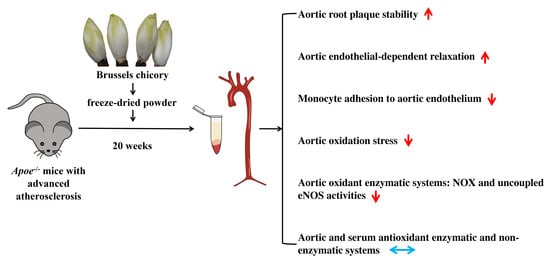
Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mouse Study
2.2. Advanced Plaque Size and Stability
2.3. Cellular and Molecular Components of Advanced Plaque
2.4. Aortic Relaxation
2.5. Detection of Monocyte Adherence to Aortic Endothelium
2.6. Oxidative Stress Burden in Advanced Plaque
2.7. Oxidative Products ox-LDL and 4-Hydroxynonenal (4-HNE) in Advanced Plaque
2.8. Measurement of Superoxide
2.9. Aortic Oxidant ENZYME Activity
2.10. Aortic and Serum Antioxidant Enzyme Activity
2.11. qRT-PCR
2.12. Aortic and Serum Non-Enzymatic Antioxidants
2.13. Statistical Analyses
3. Results
3.1. Brussels Chicory Stabilizes Atherosclerotic Plaques at the Site of Aortas
3.2. Brussels Chicory Improves Aortic Endothelium Functions
3.3. Brussels Chicory Alleviates Oxidative Stress in Atherosclerotic Plaques
3.4. Brussels Chicory Inhibits NADPH Oxidase- and Uncoupled eNOS-Mediated Oxidative Stress Production in Atherosclerotic Plaques
3.5. Brussels Chicory Does Not Affect Enzymatic and Non-Enzymatic Antioxidant System in Atherosclerotic Plaques
3.6. Brussels Chicory DOES Not Alter Enzymatic and Non-Enzymatic Antioxidant System in Blood Circulation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, X.; Du, Y.; Liu, X.; Chen, G.; Xiang, P.; Wu, H.; Liu, C.; Wang, D. Brussels Chicory Stabilizes Unstable Atherosclerotic Plaques and Reshapes the Gut Microbiota in Apoe−/− Mice. J. Nutr. 2022, 152, 2209–2217. [Google Scholar] [CrossRef]
- VanderLaan, P.A.; Reardon, C.A.; Getz, G.S. Site specificity of atherosclerosis: Site-selective responses to atherosclerotic modulators. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 12–22. [Google Scholar] [CrossRef]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef]
- Lorenzon Dos Santos, J.; Quadros, A.S.; Weschenfelder, C.; Garofallo, S.B.; Marcadenti, A. Oxidative Stress Biomarkers, Nut-Related Antioxidants, and Cardiovascular Disease. Nutrients 2020, 12, 682. [Google Scholar] [CrossRef] [PubMed]
- Simsek, B.; Selte, A.; Egeli, B.H.; Cakatay, U. Effects of vitamin supplements on clinical cardiovascular outcomes: Time to move on!—A comprehensive review. Clin. Nutr. ESPEN 2021, 42, 1–14. [Google Scholar] [CrossRef]
- Sureda, A.; Bibiloni, M.D.; Martorell, M.; Buil-Cosiales, P.; Marti, A.; Pons, A.; Tur, J.A.; Martinez-Gonzalez, M.A.; Investigators, P.S. Mediterranean diets supplemented with virgin olive oil and nuts enhance plasmatic antioxidant capabilities and decrease xanthine oxidase activity in people with metabolic syndrome: The PREDIMED study. Mol. Nutr. Food Res. 2016, 60, 2654–2664. [Google Scholar] [CrossRef]
- Puhlmann, M.L.; de Vos, W.M. Back to the Roots: Revisiting the Use of the Fiber-Rich Cichorium intybus L. Taproots. Adv. Nutr. 2020, 11, 878–889. [Google Scholar] [CrossRef]
- Amer, A.M. Antimicrobial Effects of Egyptian Local Chicory, Cichorium endivia subsp. pumilum. Int. J. Microbiol. 2018, 2018, 6475072. [Google Scholar] [CrossRef] [Green Version]
- Perovic, J.; Tumbas Saponjac, V.; Kojic, J.; Krulj, J.; Moreno, D.A.; Garcia-Viguera, C.; Bodroza-Solarov, M.; Ilic, N. Chicory (Cichorium intybus L.) as a food ingredient-Nutritional composition, bioactivity, safety, and health claims: A review. Food Chem. 2021, 336, 127676. [Google Scholar] [CrossRef]
- Pouille, C.L.; Ouaza, S.; Roels, E.; Behra, J.; Tourret, M.; Molinie, R.; Fontaine, J.X.; Mathiron, D.; Gagneul, D.; Taminiau, B.; et al. Chicory: Understanding the Effects and Effectors of This Functional Food. Nutrients 2022, 14, 957. [Google Scholar] [CrossRef] [PubMed]
- Street, R.A.; Sidana, J.; Prinsloo, G. Cichorium intybus: Traditional Uses, Phytochemistry, Pharmacology, and Toxicology. Evid. Based Complement. Altern. Med. 2013, 2013, 579319. [Google Scholar] [CrossRef] [PubMed]
- Rothwell, J.A.; Urpi-Sarda, M.; Boto-Ordonez, M.; Knox, C.; Llorach, R.; Eisner, R.; Cruz, J.; Neveu, V.; Wishart, D.; Manach, C.; et al. Phenol-Explorer 2.0: A major update of the Phenol-Explorer database integrating data on polyphenol metabolism and pharmacokinetics in humans and experimental animals. Database 2012, 2012, bas031. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, M.; Lante, A.; Vanzani, P.; Spettoli, P.; Scarpa, M.; Rigo, A. Red chicories as potent scavengers of highly reactive radicals: A study on their phenolic composition and peroxyl radical trapping capacity and efficiency. J. Agric. Food Chem. 2005, 53, 8169–8175. [Google Scholar] [CrossRef]
- Perez-Ternero, C.; Werner, C.M.; Nickel, A.G.; Herrera, M.D.; Motilva, M.J.; Bohm, M.; Alvarez de Sotomayor, M.; Laufs, U. Ferulic acid, a bioactive component of rice bran, improves oxidative stress and mitochondrial biogenesis and dynamics in mice and in human mononuclear cells. J. Nutr. Biochem. 2017, 48, 51–61. [Google Scholar] [CrossRef]
- Kim, M.; Yoo, G.; Randy, A.; Kim, H.S.; Nho, C.W. Chicoric acid attenuate a nonalcoholic steatohepatitis by inhibiting key regulators of lipid metabolism, fibrosis, oxidation, and inflammation in mice with methionine and choline deficiency. Mol. Nutr. Food Res. 2017, 61, 1600632. [Google Scholar] [CrossRef]
- Choi, J.R.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Protective effects of protocatechuic acid against cognitive impairment in an amyloid beta-induced Alzheimer’s disease mouse model. Food Chem. Toxicol. 2020, 144, 111571. [Google Scholar] [CrossRef]
- Lin, W.; Liu, C.; Yang, H.; Wang, W.; Ling, W.; Wang, D. Chicory, a typical vegetable in Mediterranean diet, exerts a therapeutic role in established atherosclerosis in apolipoprotein E-deficient mice. Mol. Nutr. Food Res. 2015, 59, 1803–1813. [Google Scholar] [CrossRef]
- Rosenfeld, M.E.; Polinsky, P.; Virmani, R.; Kauser, K.; Rubanyi, G.; Schwartz, S.M. Advanced atherosclerotic lesions in the innominate artery of the ApoE knockout mouse. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 2587–2592. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Wang, W.; Lin, W.; Yang, W.; Zhang, P.; Chen, M.; Ding, D.; Liu, C.; Zheng, J.; Ling, W. Apoptotic cell induction of miR-10b in macrophages contributes to advanced atherosclerosis progression in ApoE−/− mice. Cardiovasc. Res. 2018, 114, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, W.; Lin, W.; Ling, W.; Wang, D. Established atherosclerosis might be a prerequisite for chicory and its constituent protocatechuic acid to promote endothelium-dependent vasodilation in mice. Mol. Nutr. Food Res. 2016, 60, 2141–2150. [Google Scholar] [CrossRef]
- Weng, H.; He, L.; Liu, X.; Li, Q.; Du, Y.; Zheng, J.; Wang, D. Natural lactucopicrin alleviates importin-alpha3-mediated NF-kappaB activation in inflammated endothelial cells and improves sepsis in mice. Biochem. Pharmacol. 2021, 186, 114501. [Google Scholar] [CrossRef] [PubMed]
- Satoh, K.; Nigro, P.; Matoba, T.; O’Dell, M.R.; Cui, Z.; Shi, X.; Mohan, A.; Yan, C.; Abe, J.; Illig, K.A.; et al. Cyclophilin A enhances vascular oxidative stress and the development of angiotensin II-induced aortic aneurysms. Nat. Med. 2009, 15, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, C.A.; Heistad, D.D.; Piegors, D.J.; Miller, F.J., Jr. Regression of atherosclerosis in monkeys reduces vascular superoxide levels. Circ. Res. 2002, 90, 277–283. [Google Scholar] [CrossRef]
- Beckman, J.S.; Parks, D.A.; Pearson, J.D.; Marshall, P.A.; Freeman, B.A. A sensitive fluorometric assay for measuring xanthine dehydrogenase and oxidase in tissues. Free Radic. Biol. Med. 1989, 6, 607–615. [Google Scholar] [CrossRef]
- Wendel, A. Glutathione peroxidase. Methods Enzymol. 1981, 77, 325–333. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Force, U.S.P.S.T.; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davis, E.M.; Donahue, K.E.; Doubeni, C.A.; et al. Vitamin, Mineral, and Multivitamin Supplementation to Prevent Cardiovascular Disease and Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2022, 327, 2326–2333. [Google Scholar] [CrossRef]
- Behrendt, I.; Eichner, G.; Fasshauer, M. Association of Antioxidants Use with All-Cause and Cause-Specific Mortality: A Prospective Study of the UK Biobank. Antioxidants 2020, 9, 1287. [Google Scholar] [CrossRef]
- Morelli, M.B.; Gambardella, J.; Castellanos, V.; Trimarco, V.; Santulli, G. Vitamin C and Cardiovascular Disease: An Update. Antioxidants 2020, 9, 1227. [Google Scholar] [CrossRef] [PubMed]
- Petrucci, G.; Rizzi, A.; Hatem, D.; Tosti, G.; Rocca, B.; Pitocco, D. Role of Oxidative Stress in the Pathogenesis of Atherothrombotic Diseases. Antioxidants 2022, 11, 1408. [Google Scholar] [CrossRef]
- Hertog, M.G.; Feskens, E.J.; Hollman, P.C.; Katan, M.B.; Kromhout, D. Dietary antioxidant flavonoids and risk of coronary heart disease: The Zutphen Elderly Study. Lancet 1993, 342, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Mukamal, K.J.; Liu, L.; Franz, M.; Eliassen, A.H.; Rimm, E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013, 127, 188–196. [Google Scholar] [CrossRef]
- Ros, E.; Martinez-Gonzalez, M.A.; Estruch, R.; Salas-Salvado, J.; Fito, M.; Martinez, J.A.; Corella, D. Mediterranean diet and cardiovascular health: Teachings of the Predimed study. Adv. Nutr. 2014, 5, 330S–336S. [Google Scholar] [CrossRef]
- Xia, X.; Ling, W.; Ma, J.; Xia, M.; Hou, M.; Wang, Q.; Zhu, H.; Tang, Z. An anthocyanin-rich extract from black rice enhances atherosclerotic plaque stabilization in apolipoprotein E-deficient mice. J. Nutr. 2006, 136, 2220–2225. [Google Scholar] [CrossRef]
- Millar, C.L.; Norris, G.H.; Jiang, C.; Kry, J.; Vitols, A.; Garcia, C.; Park, Y.K.; Lee, J.Y.; Blesso, C.N. Long-Term Supplementation of Black Elderberries Promotes Hyperlipidemia, but Reduces Liver Inflammation and Improves HDL Function and Atherosclerotic Plaque Stability in Apolipoprotein E-Knockout Mice. Mol. Nutr. Food Res. 2018, 62, e1800404. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Li, Y.; Shi, H.; Wang, H.; Chen, B.; Wang, F.; Wang, Z.; Yang, Z.; Wang, L. Green tea polyphenol epigallocatechin-3-gallate increases atherosclerotic plaque stability in apolipoprotein E-deficient mice fed a high-fat diet. Kardiol. Pol. 2018, 76, 1263–1270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Li, Q.; He, L.; Weng, H.; Su, D.; Liu, X.; Ling, W.; Wang, D. Protocatechuic Acid Inhibits Vulnerable Atherosclerotic Lesion Progression in Older Apoe−/− Mice. J. Nutr. 2020, 150, 1167–1177. [Google Scholar] [CrossRef]
- Manickam, V.; Dhawan, U.K.; Singh, D.; Gupta, M.; Subramanian, M. Pomegranate Peel Extract Decreases Plaque Necrosis and Advanced Atherosclerosis Progression in Apoe−/− Mice. Front. Pharmacol. 2022, 13, 888300. [Google Scholar] [CrossRef]
- Callegari, A.; Liu, Y.; White, C.C.; Chait, A.; Gough, P.; Raines, E.W.; Cox, D.; Kavanagh, T.J.; Rosenfeld, M.E. Gain and loss of function for glutathione synthesis: Impact on advanced atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 2473–2482. [Google Scholar] [CrossRef] [PubMed]
- Aviram, M.; Rosenblat, M.; Gaitini, D.; Nitecki, S.; Hoffman, A.; Dornfeld, L.; Volkova, N.; Presser, D.; Attias, J.; Liker, H.; et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004, 23, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric Acid Ameliorates Lipopolysaccharide-Induced Oxidative Stress via Promoting the Keap1/Nrf2 Transcriptional Signaling Pathway in BV-2 Microglial Cells and Mouse Brain. J. Agric. Food Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ji, N.; Gong, X.; Ni, S.; Xu, L.; Zhang, H. Thioredoxin-1 attenuates atherosclerosis development through inhibiting NLRP3 inflammasome. Endocrine 2020, 70, 65–70. [Google Scholar] [CrossRef]
- Sung, J.H.; Gim, S.A.; Koh, P.O. Ferulic acid attenuates the cerebral ischemic injury-induced decrease in peroxiredoxin-2 and thioredoxin expression. Neurosci. Lett. 2014, 566, 88–92. [Google Scholar] [CrossRef]
- Sullivan, J.C.; Pollock, J.S. Coupled and uncoupled NOS: Separate but equal? Uncoupled NOS in endothelial cells is a critical pathway for intracellular signaling. Circ. Res. 2006, 98, 717–719. [Google Scholar] [CrossRef]
- Prasad, A.; Andrews, N.P.; Padder, F.A.; Husain, M.; Quyyumi, A.A. Glutathione reverses endothelial dysfunction and improves nitric oxide bioavailability. J. Am. Coll. Cardiol. 1999, 34, 507–514. [Google Scholar] [CrossRef]
- Rosenblat, M.; Coleman, R.; Aviram, M. Increased macrophage glutathione content reduces cell-mediated oxidation of LDL and atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 2002, 163, 17–28. [Google Scholar] [CrossRef]
- Rom, O.; Liu, Y.; Finney, A.C.; Ghrayeb, A.; Zhao, Y.; Shukha, Y.; Wang, L.; Rajanayake, K.K.; Das, S.; Rashdan, N.A.; et al. Induction of glutathione biosynthesis by glycine-based treatment mitigates atherosclerosis. Redox Biol. 2022, 52, 102313. [Google Scholar] [CrossRef]
- Vari, R.; D’Archivio, M.; Filesi, C.; Carotenuto, S.; Scazzocchio, B.; Santangelo, C.; Giovannini, C.; Masella, R. Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. J. Nutr. Biochem. 2011, 22, 409–417. [Google Scholar] [CrossRef]
- Marcil, V.; Delvin, E.; Sane, A.T.; Tremblay, A.; Levy, E. Oxidative stress influences cholesterol efflux in THP-1 macrophages: Role of ATP-binding cassette A1 and nuclear factors. Cardiovasc. Res. 2006, 72, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.Z.; Rabinovitch, P.S.; Tabas, I. Macrophage mitochondrial oxidative stress promotes atherosclerosis and nuclear factor-kappaB-mediated inflammation in macrophages. Circ. Res. 2014, 114, 421–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Du, Y.; Xiang, P.; Chen, G.; Qian, X.; Li, S.; Mao, Y.; Ling, W.; Wang, D. Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets. Nutrients 2023, 15, 832. https://doi.org/10.3390/nu15040832
Li Q, Du Y, Xiang P, Chen G, Qian X, Li S, Mao Y, Ling W, Wang D. Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets. Nutrients. 2023; 15(4):832. https://doi.org/10.3390/nu15040832
Chicago/Turabian StyleLi, Qing, Yushi Du, Panyin Xiang, Guanyu Chen, Xiaoxian Qian, Shuangshuang Li, Yihui Mao, Wenhua Ling, and Dongliang Wang. 2023. "Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets" Nutrients 15, no. 4: 832. https://doi.org/10.3390/nu15040832
APA StyleLi, Q., Du, Y., Xiang, P., Chen, G., Qian, X., Li, S., Mao, Y., Ling, W., & Wang, D. (2023). Re-Visiting Antioxidant Therapy in Murine Advanced Atherosclerosis with Brussels Chicory, a Typical Vegetable in Mediterranean Diets. Nutrients, 15(4), 832. https://doi.org/10.3390/nu15040832