Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials
Abstract
:1. Introduction
2. Ketogenic Diet
3. Diabetes Mellitus
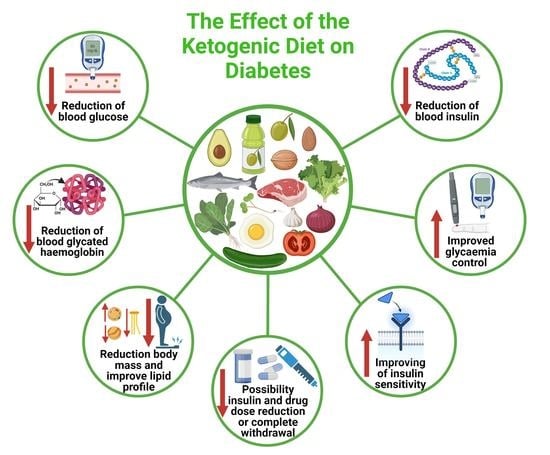
4. The Effect of the Ketogenic Diet on the Pharmacotherapy of Type 1 and Type 2 Diabetes
5. The Effect of the Ketogenic Diet on the Course of Type 1 Diabetes
5.1. Possible Mechanisms of Therapeutic Ketogenic Diet Activity in Type 1 Diabetes
5.2. The Ketogenic Diet in the Treatment of Type 1 Diabetes in Children
5.3. The Ketogenic Diet in the Treatment of Type 1 Diabetes in Adults
6. The Effect of the Ketogenic Diet on the Prevention and Treatment of Type 2 Diabetes
6.1. The Effect of the Ketogenic Diet in the Therapy of Type 2 Diabetes—Meta-Analyses and Systematic Reviews
6.2. The Effect of the Ketogenic Diet in the Therapy of Diabetes Type 2—Randomized Controlled Trials (RCT)
6.3. The Effect of the Ketogenic Diet in the Therapy of Diabetes Type 2—Additional Studies
7. The Ketogenic Diet and Standard Recommended Diabetes Diets
8. The Ketogenic Diet in Practice
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilder, R.M.A. The effects of ketonemia on the course of epilepsy. Mayo Clin. Bull. 1921, 2, 307. [Google Scholar]
- Ko, A.; Kwon, H.E.; Kim, H.D. Updates on the ketogenic diet therapy for pediatric epilepsy. Biomed. J. 2021. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Chen, L.; She, D.; Chung, Y.; Ge, L.; Han, L. Ketogenic diet for epilepsy: An overview of systematic review and meta-analysis. Eur. J. Clin. Nutr. 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Pizzo, F.; Collotta, A.D.; Di Nora, A.; Costanza, G.; Ruggieri, M.; Falsaperla, R. Ketogenic diet in pediatric seizures: A randomized controlled trial review and meta-analysis. Expert Rev. Neurother. 2022, 22, 169–177. [Google Scholar] [CrossRef]
- Weber, D.D.; Aminazdeh-Gohari, S.; Kofler, B. Ketogenic diet in cancer therapy. Aging 2018, 10, 164–165. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.C.L.; Deprez, L.M.; Mortimer, G.M.N.; Murtagh, D.K.J.; McCoy, S.; Mylchreest, R.; Gilbertson, L.J.; Clark, K.M.; Simpson, P.V.; McManus, E.J.; et al. Randomized crossover trial of a modified ketogenic diet in Alzheimer’s disease. Alzheimer’s Res. Ther. 2021, 13, 51. [Google Scholar] [CrossRef]
- Dowis, K.; Banga, S. The Potential Health Benefits of the Ketogenic Diet: A Narrative Review. Nutrients 2021, 13, 1654. [Google Scholar] [CrossRef] [PubMed]
- Joslin, E.P. The Treatment of Diabetes Mellitus. Can. Med. Assoc. J. 1916, 6, 673–684. [Google Scholar]
- Henwood, M.J.; Thornton, P.S.; Preis, C.M.; Chee, C.; Grimberg, A. Reconciling diabetes management and the ketogenic diet in a child with pyruvate dehydrogenase deficiency. J. Child Neurol. 2006, 21, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Westman, E.C.; Yancy, W.S., Jr.; Humphreys, M. Dietary treatment of diabetes mellitus in the pre-insulin era (1914–1922). Perspect. Biol. Med. 2006, 49, 77–83. [Google Scholar] [CrossRef]
- Gumbiner, B.; Wendel, J.A.; McDermott, M.P. Effects of diet composition and ketosis on glycemia during very-low-energy-diet therapy in obese patients with non-insulin-dependent diabetes mellitus. Am. J. Clin. Nutr. 1996, 63, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Bolla, A.M.; Caretto, A.; Laurenzi, A.; Scavini, M.; Piemonti, L. Low-Carb and Ketogenic Diets in Type 1 and Type 2 Diabetes. Nutrients 2019, 11, 962. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.A.; Mathew, T.C.; Dashti, A.A.; Asfar, S.; Al-Zaid, N.; Dashti, H.M. Effect of low-calorie versus low-carbohydrate ketogenic diet in type 2 diabetes. Nutrition 2012, 28, 1016–1021. [Google Scholar] [CrossRef]
- Brouns, F. Overweight and diabetes prevention: Is a low-carbohydrate–high-fat diet recommendable? Eur. J. Nutr. 2018, 57, 1301–1312. [Google Scholar] [CrossRef] [Green Version]
- Schiavo, L.; Pilone, V.; Rossetti, G.; Barbarisi, A.; Cesaretti, M.; Iannelli, A. A 4-Week Preoperative Ketogenic Micronutrient Enriched Diet Is Effective in Reducing Body Weight, Left Hepatic Lobe Volume, and Micronutrient Deficiencies in Patients Undergoing Bariatric Surgery: A Prospective Pilot Study. Obes. Surg. 2018, 28, 2215–2224. [Google Scholar] [CrossRef]
- Alarim, R.A.; Alasmre, F.A.; Alotaibi, H.A.; Alshehri, M.A.; Hussain, S.A. Effects of the Ketogenic Diet on Glycemic Control in Diabetic Patients: Meta-Analysis of Clinical Trials. Cureus 2020, 12, e10796. [Google Scholar] [CrossRef]
- Michalczyk, M.M.; Klonek, G.; Maszczyk, A.; Zajac, A. The Effects of a Low Calorie Ketogenic Diet on Glycaemic Control Variables in Hyperinsulinemic Overweight/Obese Females. Nutrients 2020, 12, 1854. [Google Scholar] [CrossRef]
- Pondel, N.; Liśkiewicz, D.; Liśkiewicz, A. Dieta ketogeniczna–mechanizm działania i perspektywy zastosowania w terapii: Dane z badań klinicznych. Postępy Biochem. 2020, 66, 270–286. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional Roles of Ketone Bodies in Fuel Metabolism, Signaling, and Therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [Green Version]
- Alharbi, A.; Al-Sowayan, N. The Effect of Ketogenic-Diet on Health. Jan. Food Nutr. Sci. 2020, 11, 301–313. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.; Lowery, R. The Ketogenic Bible; Victory Belt Publishing Inc.: Las Vegas, NV, USA, 2017; ISBN 13:978-1-628601-04-6. [Google Scholar]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cloete, L. Diabetes mellitus: An overview of the types, symptoms, complications and management. Nurs. Stand. 2022, 37, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Katsarou, A.; Gudbjörnsdottir, S.; Rawshani, A.; Dabelea, D.; Bonifacio, E.; Anderson, B.J.; Jacobsen, L.M.; Schatz, D.A.; Lernmark, Å. Type 1 diabetes mellitus. Nat. Rev. Dis. Primers 2017, 3, 17016. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cdc.gov/diabetes/basics/what-is-type-1-diabetes.html (accessed on 14 January 2023).
- Divers, J.; Mayer-Davis, E.J.; Lawrence, J.; Isom, S.; Dabelea, D.; Dolan, L.; Imperatore, G.; Marcovina, S.; Pettitt, D.J.; Pihoker, C.; et al. Trends in Incidence of Type 1 and Type 2 Diabetes Among Youths-Selected Counties and Indian Reservations, United States, 2002–2015. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Kotwas, A.; Karakiewicz, B.; Zabielska, P.; Wieder-Huszla, S.; Jurczak, A. Epidemiological factors for type 2 diabetes mellitus: Evidence from the Global Burden of Disease. Arch. Public Health 2021, 79, 110. [Google Scholar] [CrossRef]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2. In StatPearls [Internet]; [Updated 28 September 2021]; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (accessed on 14 January 2023).
- American Diabetes Association. Standards of Medical Care in Diabetes—2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [Google Scholar] [CrossRef]
- Dashti, H.M.; Mathew, T.C.; Al-Zaid, N.S. Efficacy of Low-Carbohydrate Ketogenic Diet in the Treatment of Type 2 Diabetes. Med. Princ. Pract. 2021, 30, 223–235. [Google Scholar] [CrossRef]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Holst, J.J.; Madsbad, S.; Nørgaard, K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes Obes. Metab. 2017, 19, 1479–1484. [Google Scholar] [CrossRef]
- Westman, E.C.; Yancy, W.S.; Mavropoulos, J.C.; Marquart, M.; McDuffie, J.R. The effect of a low-carbohydrate, ketogenic diet versus a lowglycemic index diet on glycemic control in type 2 diabetes mellitus. Nutr. Metab. 2008, 5, 36. [Google Scholar] [CrossRef] [Green Version]
- Handelsman, Y.; Henry, R.R.; Bloomgarden, Z.T.; Dagogo-Jack, S.; DeFronzo, R.A.; Einhorn, D.; Ferrannini, E.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Position Statement on the association of SGLT-2 inhibitors and diabetic ketoacidosis. Endocr. Pract. 2016, 22, 753–762. [Google Scholar] [CrossRef] [Green Version]
- Ahrén, B.; Hirsch, I.B.; Pieber, T.R.; Mathieu, C.; Gómez-Peralta, F.; Hansen, T.K.; Philotheou, A.; Birch, S.; Christiansen, E.; Jensen, T.J.; et al. ADJUNCT TWO Investigators. Efficacy and safety of liraglutide added to capped insulin treatment in subjects with type 1 diabetes: The ADJUNCT TWO randomized trial. Diabetes Care 2016, 39, 1693–1701. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, C.; Zinman, B.; Hemmingsson, J.U.; Woo, V.; Colman, P.; Christiansen, E.; Linder, M.; Bode, B. ADJUNCT ONE Investigators. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: The ADJUNCT ONE Treat-To-Target randomized trial. Diabetes Care 2016, 39, 1702–1710. [Google Scholar] [CrossRef]
- Moriconi, E.; Camajani, E.; Fabbri, A.; Lenzi, A.; Caprio, M. Very-low-calorie ketogenic diet as a safe and valuable tool for long-term glycemic management in patients with obesity and type 2 diabetes. Nutrients 2021, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Krejčí, H.; Vyjídák, J.; Kohutiar, M. Low-carbohydrate diet in diabetes mellitus treatment. Vnitr. Lek. 2018, 64, 742–752. (In English) [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S., Jr.; Foy, M.; Chalecki, A.M.; Vernon, M.C.; Westman, E.C. A low-carbohydrate, ketogenic diet to treat type 2 diabetes. Nutr. Metab. 2005, 2, 34. [Google Scholar] [CrossRef]
- Tinguely, D.; Gross, J.; Kosinski, C. Efficacy of Ketogenic Diets on Type 2 Diabetes: A Systematic Review. Curr. Diab. Rep. 2021, 21, 32. [Google Scholar] [CrossRef]
- Wong, K.; Raffray, M.; Roy-Fleming, A.; Blunden, S.; Brazeau, A.S. Ketogenic Diet as a Normal Way of Eating in Adults With Type 1 and Type 2 Diabetes: A Qualitative Study. Can. J. Diabetes 2021, 45, 137–143. [Google Scholar] [CrossRef]
- Krebs, H.A. The regulation of the release of ketone bodies by the liver. Adv. Enzym. Regul. 1966, 4, 339–354. [Google Scholar] [CrossRef]
- Volek, J.S.; Phinney, S.D. The Art and Science of Low Carbohydrate Performance; Beyond Obesity LLC: London, UK, 2012. [Google Scholar]
- Oh, R.; Gilani, B.; Uppaluri, K.R. Low Carbohydrate Diet. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Sukkari, M.A.; Cotten, L.; Alam, M.; Temponi, E.; John, P.D.; Davis, G.; Vellanki, P. Ketogenic Diet in a Patient With Type 1 Diabetes Mellitus With Hypoglycemia Unawareness. J. Endocr. Soc. 2021, 5, A460. [Google Scholar] [CrossRef]
- Buehler, L.A.; Noe, D.; Knapp, S.; Isaacs, D.; Pantalone, K.M. Ketogenic diets in the management of type 1 diabetes: Safe or safety concern? Clevel. Clin. J. Med. 2021, 88, 547–555. [Google Scholar] [CrossRef]
- De Goffau, M.C.; Luopajärvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef] [Green Version]
- Endesfelder, D.; Engel, M.; Davis-Richardson, A.G.; Ardissone, A.N.; Achenbach, P.; Hummel, S.; Winkler, C.; Atkinson, M.; Schatz, D.; Triplett, E.; et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 2016, 4, 17. [Google Scholar] [CrossRef]
- Ang, Q.Y.; Alexander, M.; Newman, J.C.; Tian, Y.; Cai, J.; Upadhyay, V.; Turnbaugh, J.A.; Verdin, E.; Hall, K.D.; Leibel, R.L.; et al. Ketogenic Diets Alter the Gut Microbiome Resulting in Decreased Intestinal Th17 Cells. Cell 2020, 181, 1263–1275. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Sasaki, D.; Hannya, A.; Tsubota, J.; Kondo, A. In vitro human colonic microbiota utilises D-β-hydroxybutyrate to increase butyrogenesis. Sci. Rep. 2020, 10, 8516. [Google Scholar] [CrossRef]
- Olson, C.A.; Lum, G.R.; Hsiao, E.Y. Ketone Bodies Exert Ester-Ordinary Suppression of Bifidobacteria and Th17 Cells. Cell Metab. 2020, 31, 1049–1051. [Google Scholar] [CrossRef] [PubMed]
- Paoli, A.; Mancin, L.; Bianco, A.; Thomas, E.; Mota, J.F.; Piccini, F. Ketogenic Diet and Microbiota: Friends or Enemies? Genes 2019, 10, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.Y.; Lee, C.; Longo, V.D. Nutrition and fasting mimicking diets in the prevention and treatment of autoimmune diseases and immunosenescence. Mol. Cell. Endocrinol. 2017, 455, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.-W.; Villani, V.; Buono, R.; Wei, M.; Kumar, S.; Yilmaz, O.H.; Cohen, P.; Sneddon, J.B.; Perin, L.; Longo, V.D. Fasting-mimicking diet promotes ngn3-driven β-cell regeneration to reverse diabetes. Cell 2017, 168, 775–788. [Google Scholar] [CrossRef] [Green Version]
- Tóth, C.; Clemens, Z. Type 1 diabetes mellitus successfully managed with the paleolithic ketogenic diet. Int. J. Case Rep. Images 2014, 5, 699–703. [Google Scholar] [CrossRef]
- McClean, A.M.; Montorio, L.; McLaughlin, D.; McGovern, S.; Flanagan, N. Can a ketogenic diet be safely used to improve glycaemic control in a child with type 1 diabetes? Arch. Dis. Child. 2019, 104, 501–504. [Google Scholar] [CrossRef]
- Aylward, N.M.; Shah, N.; Sellers, E.A. The ketogenic diet for the treatment of myoclonic astatic epilepsy in a child with type 1 diabetes mellitus. Can. J. Diabetes 2014, 38, 223–224. [Google Scholar] [CrossRef]
- Cogen, F.R. Incorporation of the Ketogenic Diet in a Youth With Type 1 Diabetes. Clin. Diabetes 2020, 38, 412–415. [Google Scholar] [CrossRef] [PubMed]
- Dressler, A.; Reithofer, E.; Trimmel-Schwahofer, P.; Klebermasz, K.; Prayer, D.; Kasprian, G.; Rami, B.; Schober, E.; Feucht, M. Type 1 diabetes and epilepsy: Efficacy and safety of the ketogenic diet. Epilepsia 2010, 51, 1086–1089. [Google Scholar] [CrossRef] [PubMed]
- Tóth, C.; Clemens, Z. A child with type 1 diabetes mellitus (T1DM) successfully treated with the Paleolithic ketogenic diet: A 19-month insulin freedom. Int. J. Case Rep. Images 2015, 6, 753–758. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, R.L.A.; Mack, K.J.; Lteif, A. Successful treatment of type 1 diabetes and seizures with combined ketogenic diet and insulin. Pediatrics 2012, 129, e511–e514. [Google Scholar] [CrossRef]
- de Bock, M.; Lobley, K.; Anderson, D.; Davis, E.; Donaghue, K.; Pappas, M.; Siafarikas, A.; Cho, Y.H.; Jones, T.; Smart, C. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: An illustrative case series. Pediatr. Diabetes 2018, 19, 129–137. [Google Scholar] [CrossRef]
- Nielsen, J.V.; Jönsson, E.; Ivarsson, A. A low carbohydrate diet in type 1 diabetes: Clinical experience—A brief report. Ups. J. Med. Sci. 2005, 110, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Leow, Z.Z.X.; Guelfi, K.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet. Med. 2018. Epub ahead of print. [Google Scholar] [CrossRef]
- Krebs, J.D.; Strong, A.P.; Cresswell, P.; Reynolds, A.N.; Hanna, A.; Haeusler, S. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac. J. Clin. Nutr. 2016, 25, 78–84. [Google Scholar] [CrossRef]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S., Jr.; et al. Management of Type 1 Diabetes with a Very Low-Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.; Rush, A.; Kaye, J. Glycaemic stability of a cyclist with Type 1 diabetes: 4011 km in 20 days on a ketogenic diet. Diabet. Med. 2019, 36, 1503–1507. [Google Scholar] [CrossRef]
- Charoensri, S.; Sothornwit, J.; Trirattanapikul, A.; Pongchaiyakul, C. Ketogenic Diet-Induced Diabetic Ketoacidosis in a Young Adult with Unrecognized Type 1 Diabetes. Case Rep. Endocrinol. 2021, 2021, 6620832. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.J.; Jeon, S.M.; Shin, S. Impact of a Ketogenic Diet on Metabolic Parameters in Patients with Obesity or Overweight and with or without Type 2 Diabetes: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2005. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wang, J.; Yang, S.; Gao, M.; Cao, L.; Li, X.; Hong, D.; Tian, S.; Sun, C. Effect of the ketogenic diet on glycemic control, insulin resistance, and lipid metabolism in patients with T2DM: A systematic review and meta-analysis. Nutr. Diabetes 2020, 10, 38. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, M.; Liang, J.; He, G.; Chen, N. Ketogenic Diet Benefits to Weight Loss, Glycemic Control, and Lipid Profiles in Overweight Patients with Type 2 Diabetes Mellitus: A Meta-Analysis of Randomized Controlled Trails. Int. J. Environ. Res. Public Health 2022, 19, 10429. [Google Scholar] [CrossRef]
- Parry-Strong, A.; Wright-McNaughton, M.; Weatherall, M.; Hall, R.M.; Coppell, K.J.; Barthow, C.; Krebs, J.D. Very low carbohydrate (ketogenic) diets in type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 2431–2442. [Google Scholar] [CrossRef]
- Rafiullah, M.; Musambil, M.; David, S.K. Effect of a very low-carbohydrate ketogenic diet vs recommended diets in patients with type 2 diabetes: A meta-analysis. Nutr. Rev. 2022, 80, 488–502. [Google Scholar] [CrossRef]
- Zaki, H.A.; Iftikhar, H.; Bashir, K.; Gad, H.; Fahmy, A.S.; Elmoheen, A. A Comparative Study Evaluating the Effectiveness Between Ketogenic and Low-Carbohydrate Diets on Glycemic and Weight Control in Patients With Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e25528. [Google Scholar] [CrossRef]
- Skow, S.L.; Jha, R.K. A Ketogenic Diet is Effective in Improving Insulin Sensitivity in Individuals with Type 2 Diabetes. Curr. Diabetes Rev. 2022. Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Charlot, A.; Zoll, J. Beneficial Effects of the Ketogenic Diet in Metabolic Syndrome: A Systematic Review. Diabetology 2022, 3, 292–309. [Google Scholar] [CrossRef]
- Li, M.; Yuan, J. Effects of very low-carbohydrate ketogenic diet on lipid metabolism in patients with type II diabetes mellitus: A meta-analysis. Nutr. Hosp. 2022, 39, 916–923. (In English) [Google Scholar] [CrossRef]
- Wolff, K.; Cavanaugh, K.; Malone, R.; Hawk, V.; Gregory, B.P.; Davis, D.; Wallston, K.; Rothman, R.L. The Diabetes Literacy and Numeracy Education Toolkit (DLNET): Materials to facilitate diabetes education and management in patients with low literacy and numeracy skills. Diabetes Educ. 2009, 35, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Saslow, L.R.; Mason, A.E.; Kim, S.; Goldman, V.; Ploutz-Snyder, R.; Bayandorian, H.; Daubenmier, J.; Hecht, F.M.; Moskowitz, J.T. An Online Intervention Comparing a Very Low-Carbohydrate Ketogenic Diet and Lifestyle Recommendations Versus a Plate Method Diet in Overweight Individuals With Type 2 Diabetes: A Randomized Controlled Trial. J. Med. Internet Res. 2017, 19, e36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goday, A.; Bellido, D.; Sajoux, I.; Crujeiras, A.B.; Burguera, B.; García-Luna, P.P.; Oleaga, A.; Moreno, B.; Casanueva, F.F. Short-term safety, tolerability and efficacy of a very low-calorie-ketogenic diet interventional weight loss program versus hypocaloric diet in patients with type 2 diabetes mellitus. Nutr. Diabetes 2016, 6, e230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Lin, G.; Chen, J.; Chen, Z.; Xu, F.; Zhu, F.; Zhang, J.; Yuan, S. The effect of periodic ketogenic diet on newly diagnosed overweight or obese patients with type 2 diabetes. BMC Endocr. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Gardner, C.D.; Landry, M.J.; Perelman, D.; Petlura, C.; Durand, L.R.; Aronica, L.; Crimarco, A.; Cunanan, K.M.; Chang, A.; Dant, C.C.; et al. Effect of a ketogenic diet versus Mediterranean diet on glycated hemoglobin in individuals with prediabetes and type 2 diabetes mellitus: The interventional Keto-Med randomized crossover trial. Am. J. Clin. Nutr. 2022, 116, 640–652, Erratum in Am. J. Clin. Nutr. 2022 Nov. 09. [Google Scholar] [CrossRef]
- Saslow, L.R.; Kim, S.; Daubenmier, J.J.; Moskowitz, J.T.; Phinney, S.D.; Goldman, V.; Murphy, E.J.; Cox, R.M.; Moran, P.; Hecht, F.M. A randomized pilot trial of a moderate carbohydrate diet compared to a very low carbohydrate diet in overweight or obese individuals with type 2 diabetes mellitus or prediabetes. PLoS ONE 2014, 9, e91027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saslow, L.R.; Daubenmier, J.J.; Moskowitz, J.T.; Kim, S.; Murphy, E.J.; Phinney, S.D.; Ploutz-Snyder, R.; Goldman, V.; Cox, R.M.; Mason, A.E.; et al. Twelve-month outcomes of a randomized trial of a moderate-carbohydrate versus very low-carbohydrate diet in overweight adults with type 2 diabetes mellitus or prediabetes. Nutr. Diabetes 2017, 7, 304. [Google Scholar] [CrossRef] [Green Version]
- Landry, M.J.; Crimarco, A.; Perelman, D.; Durand, L.R.; Petlura, C.; Aronica, L.; Robinson, J.L.; Kim, S.H.; Gardner, C.D. Adherence to Ketogenic and Mediterranean Study Diets in a Crossover Trial: The Keto-Med Randomized Trial. Nutrients 2021, 13, 967. [Google Scholar] [CrossRef]
- Myette-Côté, É.; Durrer, C.; Neudorf, H.; Bammert, T.D.; Botezelli, J.D.; Johnson, J.D.; DeSouza, C.A.; Little, J.P. The effect of a short-term low-carbohydrate, high-fat diet with or without postmeal walks on glycemic control and inflammation in type 2 diabetes: A randomized trial. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R1210–R1219. [Google Scholar] [CrossRef] [Green Version]
- Jayedi, A.; Zeraattalab-Motlagh, S.; Jabbarzadeh, B.; Hosseini, Y.; Jibril, A.T.; Shahinfar, H.; Mirrafiei, A.; Hosseini, F.; Bidar, S.S. Dose-dependent effect of carbohydrate restriction for type 2 diabetes management: A systematic review and dose-response meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2022, 116, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, S.J.; McKenzie, A.L.; Williams, P.T.; Bhanpuri, N.H.; Peters, A.L.; Campbell, W.W.; Hazbun, T.L.; Volk, B.M.; McCarter, J.P.; Phinney, S.D.; et al. Effectiveness and Safety of a Novel Care Model for the Management of Type 2 Diabetes at 1 Year: An Open-Label, Non-Randomized, Controlled Study. Diabetes Ther. 2018, 9, 583–612. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Horgan, G.W.; Murison, S.D.; Bremner, D.M.; Lobley, G.E. Effects of a high-protein ketogenic diet on hunger, appetite, and weight loss in obese men feeding ad libitum. Am. J. Clin. Nutr. 2008, 87, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Sato, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y. Influence of fat and carbohydrate proportions on the metabolic profile in patients with type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.; Di, H.; Chen, G.; Mao, X.; Liu, C. Effects of low carbohydrate diets in individuals with type 2 diabetes: Systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2016, 9, 11166–11174. [Google Scholar]
- Snorgaard, O.; Poulsen, G.M.; Andersen, H.K.; Astrup, A. Systematic review and meta-analysis of dietary carbohydrate restriction in patients with type 2 diabetes. BMJ Open Diabetes Res. Care 2017, 5, e000354. [Google Scholar] [CrossRef]
- van Zuuren, E.J.; Fedorowicz, Z.; Kuijpers, T.; Pijl, H. Effects of low-carbohydrate-compared with low-fat-diet interventions on metabolic control in people with type 2 diabetes: A systematic review including GRADE assessments. Am. J. Clin. Nutr. 2018, 108, 300–331. [Google Scholar] [CrossRef] [Green Version]
- McArdle, P.; Greenfield, S.; Rilstone, S.; Narendran, P.; Haque, M.S.; Gill, P. Carbohydrate restriction for glycaemic control in type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2019, 36, 335–348. [Google Scholar] [CrossRef] [Green Version]
- Czupryniak, L. Current Topics in Diabetes. Off. J. Diabetes Pol. 2022, 2, 1–134. [Google Scholar]
- Nuttall, F.Q.; Almokayyad, R.M.; Gannon, M.C. Comparison of a carbohydrate-free diet vs. fasting on plasma glucose, insulin and glucagon in type 2 diabetes. Metabolism 2015, 64, 253–262. [Google Scholar] [CrossRef]



| Gender and Age | Disease | Dietary Intervention | Benefits | Adverse Effects | References |
|---|---|---|---|---|---|
| Boy, 4 years | Type 1 diabetes and myoclonic-astatic epilepsy | Ketogenic diet | 1. Acceptable control of epileptic seizures 2. Improvement of cognitive functions 3. Maintenance of the target glycemia values | No severe episodes of hypoglycemia or ketoacidosis were observed | [56] |
| Girl, 14 years | Type 1 diabetes | Ketogenic diet | 1. Significant improvement of subjective sensations 2. Significant improvement in glycemia | Not observed | [57] |
| Girl, 3.5 years | Type 1 diabetes, right hemiparesis, epilepsy | Ketogenic diet | 1. Absence of epileptic seizures 2. Improvement of development, motor functions, and activity 3. Proper glycemic control and improvement in the HbA1c value | 1 episode of ketoacidosis, apart from which no adverse effects were observed | [58] |
| A 9-year-old child (no information on gender) | Type 1 diabetes | Paleolithic ketogenic diet | 1. Improvement of insulin and glucose levels 2. Discontinuation of insulin treatment | Not observed | [59] |
| Girl, 2 years | Type 1 diabetes and epilepsy | Ketogenic diet | 1. No episodes of epileptic seizures 2. Improvement (reduction) of glycated hemoglobin level 3. No new episodes of diabetic ketoacidosis | Mild hypoglycemia episodes | [60] |
| Girl, 4 years | Pyruvate dehydrogenase deficiency, diabetic ketoacidosis, static encephalopathy, convulsive disorders | Ketogenic diet | 1. Proper glycemic control 2. Improvement of activity level 3. Significant developmental achievement 4. Compensation of linear growth from < 5th to the 50th percentile | No major adverse effects were observed | [9] |
| Gender and Age | Disease | Dietary Intervention | Benefits | Adverse Effects | References |
|---|---|---|---|---|---|
| Group of 17 women and 7 men. Mean age 51 ± 10 years | Type 1 diabetes | Low-carbohydrate diet (70–90 g carbohydrates) | 1. Significant reduction in hypoglycemia episodes and glycated hemoglobin concentration (from 7.5% to 6.4%), 2. Significant reduction in the postprandial requirement for insulin (from 21.1 IU daily to 12.4 IU daily) 3. Reduction of triglyceride concentration by 16% on average | Diabetic gastroparesis was observed in 6 individuals | [62] |
| Group of 7 men and 4 women. Mean age 36.1 ± 6.8 years | Type 1 diabetes | Ketogenic diet | 1. Normal HbA1c concentration (The mean HbA1c levels were 35 ± 4 mmol/mol (5.3 ± 0.4%)) 2. Slight changes in glycemia values (little daily glycemic variability (1.5 ± 0.7 mmol/L) | Hypoglycemia episodes and dyslipidemia were observed | [63] |
| Group of 4 men and 1 woman. Mean age 44.5 ± 10.4 years (in the normal carbohydrate amount group, there were an additional 3 men and 2 women, mean age 44.8 ± 8.3 years) | Type 1 diabetes | Target: ketogenic diet, 50–75 g carbohydrates as a result: low-carbohydrate diet, up to 100 g of carbohydrates | 1. Improved glycemic control (significant reductions in HbA1c (63 to 55 mmol/mol)) 2. Reduction of insulin doses (significant reductions in daily insulin use (64.4 to 44.2 units/day)) 3. Body mass reduction (83.2 to 78.0 kg) | One participant reported a higher irritability and another one a greater number of minor diseases | [64] |
| Group of 316 women and men. Mean age 27 ± 19 | Type 1 diabetes | Carbohydrates of 36 g on average, i.e., a ketogenic diet | 1. Improved glycemic control (mean HbA1c was 5.67% ± 0.66%) 2. Lower requirement for insulin | A group of 7 individuals in a year, who reported a hospitalization associated with diabetes (including ketoacidosis and hypoglycemia); in the remaining majority of cases, there were no adverse effects | [65] |
| Man. 37 years | Type 1 diabetes | Ketogenic diet | 1. Maintenance of glycemic stability (average interstitial glucose 6.1 mmol/L) and 80.4% of the time spent within a range of 3.9–10 mmol/L. Interstitial glucose was < 3 mmol/L for 2.1% of this time 2. No problems with riding 4011 km on a bicycle over 20 days | 1 episode of major hypoglycemia | [66] |
| Woman. 22 years | Undiagnosed type 1 diabetes | Ketogenic diet | None | Development of diabetic ketoacidosis in 4 days | [67] |
| Year and Type of Publication | Number of Studies Considered | Diet Type | Publication Aim | Results | References |
|---|---|---|---|---|---|
| 2020 Meta-analysis | 14 RCT | Ketogenic diet | Assessment of the effectiveness of a ketogenic diet in metabolic compensation in patients who are overweight/obese, with and without type 2 diabetes, compared with a low-fat diet | Advantages of a ketogenic diet over a low-fat diet in the control of glycemia (lower HbA1c levels (SMD −0.62), body mass (SMD −0.46) and lipid profile (reduction in triglyceride concentration (mean -0.45, increase in HDL (SMD 0.31) concentration) | [68] |
| 2020 Systematic review and meta-analysis | 13 | Ketogenic diet | Assessment of the effect of a ketogenic diet on the control of glycemia, insulin resistance, and lipid metabolism in type 2 diabetes patients | Reduction in the concentrations of glucose (by 1.29 mmol/L on average), glycated hemoglobin (by 1.07% HbA1c on average), total cholesterol (by 0.33 mmol/L on average, LDL (by 0.05 mmol/L on average), and the reduction of body mass (by 8.66 kg on average), waist circumference (by 9.17 cm on average), and BMI (by 3.13 kg/m2 on average) HDL concentration increased (by 0.14 mmol/L, on average) | [69] |
| 2022 Meta-analysis | 8 RCT | Ketogenic diet | Studying the role of the ketogenic diet in controlling body mass and glycemia in patients with type 2 diabetes who are overweight | Reduction in body mass (by 5.63 kg on average), waist circumference (by 2.32 cm on average), glycated hemoglobin concentration (by 0.38% HbA1c on average), triglycerides (by 0.36 mmol/L on average) and an increase in HDL cholesterol concentration (by 0.28 mmol/L, on average) | [70] |
| 2022 Meta-analysis | 10 RCT | Ketogenic diet | Assessment of the effect of a ketogenic diet on lipid metabolism in patients with type 2 diabetes (compared with the effects of the standard diets) | Advantage of the ketogenic diet in reducing triglyceride concentration, particularly in the 3rd month (compared with the control group, with TG reduction in the 3rd, 6th, and 12th months of treatment, by 0.49 mmol/L, −0.82 mmol/L, and −0.18 mmol/L, on average) No significant differences in total cholesterol, LDL, and HDL concentrations | [76] |
| 2022 Systematic review and meta-analysis | 8 RCT | Ketogenic diet | Estimation of the effect of a ketogenic diet in type 2 diabetes patients and individuals with pre-diabetic conditions (compared with the diets with a higher content of carbohydrates than in a ketogenic diet) | In individuals on a ketogenic diet, compared with the control group, a reduction of triglyceride concentration by 0.28 mmol/L and an increase in HDL cholesterol level by 0.04 mmol/L. In total, 4 studies demonstrated changes in HbA1c concentration after 12 months, with the estimation of persistent effects (VLC/KD minus control group) at 0.01% level (−0.22 to 0.25). In addition, 2 studies demonstrated a change in HbA1c from the initial value: −0.65% (−0.99; −0.31) | [71] |
| 2022 Meta-analysis | 8RCT | Ketogenic diet | Comparison of a ketogenic diet vs. standard diet recommended in patients with type 2 diabetes in the context of parameter changes, i.e.: glycemia, body mass, lipid profile, drug taking, and the discontinuation of drug taking | Compared with the standard recommended diets, in patients on a ketogenic diet, a reduction in HbA1c after 3 and 6 months (by 6.7 mmol/L and 6.3 mmol/L, on average, respectively) and a body mass reduction after 3 and 6 months (by 2.91 kg and 2.84 kg on average, respectively) were observed. In a 12-month period, an advantage of a ketogenic diet over the control diets was seen in respect of triglyceride concentration reduction and a reduction in the requirements for drugs, as well as an increase in HDL concentration | [72] |
| 2022 Systematic review and meta-analysis | 15 | Ketogenic diet and low-carbohydrate diet | Assessment of the effectiveness of a ketogenic diet (and low-carbohydrate diet) in controlling glycemia and body mass in patients with type 2 diabetes | Patients using a ketogenic diet, compared with control diets, reduced their glycated hemoglobin values by 1.45% HbA1c, on average, and their body mass by 2.67 kg, on average | [73] |
| 2021 Systematic review | 14 | Ketogenic diet | Assessment of the pleiotropic effect of a ketogenic diet on glycemic control, drug changes, and body mass loss in patients with type 2 diabetes | Improvement of glycated hemoglobin concentrations in patients with type 2 diabetes within three weeks and the persistence of the effect for at least one year. Effectiveness in the long-term maintenance of a reduced body mass | [39] |
| Year and Type of Publication | Number of Patients and Duration | Diet Type | Publication Aim | Main Outcome(for Ketogenic Diet) | Percentage of Drop-Outs | References |
|---|---|---|---|---|---|---|
| 2017, RCT | 25 (12 in the intervention, 13 control). 32 weeks | Ketogenic diet ad libitum vs. a diet program based on the American Diabetes Association’s “Create Your Plate” website | Comparison of the online intervention of a ketogenic diet ad libitum vs. an online diet program based on the American Diabetes Association’s “Create Your Plate” website on glycemic control and other health outcomes among overweight individuals with type 2 diabetes | 1. Greater HbA1c decrease (−0.8% vs. −0.6%), 2. Lowering HbA1c to less than 6.5% (55% of participants vs. 0% of participants), 3. Greater weight loss (−12.7 kg vs. −3.0 kg), 4. A greater percentage of participants lost at least 5% of their body weight (90% of participants vs. 29% of participants) 5. Greater reduction in triglyceride levels (−60,1mg/dl vs. −28,9mg/dl) 6. Lower numbers of dropouts (8% of participants vs. 46% of participants) | Intervention group: 8% (1/12) and control group: 46% (6/13) | [78] |
| 2016, RCT | 89 (45 in the intervention, 44 control). 4 months | Low-calorie ketogenic diet (VLC/KD) vs. a standard low-calorie diet | Evaluating the short-term safety and tolerability of a very low-calorie-ketogenic diet (VLCKD) (< 50 g of carbohydrate daily) in an interventional weight loss program including lifestyle and behavioral modification support (Diaprokal method) in subjects with T2DM. | 1. Greater body mass reduction (−14.7 kg vs. −5.05 kg) 2. Greater percentage of participants lost more than 5% and 10% of their body weight (97.6% vs. 50% and 85.4% vs. 16.7%, respectively) 3. Greater reduction in waist circumference (−12 cm vs. −5.4 cm) 4. Greater reduction in HbA1c levels (−0.9% vs. −0.4%) 5. Greater reduction in oral diabetes medication (from 73.3% to 50% of participants vs. from 86.4% to 83.3% of participants) | VLCKD: 11.1% (5/45), LC diet: 18.2% (8/44) | [79] |
| 2022, RCT | 60 (30 intervention, 30 control). 12 weeks | Ketogenic diet vs. the routine diet for diabetes | Observation of a periodic ketogenic diet for its effect on overweight or obese patients newly diagnosed with T2DM, with a comparison with the routine diet for diabetes | 1. Greater reduction in HbA1c levels (−0.92% vs.–0.27%) 2. Greater reduction in fasting glucose concentration (−1.39 mmol/L vs. −0.56 mmol/L) 3. Greater reduction in fasting insulin concentration (−48.23 pmol/L vs. −3.7 pmol/L) 4. Greater body mass reduction (−8.06 kg vs. −0.61 kg) 5. Greater reduction in waist circumference (−9.29 cm vs. −0.77 cm) 6. Increase HDL concentration (+0.13 mmol/L vs. + 0.03 mmol/L) and decrease of LDL concentration (−0.41 mmol/L vs. −0.18 mmol/L) | Ketogenic diet group: 20% (6/30), Control group: 3.3% (1/30) | [80] |
| 2022, RCT | 40 (20 + 20) (33 after drop-outs (16 on the ketogenic diet vs. 17 on the Mediterranean diet)). 12 weeks | Ketogenic diet vs. a low-carb Mediterranean diet | Comparison of 2 low-carbohydrate diets with 3 key similarities (incorporating nonstarchy vegetables and avoiding added sugars and refined grains) and 3 key differences (incorporating, compared with avoiding, legumes, fruits, and whole, intact grains) for their effects on glucose control and cardiometabolic risk factors in individuals with pre-diabetes and T2DM | 1. Decrease in triglyceride concentration (−16% vs. −5%) 2. Increase in HDL concentration (+11% vs. +7%) 3. Greater reduction in body mass (−8% vs. −7%) 4. Increase in LDL concentration (+10% vs. −5%) | 17.5% (7/40) | [81] |
| 2021, RCT | 40 (20 + 20) 24 weeks (12 + 12) | Ketogenic diet vs. a low-carb Mediterranean diet | Detailed examination and comparison of the adherence to the two study diets (well-formulated ketogenic diet (WFKD) and Mediterranean Plus (Med-Plus) under two conditions: all food being provided (delivered) and all food being obtained by individual participants (self-provided) | 1. Higher adherence to the WFKD diet in the food delivery phase (7.6 vs. 7.3) and self-provided food (5.7 vs. 5.4) on a 10-point scale. 2. After study completion, a clear relationship with diet preference was observed—participants preferred the diet that they were assigned first | 12.5% (5/40) | [84] |
| 2014, RCT | 34 (16 ketogenic diet + 18 standard ADA diet). 3 months | Ketogenic diet vs. a diet consistent with guidelines from the American Diabetes Association | Comparison of the effects of each diet on glycemic control, medication use, and weight loss among overweight or obese individuals with type 2 diabetes mellitus or prediabetes + testing the feasibility of research design for conducting a larger scale | 1. Decrease in HbA1c levels (−0.6% vs. 0%) 2. Higher percentage of participants discontinued one or more diabetes medications (44% vs. 11%) 3. Higher percentage of participants discontinued sulfonylureas (31% vs. 5%) 4. Greater body mass reduction (−5.5 kg vs. −2.6 kg) | LCK group: 6.25% (1/16), MCCR group: 5.55% (1/18) | [82] |
| 2017, RCT | 34 (16 ketogenic diet vs. 18 standard diet). 12 months | Ketogenic diet vs. a low fat, moderate carbohydrate, calorie-restricted diet | Comparison of the effects of each diet on glycemic control, medication use, and weight loss among overweight or obese individuals with type 2 diabetes mellitus or pre-diabetes | 1. Greater reduction in HbA1c levels (−0.5% vs. −0.2%) 2. Greater body mass reduction (−7.9 kg vs. −1.7 kg) 3. Discontinued metformin medication (30% of participants vs. 0% of participants) 4. Discontinued sulfonylureas or dipeptidyl peptidase-4 inhibitor medications (60% of participants vs. 0% of participants) | LCK group: 12.5% (2/16), MCCR group: 16.7% (3/18) | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dyńka, D.; Kowalcze, K.; Ambrozkiewicz, F.; Paziewska, A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients 2023, 15, 500. https://doi.org/10.3390/nu15030500
Dyńka D, Kowalcze K, Ambrozkiewicz F, Paziewska A. Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients. 2023; 15(3):500. https://doi.org/10.3390/nu15030500
Chicago/Turabian StyleDyńka, Damian, Katarzyna Kowalcze, Filip Ambrozkiewicz, and Agnieszka Paziewska. 2023. "Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials" Nutrients 15, no. 3: 500. https://doi.org/10.3390/nu15030500
APA StyleDyńka, D., Kowalcze, K., Ambrozkiewicz, F., & Paziewska, A. (2023). Effect of the Ketogenic Diet on the Prophylaxis and Treatment of Diabetes Mellitus: A Review of the Meta-Analyses and Clinical Trials. Nutrients, 15(3), 500. https://doi.org/10.3390/nu15030500