Effect of Milling on Nutritional Components in Common and Zinc-Biofortified Wheat
Abstract
:1. Introduction
2. Materials and Methods
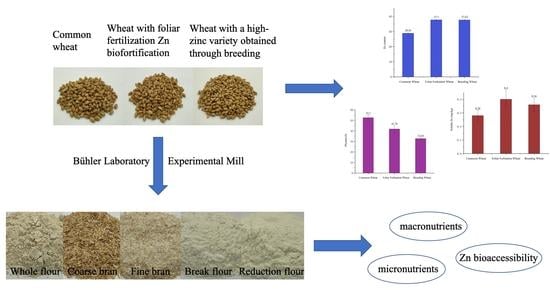
2.1. Materials
2.2. Milling
2.3. Analysis of Basic Nutrients
2.4. Analysis of Mineral Contents
2.5. Analysis of Phenolic Content
2.6. Analysis of Phytic Acid Content
2.7. Analysis of Vitamin B Contents
2.8. Estimation of Zn Bioaccessibility by In Vitro Gastrointestinal Digestion
2.9. Statistical Analysis
3. Results and Discussion
3.1. Basic Nutritional Components of Different Wheat Samples
3.2. Vitamin B Contents of Different Wheat Samples
3.3. Mineral Composition of Different Wheat Samples
3.4. Polyphenol and Phytate Contents of Different Wheat Samples
3.5. Bioaccessibility of Zinc in Different Wheat Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The Physiological, Biochemical, and Molecular Roles of Zinc Transporters in Zinc Homeostasis and Metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Wang, C.; Li, B.; Ao, J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC-MS/MS. Food Chem. 2012, 134, 1231–1238. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Secur. -Agric. Policy Econ. Environ. 2017, 12, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zou, C.; He, Z.; Shi, R.; Ortiz-Monasterio, I.; Qu, Y.; Zhang, Y. Mineral element distributions in milling fractions of Chinese wheats. J. Cereal Sci. 2008, 48, 821–828. [Google Scholar] [CrossRef]
- Drakos, A.; Kyriakakis, G.; Evageliou, V.; Protonotariou, S.; Mandala, I.; Ritzoulis, C. Influence of jet milling and particle size on the composition, physicochemical and mechanical properties of barley and rye flours. Food Chem. 2017, 215, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Previtali, M.A.; Mastromatteo, M.; Conte, A.; De Vita, P.; Ficco, D.B.M.; Del Nobile, M.A. Optimization of durum wheat bread from a selenium-rich cultivar fortified with bran. J. Food Sci. Technol. -Mysore 2016, 53, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Ortega, R.M.; Maldonado, J. Wholegrain cereals and bread: A duet of the Mediterranean diet for the prevention of chronic diseases. Public Health Nutr. 2011, 14, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Baye, K.; Guyot, J.P.; Mouquet-Rivier, C. The unresolved role of dietary fibers on mineral absorption. Crit. Rev. Food Sci. Nutr. 2017, 57, 949–957. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Liu, Y.; Zhang, W.; Chen, X.; Zou, C. Agronomic Approach of Zinc Biofortification Can Increase Zinc Bioavailability in Wheat Flour and thereby Reduce Zinc Deficiency in Humans. Nutrients 2017, 9, 465. [Google Scholar] [CrossRef]
- Swieca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Altundag, H.; Tuzen, M. Comparison of dry, wet and microwave digestion methods for the multi element determination in some dried fruit samples by ICP-OES. Food Chem. Toxicol. 2011, 49, 2800–2807. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Johnson, S.K.; Bornman, J.F.; Bennett, S.J.; Fang, Z. Changes in whole grain polyphenols and antioxidant activity of six sorghum genotypes under different irrigation treatments. Food Chem. 2017, 214, 199–207. [Google Scholar] [CrossRef]
- Haug, W.; Lantzsch, H.J. Sensitive Method for The Rapid-Determination of Phytate In Cereals and Cereal Products. J. Sci. Food Agric. 1983, 34, 1423–1426. [Google Scholar] [CrossRef]
- Sami, R.; Li, Y.; Qi, B.; Wang, S.; Zhang, Q.; Han, F.; Ma, Y.; Jing, J.; Jiang, L. HPLC Analysis of Water-Soluble Vitamins (B2, B3, B6, B12, and C) and Fat-Soluble Vitamins (E, K, D, A, and beta-Carotene) of Okra (Abelmoschus esculentus). J. Chem. 2014, 2014, 831357. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assuncao, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carriere, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Lopez-Morales, D.; de la Cruz-lazaro, E.; Sanchez-Chavez, E.; Preciado-Rangel, P.; Marquez-Quiroz, C.; Osorio-Osorio, R. Impact of Agronomic Biofortification with Zinc on the Nutrient Content, Bioactive Compounds, and Antioxidant Capacity of Cowpea Bean (Vigna unguiculata L. Walpers). Agron. -Basel 2020, 10, 1460. [Google Scholar] [CrossRef]
- Sidhu, J.S.; Al-Hooti, S.N.; Al-Saqer, J.M. Effect of adding wheat bran and germ fractions on the chemical composition of high-fiber toast bread. Food Chem. 1999, 67, 365–371. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Maqsood, M.; Hussain, S.; Anwar-ul-Haq, M. Iron Nutrition Improves Productivity, Profitability, and Biofortification of Bread Wheat under Conventional and Conservation Tillage Systems. J. Soil Sci. Plant Nutr. 2020, 20, 1298–1310. [Google Scholar] [CrossRef]
- Zhao, F.J.; Su, Y.H.; Dunham, S.J.; Rakszegi, M.; Bedo, Z.; McGrath, S.P.; Shewry, P.R. Variation in mineral micronutrient concentrations in grain of wheat lines of diverse origin. J. Cereal Sci. 2009, 49, 290–295. [Google Scholar] [CrossRef]
- Shi, K.; Yin, T.; Zhu, Y.; Liu, B.; Tang, L.; Cao, W.; Liu, L. Estimating the effect of low-temperature stress on the spatial distribution patterns of protein in wheat grains. J. Cereal Sci. 2022, 105, 103461. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Oniszczuk, A.; Kasprzak, K.; Olech, M.; Mitrus, M.; Oniszczuk, T. Chemical composition and selected quality characteristics of new types of precooked wheat and spelt pasta products. Food Chem. 2020, 309, 125673. [Google Scholar] [CrossRef]
- Rousseau, S.; Kyomugasho, C.; Celus, M.; Hendrickx, M.E.G.; Grauwet, T. Barriers impairing mineral bioaccessibility and bioavailability in plant-based foods and the perspectives for food processing. Crit. Rev. Food Sci. Nutr. 2020, 60, 826–843. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, L.; Phillips, F.; O’Sullivan, K.; Walton, J. Wheat bran: Its composition and benefits to health, a European perspective. Int. J. Food Sci. Nutr. 2012, 63, 1001–1013. [Google Scholar] [CrossRef]
- Hafeez, M.B.; Ramzan, Y.; Khan, S.; Ibrar, D.; Bashir, S.; Zahra, N.; Rashid, N.; Nadeem, M.; Rahman, S.U.; Shair, H.; et al. Application of Zinc and Iron-Based Fertilizers Improves the Growth Attributes, Productivity, and Grain Quality of Two Wheat (Triticum aestivum) Cultivars. Front. Nutr. 2021, 8, 1036. [Google Scholar] [CrossRef] [PubMed]
- Nurit, E.; Lyan, B.; Pujos-Guillot, E.; Branlard, G.; Piquet, A. Change in B and E vitamin and lutein, beta-sitosterol contents in industrial milling fractions and during toasted bread production. J. Cereal Sci. 2016, 69, 290–296. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, K.; Shariful, I.; Ye, X.; Zhang, C. Folate content and retention in wheat grains and wheat-based foods: Effects of storage, processing, and cooking methods. Food Chem. 2020, 333, 127459. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets-iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, R.; Rezaul, K.M.; Zhang, F.; Zou, C. Iron and Zinc Concentrations in Grain and Flour of Winter Wheat As Affected by Foliar Application. J. Agric. Food Chem. 2010, 58, 12268–12274. [Google Scholar] [CrossRef] [PubMed]
- Borrill, P.; Connorton, J.M.; Balk, J.; Miller, A.J.; Sanders, D.; Uauy, C. Biofortification of wheat grain with iron and zinc: Integrating novel genomic resources and knowledge from model crops. Front. Plant Sci. 2014, 5, 53. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, L.; Ning, P.; Chen, Q.; Wang, B.; Zhang, F.; Yang, X.; Zhang, Y. Zinc in cereal grains: Concentration, distribution, speciation, bioavailability, and barriers to transport from roots to grains in wheat. Crit. Rev. Food Sci. Nutr. 2021, 62, 7917–7928. [Google Scholar]
- Pongrac, P.; Kreft, I.; Vogel-Mikus, K.; Regvar, M.; Germ, M.; Vavpetic, P.; Grlj, N.; Jeromel, L.; Eichert, D.; Budic, B.; et al. Relevance for food sciences of quantitative spatially resolved element profile investigations in wheat (Triticum aestivum) grain. J. R. Soc. Interface 2013, 10, 20130296. [Google Scholar] [CrossRef] [Green Version]
- Guttieri, M.J.; Seabourn, B.W.; Liu, C.; Baenziger, P.S.; Waters, B.M. Distribution of Cadmium, Iron, and Zinc in Millstreams of Hard Winter Wheat (Triticum aestivum L.). J. Agric. Food Chem. 2015, 63, 10681–10688. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, I.; Torun, A.; Millet, E.; Feldman, M.; Fahima, T.; Korol, A.; Nevo, E.; Braun, H.J.; Ozkan, H. Triticum dicoccoides: An important genetic resource for increasing zinc and iron concentration in modern cultivated wheat. Soil Sci. Plant Nutr. 2004, 50, 1047–1054. [Google Scholar] [CrossRef]
- Choukri, H.; Hejjaoui, K.; El-Baouchi, A.; El Haddad, N.; Smouni, A.; Maalouf, F.; Thavarajah, D.; Kumar, S. Heat and Drought Stress Impact on Phenology, Grain Yield, and Nutritional Quality of Lentil (Lens culinaris Medikus). Front. Nutr. 2020, 7, 596307. [Google Scholar] [CrossRef]
- Ciccolini, V.; Pellegrino, E.; Coccina, A.; Fiaschi, A.I.; Cerretani, D.; Sgherri, C.; Quartacci, M.F.; Ercoli, L. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017, 65, 5443–5452. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, Q.; Pan, F.; Yuan, L.; Yin, X. Effects of increasing rates of zinc fertilization on phytic acid and phytic acid/zinc molar ratio in zinc bio-fortified wheat. Field Crops Res. 2015, 184, 58–64. [Google Scholar] [CrossRef]
- Zhang, P.; Ma, G.; Wang, C.; Zhu, Y.; Guo, T. Mineral Elements Bioavailability in Milling Fractions of Wheat Grain Response to Zinc and Nitrogen Application. Agron. J. 2019, 111, 2504–2511. [Google Scholar] [CrossRef]
- Zhao, A.; Tian, X.; Chen, Y.; Li, S. Application of ZnSO4 or Zn-EDTA fertilizer to a calcareous soil: Zn diffusion in soil and its uptake by wheat plants. J. Sci. Food Agric. 2016, 96, 1484–1491. [Google Scholar] [CrossRef]
- Liu, Z.H.; Wang, H.Y.; Wang, X.E.; Zhang, G.P.; Chen, P.D.; Liu, D.J. Phytase activity, phytate, iron, and zinc contents in wheat pearling fractions and their variation across production locations. J. Cereal Sci. 2007, 45, 319–326. [Google Scholar] [CrossRef]
- Hemalatha, S.; Platel, K.; Srinivasan, K. Influence of heat processing on the bioaccessibility of zinc and iron from cereals and pulses consumed in India. J. Trace Elem. Med. Biol. 2007, 21, 1–7. [Google Scholar] [CrossRef]
- Lemmens, E.; De Brier, N.; Spiers, K.M.; Ryan, C.; Garrevoet, J.; Falkenberg, G.; Goos, P.; Smolders, E.; Delcour, J.A. The impact of steeping, germination and hydrothermal processing of wheat (Triticum aestivum L.) grains on phytate hydrolysis and the distribution, speciation and bio-accessibility of iron and zinc elements. Food Chem. 2018, 264, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Belen Vignola, M.; Cecilia Bustos, M.; Teresa Perez, G. In vitro dialyzability of essential minerals from white and whole grain pasta. Food Chem. 2018, 265, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Vitali, D.; Dragojevic, I.V.; Sebecic, B. Bioaccessibility of Ca, Mg, Mn and Cu from whole grain tea-biscuits: Impact of proteins, phytic acid and polyphenols. Food Chem. 2008, 110, 62–68. [Google Scholar] [CrossRef] [PubMed]

| Wheat Variety | B Flour | R Flour | Fine Bran | Coarse Bran | Whole Flour |
|---|---|---|---|---|---|
| LT2 | 18.7 | 58.3 | 10.6 | 12.4 | 100 |
| LZ2 | 20.2 | 56.5 | 11.6 | 11.7 | 100 |
| JM26 | 15.6 | 56.2 | 13.2 | 15.0 | 100 |
| Wheat Sample | Fraction | Ash | Lipids | Proteins | Starch | TDF | SDF | IDF | Carbohydrates | Calorific Value |
|---|---|---|---|---|---|---|---|---|---|---|
| LT2 | Whole flour | 1.30 ± 0.03 cB | 1.28 ± 0.09 cB | 10.00 ± 0.21 cB | 63.40 ± 0.63 cB | 10.95 ± 0.20 cA | 0.94 ± 0.03 cC | 10.01 ± 0.17 cB | 73.42 ± 0.13 aA | 326.85 ± 4.66 abA |
| B flour | 0.40 ± 0.03 dB | 0.64 ± 0.04 eC | 12.48 ± 0.64 bAB | 72.60 ± 0.57 dB | 1.31 ± 0.01 dA | 0.49 ± 0.01 dA | 0.82 ± 0.01 dA | 72.68 ± 0.28 aA | 326.43 ± 1.83 bA | |
| R flour | 0.48 ± 0.05 dAB | 0.70 ± 0.02 dB | 9.55 ± 0.50 cB | 74.20 ± 0.65 dB | 1.28 ± 0.00 eA | 0.47 ± 0.02 dA | 0.81 ± 0.02 dA | 75.27 ± 2.53 aA | 326.76 ± 2.07 bA | |
| Fine bran | 2.31 ± 0.09 bB | 2.48 ± 0.11 aB | 12.85 ± 1.25 bA | 58.65 ± 0.56 bA | 16.62 ± 0.18 bB | 1.56 ± 0.04 bB | 15.06 ± 0.14 bA | 68.36 ± 0.77 bA | 330.07 ± 0.58 aA | |
| Coarse bran | 5.03 ± 0.03 aB | 1.82 ± 0.19 bB | 14.42 ± 0.36 aA | 19.78 ± 0.35 aB | 43.49 ± 0.66 aA | 2.68 ± 0.11 aB | 40.82 ± 0.77 aA | 64.03 ± 1.05 cA | 302.47 ± 2.41 cB | |
| LZ2 | Whole flour | 1.52 ± 0.04 cA | 1.54 ± 0.02 cA | 12.75 ± 0.47 bcA | 66.20 ± 0.52 cA | 11.81 ± 0.33 cA | 1.20 ± 0.01 cB | 10.61 ± 0.32 cA | 71.94 ± 2.33 bAB | 327.64 ± 2.70 aA |
| B flour | 0.34 ± 0.01 dB | 0.79 ± 0.03 cdB | 13.94 ± 0.71 bA | 75.53 ± 0.88 dA | 1.26 ± 0.02 dA | 0.44 ± 0.01 dA | 0.82 ± 0.01 dA | 70.93 ± 0.57 bA | 328.86 ± 3.29 aA | |
| R flour | 0.34 ± 0.04 dB | 0.77 ± 0.04 cdB | 10.55 ± 1.06 dAB | 76.10 ± 0.59 dA | 1.28 ± 0.04 dA | 0.46 ± 0.02 dA | 0.82 ± 0.01 dA | 74.34 ± 0.36 aA | 327.91 ± 1.64 aA | |
| Fine bran | 2.14 ± 0.05 bC | 2.90 ± 0.18 aA | 11.74 ± 0.27 cdA | 56.81 ± 1.07 bB | 15.95 ± 0.45 bB | 1.50 ± 0.07 bB | 14.45 ± 0.38 bB | 69.22 ± 1.49 bA | 332.64 ± 5.51 aA | |
| Coarse bran | 5.36 ± 0.03 aA | 1.85 ± 0.12 bB | 16.00 ± 2.04 aA | 16.78 ± 0.33 aC | 44.47 ± 0.50 aA | 2.93 ± 0.12 aA | 41.55 ± 0.37 aA | 62.89 ± 0.50 cB | 306.59 ± 3.21 bB | |
| JM26 | Whole flour | 1.53 ± 0.01 cA | 1.61 ± 0.01 cA | 12.02 ± 0.58 bcA | 64.60 ± 0.79 cB | 11.33 ± 0.22 cA | 1.59 ± 0.04 cA | 9.74 ± 0.26 cB | 70.84 ± 1.31 aB | 328.22 ± 1.81 aA |
| B flour | 0.55 ± 0.07 dA | 0.90 ± 0.05 dA | 12.19 ± 0.77 bcB | 73.96 ± 0.62 dB | 1.31 ± 0.03 dA | 0.49 ± 0.01 dA | 0.82 ± 0.02 dA | 72.36 ± 3.96 aA | 328.21 ± 1.27 aA | |
| R flour | 0.53 ± 0.11 dA | 0.91 ± 0.08 dA | 11.03 ± 0.57 cA | 76.14 ± 0.49 dA | 1.31 ± 0.02 dA | 0.49 ± 0.01 dA | 0.82 ± 0.01 dA | 73.53 ± 0.72 aA | 328.05 ± 1.53 aA | |
| Fine bran | 2.68 ± 0.03 bA | 3.09 ± 0.08 aA | 13.02 ± 0.52 bA | 52.79 ± 0.55 bC | 17.73 ± 0.23 bA | 2.00 ± 0.12 bA | 15.23 ± 0.11 bA | 67.21 ± 0.38 bA | 331.93 ± 5.28 aA | |
| Coarse bran | 4.96 ± 0.02 aC | 2.32 ± 0.06 bA | 14.84 ± 1.20 aA | 23.74 ± 0.50 aA | 39.36 ± 0.25 aB | 2.61 ± 0.16 aB | 36.75 ± 0.40 aB | 61.88 ± 0.55 cB | 312.29 ± 3.60 bA |
| Wheat Sample | Fraction | VB1 | VB2 | VB5 | VB6 | VB9 (µg/100 g) |
|---|---|---|---|---|---|---|
| LT2 | Whole flour | 1.39 ± 0.04 bC | 0.61 ± 0.02 cA | 3.14 ± 0.14 cB | 1.21 ± 0.06 cB | 68.44 ± 1.36 cC |
| B flour | 0.38 ± 0.02 eB | 0.18 ± 0.00 dA | 1.33 ± 0.07 eB | 0.42 ± 0.03 dC | 20.75 ± 0.94 eC | |
| R flour | 0.73 ± 0.04 dB | 0.15 ± 0.01 eA | 2.13 ± 0.11 dB | 0.42 ± 0.02 dB | 24.11 ± 1.11 dC | |
| Fine bran | 3.66 ± 0.01 aB | 1.07 ± 0.02 bA | 10.00 ± 0.52 bB | 2.76 ± 0.06 bA | 120.85 ± 2.33 bA | |
| Coarse bran | 1.01 ± 0.07 cB | 5.21 ± 0.34 aA | 23.57 ± 0.10 aB | 7.09 ± 0.45 aA | 150.45 ± 0.49 aB | |
| LZ2 | Whole flour | 1.56 ± 0.05 bB | 0.44 ± 0.02 cB | 2.62 ± 0.17 cC | 1.26 ± 0.06 cB | 89.75 ± 0.66 cB |
| B flour | 0.39 ± 0.03 eB | 0.19 ± 0.01 dA | 1.01 ± 0.04 dC | 1.09 ± 0.06 dA | 48.57 ± 1.03 dA | |
| R flour | 0.70 ± 0.04 dB | 0.19 ± 0.01 dA | 0.98 ± 0.03 dC | 0.77 ± 0.03 eA | 42.35 ± 0.99 eB | |
| Fine bran | 3.89 ± 0.04 aA | 0.66 ± 0.04 bB | 14.41 ± 0.73 bA | 2.06 ± 0.09 bC | 95.50 ± 3.35 bB | |
| Coarse bran | 1.10 ± 0.02 cB | 4.84 ± 0.07 aB | 18.17 ± 1.12 aC | 7.60 ± 0.41 aA | 112.20 ± 0.42 aC | |
| JM26 | Whole flour | 1.93 ± 0.12 bA | 0.44 ± 0.00 cB | 4.96 ± 0.23 cA | 1.64 ± 0.09 cA | 115.10 ± 2.55 bA |
| B flour | 0.88 ± 0.01 eA | 0.21 ± 0.01 dA | 3.20 ± 0.12 dA | 0.53 ± 0.03 dB | 30.04 ± 0.64 eB | |
| R flour | 1.19 ± 0.07 dA | 0.19 ± 0.00 eA | 3.29 ± 0.13 dA | 0.53 ± 0.03 dB | 59.06 ± 0.80 dA | |
| Fine bran | 2.65 ± 0.00 aC | 0.75 ± 0.00 bB | 13.80 ± 0.98 bA | 2.37 ± 0.11 bB | 78.07 ± 1.07 cC | |
| Coarse bran | 1.80 ± 0.01 cA | 3.56 ± 0.09 aC | 38.21 ± 1.82 aA | 7.38 ± 0.37 aA | 225.80 ± 7.92 aA |
| Wheat Sample | Fraction | Ca | Fe | Zn |
|---|---|---|---|---|
| LT2 | Whole flour | 343.52 ± 4.95 cB | 36.10 ± 0.85 cB | 28.81 ± 0.20 cB |
| B flour | 245.81 ± 3.54d AB | 18.15 ± 0.21 dC | 8.58 ± 0.03 dC | |
| R flour | 222.39 ± 2.82 eB | 8.10 ± 0.18 eB | 7.60 ± 0.05 dC | |
| Fine bran | 510.36 ± 0.71 bB | 56.91 ± 0.14 bA | 52.72 ± 3.51 bB | |
| Coarse bran | 982.17 ± 18.38 aC | 111.72 ± 2.12 aC | 96.07 ± 0.82 aC | |
| LZ2 | Whole flour | 364.64 ± 2.12 cA | 39.05 ± 0.35 cA | 37.70 ± 0.13 cA |
| B flour | 250.87 ± 9.19 dA | 24.65 ± 0.92 dA | 10.80 ± 0.07 dB | |
| R flour | 234.90 ± 6.36 eA | 8.77 ± 0.25 eB | 9.65 ± 0.02 dB | |
| Fine bran | 493.85 ± 16.26 bA | 58.33 ± 2.12 bA | 72.23 ± 0.66 bA | |
| Coarse bran | 1085.24 ± 7.07 aA | 138.58 ± 0.71 aA | 130.95 ± 1.06 aA | |
| JM26 | Whole flour | 365.60 ± 3.54 cA | 39.30 ± 0.71 cA | 37.62 ± 0.30 cA |
| B flour | 240.83 ± 6.36 dB | 20.32 ± 0.42 dB | 11.57 ± 0.02 dA | |
| R flour | 208.33 ± 11.31 eC | 11.35 ± 0.64 eA | 10.92 ± 0.18 dA | |
| Fine bran | 492.69 ± 14.85 bA | 59.11 ± 1.98 bA | 74.64 ± 0.37 bA | |
| Coarse bran | 995.93 ± 6.36 aB | 132.42 ± 1.41 aB | 119.85 ± 1.06 aB |
| Wheat Samples | Fraction | Polyphenols (mg/g) | Phytates (mg/g) | Phytase (U/g) | Phy:Ca | Phy:Fe | Phy:Zn |
|---|---|---|---|---|---|---|---|
| LT2 | Whole flour | 1.30 ± 0.01 cB | 15.22 ± 0.76 bA | 0.48 ± 0.03 cA | 2.69 ± 0.08 bA | 36.09 ± 2.73 aA | 52.50 ± 2.75 aA |
| B flour | 1.26 ± 0.09 cA | 1.47 ± 0.04 cB | 0.27 ± 0.01 dA | 0.36 ± 0.03 dB | 6.88 ± 0.51 dA | 16.67 ± 1.28 cA | |
| R flour | 1.06 ± 0.04 dB | 1.23 ± 0.06 cA | 0.19 ± 0.01 eB | 0.33 ± 0.01 dA | 12.86 ± 0.77 cA | 15.91 ± 0.45 cA | |
| Fine bran | 1.56 ± 0.08 bA | 18.76 ± 0.79 bA | 0.59 ± 0.05 bA | 2.23 ± 0.02 cA | 27.96 ± 1.30 bA | 35.04 ± 1.32 bA | |
| Coarse bran | 2.03 ± 0.14 aA | 47.88 ± 4.71 aB | 0.80 ± 0.12 aB | 2.95 ± 0.11 aA | 36.36 ± 1.85 aA | 49.08 ± 0.66 aA | |
| LZ2 | Whole flour | 1.54 ± 0.17 bA | 15.99 ± 1.76 cA | 0.41 ± 0.05 cB | 2.66 ± 0.09 bA | 34.76 ± 0.62 aA | 41.78 ± 3.51 aB |
| B flour | 1.20 ± 0.04 cA | 1.46 ± 0.09 dB | 0.27 ± 0.03 dA | 0.35 ± 0.01 cB | 5.02 ± 0.04 dB | 13.31 ± 0.63 cB | |
| R flour | 0.98 ± 0.07 dB | 1.15 ± 0.06 dA | 0.24 ± 0.02 dA | 0.30 ± 0.03 cA | 11.11 ± 0.89 cB | 11.72 ± 0.25 dB | |
| Fine bran | 1.66 ± 0.01 bA | 19.51 ± 1.50 bA | 0.54 ± 0.01 bA | 2.39 ± 0.24 bA | 28.42 ± 2.62 bA | 26.63 ± 1.89 bB | |
| Coarse bran | 2.09 ± 0.12 aA | 54.47 ± 2.89 aA | 0.87 ± 0.08 aA | 3.04 ± 0.17 aA | 33.41 ± 0.92 aB | 40.97 ± 3.32 aB | |
| JM26 | Whole flour | 1.40 ± 0.08 bAB | 12.47 ± 0.43 cB | 0.47 ± 0.03 cA | 2.07 ± 0.12 bB | 26.99 ± 1.99 aB | 32.64 ± 1.27 bC |
| B flour | 1.33 ± 0.13 bcA | 1.70 ± 0.17 dA | 0.29 ± 0.01 dA | 0.43 ± 0.03 cA | 7.17 ± 0.56 cA | 14.49 ± 0.67 dB | |
| R flour | 1.28 ± 0.04 cA | 1.05 ± 0.17 dA | 0.25 ± 0.02 dA | 0.31 ± 0.01 dA | 7.95 ± 0.20 bC | 9.46 ± 0.08 eC | |
| Fine bran | 1.56 ± 0.05 bA | 17.43 ± 1.60 bA | 0.56 ± 0.03 bA | 2.14 ± 0.14 bA | 24.92 ± 0.39 aB | 22.97 ± 0.52 cC | |
| Coarse bran | 1.92 ± 0.04 aA | 39.33 ± 3.71 aC | 0.84 ± 0.11 aA | 2.39 ± 0.06 aB | 25.25 ± 1.73 aC | 35.26 ± 1.32 aC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Zhou, S.; Peng, Y.; Wen, X.; Ni, Y.; Li, M. Effect of Milling on Nutritional Components in Common and Zinc-Biofortified Wheat. Nutrients 2023, 15, 833. https://doi.org/10.3390/nu15040833
Jiang Z, Zhou S, Peng Y, Wen X, Ni Y, Li M. Effect of Milling on Nutritional Components in Common and Zinc-Biofortified Wheat. Nutrients. 2023; 15(4):833. https://doi.org/10.3390/nu15040833
Chicago/Turabian StyleJiang, Zefang, Shiyue Zhou, Yu Peng, Xin Wen, Yuanying Ni, and Mo Li. 2023. "Effect of Milling on Nutritional Components in Common and Zinc-Biofortified Wheat" Nutrients 15, no. 4: 833. https://doi.org/10.3390/nu15040833
APA StyleJiang, Z., Zhou, S., Peng, Y., Wen, X., Ni, Y., & Li, M. (2023). Effect of Milling on Nutritional Components in Common and Zinc-Biofortified Wheat. Nutrients, 15(4), 833. https://doi.org/10.3390/nu15040833