life Sci. 1994, 55, 2077–2082. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, C.-W.; Chen, G.Y.J.; Zhu, B.; Chai, C.; Xu, Q.-H.; Tan, E.-K.; Zhu, Q.; Lim, K.-L.; Yao, S.Q. A Sensitive Two-Photon Probe to Selectively Detect Monoamine Oxidase B Activity in Parkinson’s Disease Models. Nat. Commun. 2014, 5, 3276. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, A. Cardioprotective Signalling: Past, Present and Future. Eur. J. Pharmacol. 2018, 833, 314–319. [Google Scholar] [CrossRef]
- Zheng, Q.; Wang, H.; Hou, W.; Zhang, Y. Use of Anti-Angiogenic Drugs Potentially Associated With an Increase on Serum AST, LDH, CK, and CK-MB Activities in Patients With Cancer: A Retrospective Study. Front. Cardiovasc. Med. 2021, 8, 755191. [Google Scholar] [CrossRef]
- Hou, C.-W.; Chen, I.-C.; Shu, F.-R.; Feng, C.-H.; Hung, C.-T. Protective Effect of Supplementation with Lycium Ruthenicum Murray Extract from Exhaustive Exercise-Induced Cardiac Injury in Rats. Chin. Med. J. 2019, 132, 1005–1006. [Google Scholar] [CrossRef]
- Smart, D. Radiation Toxicity in the Central Nervous System: Mechanisms and Strategies for Injury Reduction. Semin. Radiat. Oncol. 2017, 27, 332–339. [Google Scholar] [CrossRef]
- Brizel, D.M.; Wasserman, T.H.; Henke, M.; Strnad, V.; Rudat, V.; Monnier, A.; Eschwege, F.; Zhang, J.; Russell, L.; Oster, W.; et al. Phase III Randomized Trial of Amifostine as a Radioprotector in Head and Neck Cancer. J. Clin. Oncol. 2000, 18, 3339–3345. [Google Scholar] [CrossRef]
- Kouvaris, J.R.; Kouloulias, V.E.; Vlahos, L.J. Amifostine: The First Selective-Target and Broad-Spectrum Radioprotector. Oncologist 2007, 12, 738–747. [Google Scholar] [CrossRef]
- Ramesh, A.; Kumar, S.; Brouillard, A.; Nandi, D.; Kulkarni, A. A Nitric Oxide (NO) Nanoreporter for Noninvasive Real-Time Imaging of Macrophage Immunotherapy. Adv. Mater. 2020, 32, 2000648. [Google Scholar] [CrossRef] [PubMed]
- Theivendran, S.; Gu, Z.; Tang, J.; Yang, Y.; Song, H.; Yang, Y.; Zhang, M.; Cheng, D.; Yu, C. Nanostructured Organosilica Nitric Oxide Donors Intrinsically Regulate Macrophage Polarization with Antitumor Effect. ACS Nano 2022, 16, 10943–10957. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D.; Lenz, L.L.; Harris, R.A. A Breakthrough: Macrophage-Directed Cancer Immunotherapy. Cancer Res. 2016, 76, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jing, S.; Lin, H.; Sun, W.; Jiang, W.; Yu, C.; Sun, J.; Wang, C.; Chen, J.; Li, H. Anti-Fatigue Effect of Anwulignan via the NRF2 and PGC-1α Signaling Pathway in Mice. Food Funct. 2019, 10, 7755–7766. [Google Scholar] [CrossRef] [PubMed]
- Payab, M.; Hasani-Ranjbar, S.; Baeeri, M.; Rahimifard, M.; Arjmand, B.; Haghi-Aminjan, H.; Abdollahi, M.; Larijani, B. Development of a Novel Anti-Obesity Compound with Inhibiting Properties on the Lipid Accumulation in 3T3-L1 Adipocytes. Iran. Biomed. J. 2020, 24, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-L.; Zhu, L.; Jiang, J.-G. Active Ingredients from Natural Botanicals in the Treatment of Obesity. Obes. Rev. 2014, 15, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Wadden, T.A.; Berkowitz, R.I.; Womble, L.G.; Sarwer, D.B.; Phelan, S.; Cato, R.K.; Hesson, L.A.; Osei, S.Y.; Kaplan, R.; Stunkard, A.J. Randomized Trial of Lifestyle Modification and Pharmacotherapy for Obesity. N. Engl. J. Med. 2005, 353, 2111–2120. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-Obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Lee, H.S.; Heo, C.U.; Song, Y.-H.; Lee, K.; Choi, C.-I. Naringin Promotes Fat Browning Mediated by UCP1 Activation via the AMPK Signaling Pathway in 3T3-L1 Adipocytes. Arch. Pharm. Res. 2023, 46, 192–205. [Google Scholar] [CrossRef]
- Wang, X.; Ai, X.; Zhu, Z.; Zhang, M.; Pan, F.; Yang, Z.; Wang, O.; Zhao, L.; Zhao, L. Pancreatic Lipase Inhibitory Effects of Peptides Derived from Sesame Proteins: In Silico and in Vitro Analyses. Int. J. Biol. Macromol. 2022, 222, 1531–1537. [Google Scholar] [CrossRef]
- Li, B.; Zhou, B.; Lu, H.; Ma, L.; Peng, A.-Y. Phosphaisocoumarins as a New Class of Potent Inhibitors for Pancreatic Cholesterol Esterase. Eur. J. Med. Chem. 2010, 45, 1955–1963. [Google Scholar] [CrossRef] [PubMed]
- Gaitonde, D.Y.; Moore, F.C.; Morgan, M.K. Influenza: Diagnosis and Treatment. Am. Fam. Physician 2019, 100, 751–758. [Google Scholar] [PubMed]
- Rajendran, P.; Nandakumar, N.; Rengarajan, T.; Palaniswami, R.; Gnanadhas, E.N.; Lakshminarasaiah, U.; Gopas, J.; Nishigaki, I. Antioxidants and Human Diseases. Clin. Chim. Acta 2014, 436, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-M.; Yoon, Y.; Yoon, H.; Park, H.-M.; Song, S.; Yeum, K.-J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients 2017, 9, 1089. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Dang, J.; Wang, Q.; Yu, M.; Jiang, L.; Mei, L.; Shao, Y.; Tao, Y. Optimization of Polysaccharides from Lycium Ruthenicum Fruit Using RSM and Its Anti-Oxidant Activity. Int. J. Biol. Macromol. 2013, 61, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luan, G.; Zhou, W.; Meng, J.; Wang, H.; Hu, N.; Suo, Y. Subcritical Water Extraction, UPLC-Triple-TOF/MS Analysis and Antioxidant Activity of Anthocyanins from Lycium Ruthenicum Murr. Food Chem. 2018, 249, 119–126. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, X.; Liu, C.; Dong, B.; Shao, Y.; Liu, B.; Yue, H. Ultrasonic Extraction of Anthocyanins from Lycium Ruthenicum Murr. and Its Antioxidant Activity. Food Sci. Nutr. 2020, 8, 2642–2651. [Google Scholar] [CrossRef]
- Gao, Q.; Song, Y.; Liang, Y.; Li, Y.; Chang, Y.; Ma, R.; Cao, X.; Wang, S. Dynamics of Physicochemical Properties, Functional Compounds and Antioxidant Capacity during Spontaneous Fermentation of Lycium Ruthenicum Murr. (Qinghai-Tibet Plateau) Natural Vinegar. Foods 2022, 11, 1344. [Google Scholar] [CrossRef]
- Song, J.-L.; Gao, Y.; Xu, J. Protective Effects of Methanolic Extract Form Fruits of Lycium Ruthenicum Murr on 2,2′-Azobis (2-Amidinopropane) Dihydrochloride-Induced Oxidative Stress in LLC-PK1 Cells. Pharmacogn. Mag. 2014, 10, 522–528. [Google Scholar]
- Wu, T.; Lv, H.; Wang, F.; Wang, Y. Characterization of Polyphenols from Lycium Ruthenicum Fruit by UPLC-Q-TOF/MSE and Their Antioxidant Activity in Caco-2 Cells. J. Agric. Food Chem. 2016, 64, 2280–2288. [Google Scholar] [CrossRef]


| Nutrients | g/100 g DW | Nutrients | mg/100 g DW |
|---|---|---|---|
| Total carbohydrates | 67.0 ± 1.2 | Vitamins | 12.42 ± 1.26 |
| Dietary fiber | 12.1 ± 0.1 | Macroelements | 22.1 ± 1.6 |
| Proteins | 11.5 ± 0.3 | Microelements | 129.2 ± 9.1 * |
| Ash | 6.3 ± 0.1 | Carotenoids | 1.52 ± 0.01 |
| Fat | 3.0 ± 0.1 | Anthocyanins | 25.1 ± 1.3 |
| Organic acids | 4.69 ± 0.13 | Polysaccharides | 31.3 ± 1.6 |
| Pharmacological Activity | Tested Substance | Study Model | Dose/Concentration | Study Result(s) | Ref. |
|---|---|---|---|---|---|
| Anti-inflammation | Anthocyanin | Sprague Dawley rats | 100 mg/kg | Decreased: TNF-α and IL-6 Increased: IL-10 | [13] |
| Anthocyanin | Neuro-2a cells and Male C57BL/6 mice | 10 μM (in vitro) and 50 and 100 mg/kg (in vivo) | (in vitro) Decreased: COX-2, TNF-α, IL-6, IL-1β, and p-NF-κBp65 (in vivo) Decreased: p-NF-κB, TNF-α, IL-1β, and IL-6 | [14] | |
| Fruit extract | Kunming mice | 0.05, 0.1, 0.2, and 0.5 mg/g | Decreased: TNF-α, IL-1β, IL-2, and IL-6 | [15] | |
| Fruit extract | Male ApoE−/− mice | 140 mg/kg | Decreased: Tnf-α (compared with NC and WD) Not significantly changed: Il-6 (compared with NC and WD) and Il-10 (compared with WD) Increased: Il-4 (compared with WD) | [16] | |
| Anthocyanin | Female Sprague Dawley rats | 50, 100, and 200 mg/kg | Decreased: NF-κB, IL-1β, COX-2, and TNF-α | [17] | |
| Anti-aging | Anthocyanin | Sprague Dawley rats | 100 mg/kg | Decreased: serum aging markers (AGEs and MDA) Increased: swimming speed Improved: amino acid metabolic disturbance | [13] |
| Anthocyanin | Male C57BL/6 mice | 50 and 100 mg/kg | Improved: cognitive impairment (enhanced spatial learning and memory abilities) | [14] | |
| Fruit extract | C. elegans | 2, 5, and 10 mg/mL | Decreased: mortality rate (for heat shock), motility, lipofuscin (age pigment), reproductive ability, and age-related gene expression (age-1) Increased: average lifespan, SOD, CAT, oxidative resistance, irradiation tolerance, pump rate, and age-related gene expression (daf-16, sod-2, sod-3, hsp-16.2, sir-2.1, daf-12, jnk-1) Improved: nuclear localization of DAF-16 | [18] | |
| Anticancer | Fruit extract | Human breast cancer cells | 2, 4, and 6 mg/mL | Antiproliferative activity, EC50 of free extract: 4.08 ± 0.09 mg/mL Activated: p53, p21, CDK4, Cyclin E, Bax, and Caspase3 (p53 signaling pathway) | [19] |
| Polysaccharide | AsPC-1, BxPC-3, and PANC-1 cells and BALB/cA nu/nu mice | 7.45 and 14.9 μM (in vitro) and 0.5 and 40 mg/kg (in vivo) | (in vitro) Decreased: proliferation of pancreatic cancer cells (in vivo) Decreased: tumor sizes, tumor weights, Ki67, CD31, total NF-κB, p-GSK-3β, β-Catenin, p-P38, Bcl-2, caspase-3, and caspase-9 Increased: apoptosis | [20] | |
| Polysaccharide | AsPC-1, BxPC-3, and PANC-1 cells | 4.36 and 8.71 μM | Decreased: proliferation of pancreatic cancer cells, invasion ability, p-AKT, p-GSK-3β, p-FAK, and p-p38 | [21] | |
| Polysaccharide and anthocyanin | LoVo cells and HepG2 cells | Polysaccharide 150, 300, and 500 μg/mL (with anthocyanin 20 μg/mL) | Decreased: proliferation of carcinoma cells Inhibited: replication by G0–G1 arrest Increased: apoptosis | [22] | |
| Hepatoprotective | Anthocyanin | Sprague Dawley rats | 100 mg/kg | Improved: histological damages Decreased: serum AST, ALT, and LDH levels and Fas/FasL mRNA expression level (relieved liver cell death) | [13] |
| Fruit extract | Male ApoE−/− mice | 140 mg/kg | Similar: liver morphology, weight, indices of liver/body weight, total bile acid level, serum ALT level, TC, TG, LDL, and HDL-c levels (compared to WD) Decreased: AST levels (compared to NC) and size of fat droplet in liver (compared to WD) | [16] | |
| Neuroprotective | Anthocyanin | Neuro-2a cells and Male C57BL/6 mice | 10 μM (in vitro) and 50 and 100 mg/kg (in vivo) | (in vitro) Decreased: COX-2, TNF-α, IL-6, IL-1β, and p-NF-κBp65 Increased: cell viability of CML-treated cells Improved: CML-induced apoptosis (in vivo) Decreased: p-NF-κB, TNF-α, IL-1β, IL-6, and caspase-3 (relieved hippocampus neuronal apoptosis) Improved: cognitive impairment (enhanced spatial learning and memory abilities) | [14] |
| Anthocyanin | Female Sprague Dawley rats | 50, 100, and 200 mg/kg | Decreased: d-gal-Induced neuronal apoptosis, p-JNK, Bax/Bcl-2 ratio, caspase-3, RAGE, BACE-1, Aβ42, GFAP, and Iba-1 Improved: learning, memory impairment, memory ability, and passive avoidance | [17] | |
| Polysaccharide | Primary cortical neuronal cells in Sprague Dawley rats | 0, 5, 10, and 20 μM | Increased: cell viability and expression levels of Nrf2 Inhibited: apoptosis (decreased caspase-3 activity and ratio of bax/bcl-2) | [23] | |
| Polyphenolic Glycosides | PC12 cells | 25, 50, and 100 μM | MAO-B inhibition rates (compounds 1, 2, 11, 16, and 17/1): IC50 value of 60.7 ± 1.7, 22.2 ± 0.8, 79.3 ± 2.4, 68.9 ± 1.5, and 75.5 ± 3.8 μM, respectively Decreased: apoptosis (compounds 1, 2, and 6) Improved: cell viabilities (compounds 1–3 and 5/6), morphologic changes (compounds 1 and 2) | [24] | |
| Cardioprotective | Fruit extract | ICR mice | 375 and 750 mg/kg | Decreased: CK-MB and LDH activities (amelioration of the myocardial histopathology), fibers necrosis, the number of inflammatory cells, and myocardial tissue (improved myocardial tissue damage) | [25] |
| Radiation injury protective | Fruit extract | Male Kunming mice | 2, 4, and 6 g/kg | Decreased: caspase-3, P53, and apoptosis Increased: thymus index, spleen index, and DNA content | [26] |
| Immunomodulation | Fruit extract | Male Kunming mice | 2, 4, and 6 g/kg | Increased: thymus index and spleen index | [26] |
| Polysaccharide | RAW264.7 cells | 25, 50, 100, 200, 500, and 1000 μg/mL | Not significantly changed: cell viability at concentrations of less than 200 μg/mL Increased: NO release (25, 50, 100, and 200 μg/mL; activation of macrophage) | [27] | |
| Polysaccharide | Female Kunming mice | 25, 50, and 100 mg/kg | Increased: thymus index, spleen index, T cell and B cell proliferation, macrophage phagocytosis, serum hemolysin formation, IL-2, IL-6, and TNF-α (immunosuppressed mice) | [28] | |
| Anthocyanin | Synovial fibroblasts (Isolation from RA patients) | 100, 200, and 400 μg/mL | Decreased: SF cell viability, proliferation Not significantly changed: T cells and monocyte/macrophage development | [29] | |
| Anti-fatigue | Fruit extract | Kunming mice | 0.05, 0.1, 0.2, and 0.5 mg/g | Exercise-induced oxidative stress and inflammation were reduced. Decreased: LDH, TNF-α, IL-1β, IL-2, and IL-6 level Increased: SOD level | [15] |
| Polysaccharide | Male BALB/c mice | 50, 100, and 200 mg/kg | Decreased: immobility times, BUN, TG, CPK, LDH, and MDA levels Increased: glucose, SOD, and GPx levels LRM helped mobilize TG during exercise and protected microparticles by preventing lipid oxidation by modifying several enzyme activities. | [30] | |
| Anti-obesity and antidiabetic | Fruit extract | Male ApoE−/− mice | 140 mg/kg | Decreased: Pparγ and Fasn (compared to NC) and Srebp1 (compared to WD) Not significantly changed: Scd, Lpl, and Lxrα Increased: Cpt (compared to WD) | [16] |
| Seed oil | Cell free | 0.80, 1.60, 3.20, 6.40, and 12.80 mg/mL | Pancreatic lipase inhibitory activity: IC50 value of 12.4 ± 0.1 mg/mL CEase inhibitory activity: IC50 value of 2.6 ± 0.1 mg/mL (reversible non-competitive inhibition) | [31] | |
| Phenylpropanoid derivatives | Cell free | 400 μM (Compound 13: 100, 200, 400, and 800 μM) | Most of the compounds showed weak inhibitory activity. Compound 13 showed an effect similar to that of the positive control (acarbose) and increased the inhibitory effect in a dose-dependent manner. | [32] | |
| Anti-influenza | Fruit extract | MDCK cell and influenza virus A/H3N2 | 15.625, 31.25, 62.5, 125, 250, and 500 μg/mL | Decreased: influenza activity (CC50 value was higher than 125 μg/mL) Increased: MDCK cell viability infected with the virus | [33] |
| Tyrosinase inhibitory | Fruit extract and purified anthocyanin | Cell free | 0, 0.75, 1.5, 2.25, and 3 mg/mL | Tyrosinase monophenolase inhibitory activity: IC50 value of 3.0 ± 0.02 mg/mL (extract) and 1.5 ± 0.058 mg/mL (purified), reversible competitive inhibition, and Ki = 39.8 ± 1.4 mg/mL Diphenolase inhibitory activity: 3 mg/mL, 30.8 ± 1.0% (extract) and 3 mg/mL, 42.2 ± 0.77% (purified), reversible uncompetitive inhibition, and Kis = 2.4 ± 0.10 mg/mL | [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.S.; Choi, C.-I. Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity. Nutrients 2023, 15, 4181. https://doi.org/10.3390/nu15194181
Lee HS, Choi C-I. Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity. Nutrients. 2023; 15(19):4181. https://doi.org/10.3390/nu15194181
Chicago/Turabian StyleLee, Ho Seon, and Chang-Ik Choi. 2023. "Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity" Nutrients 15, no. 19: 4181. https://doi.org/10.3390/nu15194181
APA StyleLee, H. S., & Choi, C. -I. (2023). Black Goji Berry (Lycium ruthenicum Murray): A Review of Its Pharmacological Activity. Nutrients, 15(19), 4181. https://doi.org/10.3390/nu15194181