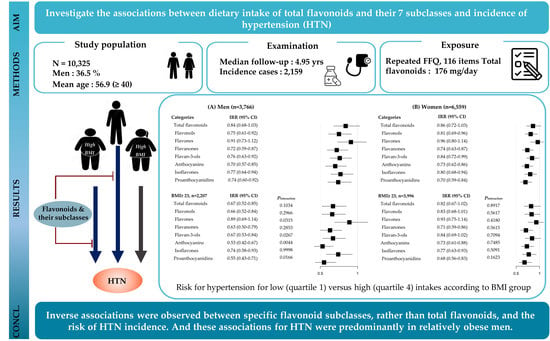
Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Assessments of Dietary Exposures and Covariates
2.3. Ascertainment of Outcome: BP Measures and Hypertension Incidence
2.4. Assessment of Non-Dietary Covariates
2.5. Statistics Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; Liu, P.; Roth, G.A.; Ng, M.; Biryukov, S.; Marczak, L.; Alexander, L.; Estep, K.; Abate, K.H.; Akinyemiju, T.F. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA 2017, 317, 165–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kjeldsen, S.E. Hypertension and cardiovascular risk: General aspects. Pharmacol. Res. 2018, 129, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.H.; Angell, S.Y.; Asma, S.; Boutouyrie, P.; Burger, D.; Chirinos, J.A.; Damasceno, A.; Delles, C.; Gimenez-Roqueplo, A.-P.; Hering, D. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: The Lancet Commission on hypertension. Lancet 2016, 388, 2665–2712. [Google Scholar] [CrossRef]
- Zhou, B.; Carrillo-Larco, R.M.; Danaei, G.; Riley, L.M.; Paciorek, C.J.; Stevens, G.A.; Gregg, E.W.; Bennett, J.E.; Solomon, B.; Singleton, R.K. Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: A pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021, 398, 957–980. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Rahaman, S. Flavonoids: A vital resource in healthcare and medicine. Pharm. Pharmacol. Int. J. 2020, 8, 91–104. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Currenti, W.; Micek, A.; Falzone, L.; Libra, M.; Giampieri, F.; Forbes-Hernández, T.Y.; Quiles, J.L.; Battino, M. The effect of dietary polyphenols on vascular health and hypertension: Current evidence and mechanisms of action. Nutrients 2022, 14, 545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Gan, R.-Y.; Li, S.; Zhou, Y.; Li, A.-N.; Xu, D.-P.; Li, H.-B. Antioxidant phytochemicals for the prevention and treatment of chronic diseases. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary polyphenol intake, blood pressure, and hypertension: A systematic review and meta-analysis of observational studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, C.D.; Hooper, L.; Kroon, P.A.; Rimm, E.B.; Cassidy, A. Relative impact of flavonoid composition, dose and structure on vascular function: A systematic review of randomised controlled trials of flavonoid-rich food products. Mol. Nutr. Food Res. 2012, 56, 1605–1616. [Google Scholar] [CrossRef] [Green Version]
- Livingstone, K.M.; McNaughton, S.A. Diet quality is associated with obesity and hypertension in Australian adults: A cross sectional study. BMC Public Health 2016, 16, 1037. [Google Scholar] [CrossRef] [Green Version]
- Dominguez, L.J.; Gea, A.; Ruiz-Estigarribia, L.; Sayon-Orea, C.; Fresan, U.; Barbagallo, M.; Ruiz-Canela, M.; Martinez-Gonzalez, M.A. Dietary magnesium and overweight/obesity in a mediterranean population: A detrimental synergy for the development of hypertension. Nutrients 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Han, B.-G.; Group, K. Cohort profile: The Korean genome and epidemiology study (KoGES) consortium. Int. J. Epidemiol. 2017, 46, e20. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Kwon, E.; Shim, J.; Park, M.; Joo, Y.; Kimm, K.; Park, C.; Kim, D. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. Eur. J. Clin. Nutr. 2007, 61, 1435–1441. [Google Scholar] [CrossRef]
- Food Values. 2009. Available online: kns.or.kr/English/Publication.asp (accessed on 20 January 2023).
- Haytowitz, D.B.; Wu, X.; Bhagwat, S. USDA Database for the Flavonoid Content of Selected Foods, Release 3.3. Available online: www.ars.usda.gov/nutrientdata/flav (accessed on 20 January 2023).
- Bhagwat, S.; Haytowitz, D.B. USDA Database for the Isoflavone Content of Selected Foods Release 2.1. Available online: https://data.nal.usda.gov/dataset/usda-database-isoflavone-content-selected-foods-release-21-november-2015 (accessed on 20 January 2023).
- Haytowitz, D.; Wu, X.; Bhagwat, S. USDA Database for the Proanthocyanidin Content of Selected Foods Release 2.1. Available online: www.ars.usda.gov (accessed on 20 January 2023).
- INRA (French National Institute for Agricultural Research). Phenol-Explorer 3.6. Database on polyphenol content in food. Available online: www.phenol-explorer.eu/ (accessed on 20 January 2023).
- Escobar-Cévoli, R.; Castro-Espín, C.; Béraud, V.; Buckland, G.; Zamora-Ros, R.; Béraud, G. An Overview of Global Flavonoid Intake and Its Food Sources; IntechOpen: London, UK, 2017. [Google Scholar]
- Hu, F.B.; Stampfer, M.J.; Rimm, E.; Ascherio, A.; Rosner, B.A.; Spiegelman, D.; Willett, W.C. Dietary fat and coronary heart disease: A comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am. J. Epidemiol. 1999, 149, 531–540. [Google Scholar] [CrossRef] [Green Version]
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo Jr, J.L.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright Jr, J.T. Seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003, 42, 1206–1252. [Google Scholar] [CrossRef] [Green Version]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y. Heart disease and stroke statistics—2022 update: A report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Tiruneh, S.A.; Bukayaw, Y.A.; Yigizaw, S.T.; Angaw, D.A. Prevalence of hypertension and its determinants in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0244642. [Google Scholar] [CrossRef]
- Lima, R.; Wofford, M.; Reckelhoff, J.F. Hypertension in postmenopausal women. Curr. Hypertens. Rep. 2012, 14, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Filippou, C.D.; Tsioufis, C.P.; Thomopoulos, C.G.; Mihas, C.C.; Dimitriadis, K.S.; Sotiropoulou, L.I.; Chrysochoou, C.A.; Nihoyannopoulos, P.I.; Tousoulis, D.M. Dietary approaches to stop hypertension (DASH) diet and blood pressure reduction in adults with and without hypertension: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2020, 11, 1150–1160. [Google Scholar] [CrossRef]
- Zou, G. A modified Poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 2004, 159, 702–706. [Google Scholar] [CrossRef] [PubMed]
- Callas, P.W.; Pastides, H.; Hosmer, D.W. Empirical comparisons of proportional hazards, poisson, and logistic regression modeling of occupational cohort data. Am. J. Ind. Med. 1998, 33, 33–47. [Google Scholar] [CrossRef]
- Woodward, M. Epidemiology: Study Design and Data Analysis; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Parmenter, B.H.; Croft, K.D.; Hodgson, J.M.; Dalgaard, F.; Bondonno, C.P.; Lewis, J.R.; Cassidy, A.; Scalbert, A.; Bondonno, N.P. An overview and update on the epidemiology of flavonoid intake and cardiovascular disease risk. Food Funct. 2020, 11, 6777–6806. [Google Scholar] [CrossRef] [PubMed]
- do Rosario, V.A.; Schoenaker, D.A.; Kent, K.; Weston-Green, K.; Charlton, K. Association between flavonoid intake and risk of hypertension in two cohorts of Australian women: A longitudinal study. Eur. J. Nutr. 2021, 60, 2507–2519. [Google Scholar] [CrossRef]
- Lajous, M.; Rossignol, E.; Fagherazzi, G.; Perquier, F.; Scalbert, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.-C. Flavonoid intake and incident hypertension in women. Am. J. Clin. Nutr. 2016, 103, 1091–1098. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, A.; O’Reilly, É.J.; Kay, C.; Sampson, L.; Franz, M.; Forman, J.; Curhan, G.; Rimm, E.B. Habitual intake of flavonoid subclasses and incident hypertension in adults. Am. J. Clin. Nutr. 2011, 93, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary polyphenol intake and risk of hypertension in the Polish arm of the HAPIEE study. Eur. J. Nutr. 2018, 57, 1535–1544. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.; Kim, E.S.; Lee, J.; Kim, Y. Trends in sodium intake and major contributing food groups and dishes in Korea: The Korea National Health and Nutrition Examination Survey 2013–2017. Nutr. Res. Pract. 2021, 15, 382–395. [Google Scholar] [CrossRef]
- Lenda, D.M.; Boegehold, M.A. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H395–H402. [Google Scholar] [CrossRef] [Green Version]
- McLean, R.M.; Farmer, V.L.; Nettleton, A.; Cameron, C.M.; Cook, N.R.; Campbell, N.R.; Consortium, T. Assessment of dietary sodium intake using a food frequency questionnaire and 24-hour urinary sodium excretion: A systematic literature review. J. Clin. Hypertens. 2017, 19, 1214–1230. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.L.; Zahradka, P.; Taylor, C.G. Efficacy of flavonoids in the management of high blood pressure. Nutr. Rev. 2015, 73, 799–822. [Google Scholar] [CrossRef] [PubMed]
- Saigo, T.; Wang, T.; Watanabe, M.; Tohge, T. Diversity of anthocyanin and proanthocyanin biosynthesis in land plants. Curr. Opin. Plant Biol. 2020, 55, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-R.; Han, X.-H.; Zhang, Y.-H.; Lee, J.-J.; Lim, Y.; Chung, J.-H.; Yun, Y.-P. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-γ2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, A.; Bertoia, M.; Chiuve, S.; Flint, A.; Forman, J.; Rimm, E.B. Habitual intake of anthocyanins and flavanones and risk of cardiovascular disease in men. Am. J. Clin. Nutr. 2016, 104, 587–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faulkner, J.L.; Belin de Chantemèle, E.J. Sex differences in mechanisms of hypertension associated with obesity. Hypertension 2018, 71, 15–21. [Google Scholar] [CrossRef]
- Fu, Q. Sex differences in sympathetic activity in obesity and its related hypertension. Ann. N. Y. Acad. Sci. 2019, 1454, 31–41. [Google Scholar] [CrossRef]
- Bruno, R.M.; Ghiadoni, L. Polyphenols, antioxidants and the sympathetic nervous system. Curr. Pharm. Des. 2018, 24, 130–139. [Google Scholar] [CrossRef]

| Characteristics | Men (n = 3766) | P diff b | Women (n = 6559) | P diff | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q1 | Q2 | Q3 | Q4 | |||
| N (%) | 941 | 942 | 942 | 941 | _ | 1639 | 1640 | 1640 | 1640 | _ |
| No. cases/ person-year | 242/4392 | 199/4992 | 186/5078 | 227/4908 | _ | 381/7568 | 306/8966 | 315/9164 | 303/8610 | _ |
| Age, y | 61.2 ± 0.31 | 59.2 ± 0.31 | 58.1 ± 0.31 | 55.6 ± 0.31 | <0.0001 | 60.9 ± 0.22 | 56.6 ± 0.22 | 54.1 ± 0.22 | 52.1 ± 0.22 | <0.0001 |
| Higher education c | 27.2 | 36.3 | 40.0 | 49.9 | <0.0001 | 21.0 | 22.9 | 29.0 | 37.7 | <0.0001 |
| Regular exercise d | 11.9 | 17.4 | 22.2 | 28.4 | <0.0001 | 13.7 | 17.3 | 24.2 | 32.1 | <0.0001 |
| Current smoker | ||||||||||
| Never-smoker | 23.8 | 22.5 | 30.0 | 32.9 | <0.0001 | 93.7 | 96.7 | 97.1 | 95.9 | <0.0001 |
| Past smoker | 31.8 | 35.2 | 33.0 | 35.6 | 0.2608 | 1.60 | 1.28 | 0.84 | 1.47 | 0.2536 |
| Current smoker | 44.4 | 42.2 | 37.0 | 31.6 | <0.0001 | 4.68 | 1.99 | 2.05 | 2.67 | <0.0001 |
| Menopausal status | _ | _ | _ | _ | _ | 67.0 | 71.0 | 72.9 | 69.4 | <0.0001 |
| Family history of hypertension | 13.8 | 13.2 | 13.1 | 15.6 | 0.3835 | 17.0 | 18.9 | 20.8 | 23.4 | <0.0001 |
| Current drinker | 63.1 | 62.8 | 61.0 | 63.5 | 0.6620 | 30.6 | 29.5 | 30.1 | 31.1 | 0.7747 |
| Alcohol consumption, ml/d | 28.3 ± 1.80 | 26.4 ± 1.78 | 25.7 ± 1.78 | 26.7 ± 1.80 | 0.7836 | 2.73 ± 0.28 | 1.98 ± 0.27 | 2.22 ± 0.27 | 2.75 ± 0.28 | 0.1143 |
| Body Mass index, kg/m2 | 23.4 ± 0.09 | 23.6 ± 0.09 | 23.7 ± 0.09 | 24.0 ± 0.09 | <0.0001 | 23.8 ± 0.08 | 24.0 ± 0.08 | 24.0 ± 0.08 | 24.2 ± 0.08 | 0.0377 |
| Total energy intake, kcal/d | 1421 ± 12.7 | 1644 ± 12.5 | 1784 ± 12.5 | 1949 ± 12.7 | <0.0001 | 1254 ± 9.38 | 1451 ± 9.05 | 1587 ± 9.09 | 1744 ± 9.24 | <0.0001 |
| Modified DASH scores e | 16.6 ± 0.10 | 17.3 ± 0.10 | 17.3 ± 0.10 | 17.7 ± 0.10 | <0.0001 | 16.9 ± 0.08 | 17.3 ± 0.08 | 17.6 ± 0.08 | 18.0 ± 0.08 | <0.0001 |
| Total flavonoids, mg/d | 45.7 ± 2.72 | 92.6 ± 2.69 | 156 ± 2.69 | 348 ± 2.72 | _ | 51.1 ± 2.75 | 103 ± 2.65 | 177 ± 2.66 | 407 ± 2.71 | <0.0001 |
| Flavonols | 10.0 ± 0.38 | 14.6 ± 0.38 | 19.1 ± 0.38 | 28.8 ± 0.38 | <0.0001 | 9.14 ± 0.31 | 14.5 ± 0.29 | 18.6 ± 0.30 | 30.0 ± 0.30 | <0.0001 |
| Flavones | 0.95 ± 0.03 | 1.40 ± 0.03 | 1.90 ± 0.03 | 2.66 ± 0.03 | <0.0001 | 0.96 ± 0.03 | 1.54 ± 0.03 | 2.03 ± 0.03 | 3.12 ± 0.03 | <0.0001 |
| Flavanones | 2.31 ± 0.26 | 4.64 ± 0.26 | 8.26 ± 0.26 | 13.2 ± 0.26 | <0.0001 | 3.21 ± 0.25 | 6.73 ± 0.24 | 11.2 ± 0.25 | 19.4 ± 0.25 | <0.0001 |
| Flavan-3-ols | 6.68 ± 2.54 | 16.5 ± 2.51 | 38.9 ± 2.51 | 161 ± 2.54 | <0.0001 | 8.63 ± 2.47 | 17.0 ± 2.38 | 40.8 ± 2.39 | 174 ± 2.43 | <0.0001 |
| Anthocyanins | 1.72 ± 0.25 | 4.44 ± 0.25 | 8.52 ± 0.25 | 14.3 ± 0.25 | <0.0001 | 2.17 ± 0.25 | 5.89 ± 0.24 | 10.8 ± 0.24 | 19.8 ± 0.24 | <0.0001 |
| Isofavones | 10.3 ± 0.48 | 19.1 ± 0.48 | 23.0 ± 0.48 | 26.3 ± 0.48 | <0.0001 | 10.0 ± 0.36 | 17.5 ± 0.34 | 22.3 ± 0.35 | 25.2 ± 0.35 | <0.0001 |
| Proanthocyanins | 13.7 ± 1.31 | 32.1 ± 1.30 | 56.3 ± 1.30 | 102 ± 1.32 | <0.0001 | 17.0 ± 1.28 | 40.1 ± 1.24 | 71.4 ± 1.24 | 136 ± 1.26 | <0.0001 |
| Flavonoids, mg/d | Men (n = 3766) | Women (n = 6559) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Plineara | Pnon-linearb | Q1 | Q2 | Q3 | Q4 | Plinear | Pnon-linear | |
| Total flavonoids | ||||||||||||
| Median (min, max) | 46.7 (3.52, 69.3) | 91.9 (69.3, 118) | 153 (118, 206) | 304 (206, 1929) | 50.1 (1.05, 75.7) | 102 (75.8, 133) | 175 (133, 236) | 345 (236, 2485) | ||||
| Age-adjusted model | 1 (ref) | 0.74 (0.62–0.89) | 0.70 (0.58–0.84) | 0.91 (0.76–1.09) | 0.9233 | <0.0001 | 1 (ref) | 0.80 (0.69–0.93) | 0.88 (0.75–1.02) | 0.99 (0.84–1.16) | 0.3674 | <0.0001 |
| Multivariable model 1 c | 1 (ref) | 0.70 (0.58–0.84) | 0.70 (0.57–0.85) | 0.84 (0.68–1.03) | 0.8302 | <0.0001 | 1 (ref) | 0.73 (0.63–0.85) | 0.80 (0.68–0.93) | 0.86 (0.72–1.03) | 0.7528 | 0.0002 |
| Multivariable model 2 d | 1 (ref) | 0.70 (0.58–0.85) | 0.70 (0.58–0.86) | 0.85 (0.69–1.04) | 0.8853 | 0.0001 | 1 (ref) | 0.74 (0.63–0.86) | 0.80 (0.68–0.94) | 0.87 (0.72–1.04) | 0.8415 | 0.0002 |
| Flavonols | ||||||||||||
| Median (min, max) | 7.09 (0.57, 9.81) | 12.4 (9.83, 15.0) | 18.1 (15, 22.3) | 29.6 (22.4, 246) | 6.33 (0.11, 8.98) | 11.6 (8.99, 14.5) | 17.7 (14.5, 22.6) | 31.4 (22.6, 219) | ||||
| Age-adjusted model | 1 (ref) | 0.68 (0.56–0.81) | 0.76 (0.63–0.91) | 0.85 (0.71–1.01) | 0.4899 | <0.0001 | 1 (ref) | 0.78 (0.68–0.91) | 0.77 (0.66–0.90) | 0.92 (0.79–1.08) | 0.8456 | <0.0001 |
| Multivariable model 1 | 1 (ref) | 0.63 (0.53–0.76) | 0.69 (0.57–0.84) | 0.75 (0.61–0.92) | 0.1165 | <0.0001 | 1 (ref) | 0.73 (0.63–0.84) | 0.70 (0.60–0.82) | 0.81 (0.69–0.96) | 0.2043 | <0.0001 |
| Multivariable model 2 | 1 (ref) | 0.63 (0.52–0.75) | 0.68 (0.56–0.82) | 0.73 (0.59–0.89) | 0.0671 | <0.0001 | 1 (ref) | 0.72 (0.62–0.84) | 0.70 (0.59–0.81) | 0.80 (0.68–0.95) | 0.1692 | <0.0001 |
| Flavones | ||||||||||||
| Median (min, max) | 0.7 (0, 0.97) | 1.22 (0.97, 1.49) | 1.78 (1.49, 2.16) | 2.86 (2.16, 11.6) | 0.70 (0.01, 1.01) | 1.30 (1.02, 1.60) | 1.96 (1.60, 2.43) | 3.27 (2.43, 18.3) | ||||
| Age-adjusted model | 1 (ref) | 0.86 (0.72–1.03) | 0.72 (0.59–0.87) | 0.99 (0.82–1.19) | 0.9740 | 0.0002 | 1 (ref) | 0.93 (0.80–1.08) | 0.85 (0.73–0.99) | 1.07 (0.92–1.26) | 0.3307 | 0.0001 |
| Multivariable model 1 | 1 (ref) | 0.78 (0.65–0.93) | 0.66 (0.55–0.81) | 0.91 (0.73–1.12) | 0.6525 | <0.0001 | 1 (ref) | 0.91 (0.78–1.05) | 0.78 (0.67–0.92) | 0.96 (0.80–1.14) | 0.7084 | 0.0037 |
| Multivariable model 2 | 1 (ref) | 0.77 (0.64–0.93) | 0.66 (0.54–0.80) | 0.89 (0.72–1.10) | 0.5638 | <0.0001 | 1 (ref) | 0.91 (0.78–1.05) | 0.78 (0.66–0.91) | 0.95 (0.80–1.14) | 0.6656 | 0.0038 |
| Flavanones | ||||||||||||
| Median (min, max) | 0.77 (0, 1.82) | 3.11 (1.82, 4.58) | 6.47 (4.58, 9.21) | 14.3 (9.21, 133) | 1.25 (0, 2.95) | 4.7 (2.95, 6.79) | 9.3 (6.79, 13.3) | 19.7 (13.3, 192) | ||||
| Age-adjusted model | 1 (ref) | 0.69 (0.58–0.82) | 0.60 (0.50–0.72) | 0.74 (0.61–0.89) | 0.0432 | <0.0001 | 1 (ref) | 0.80 (0.69–0.93) | 0.81 (0.69–0.94) | 0.83 (0.71–0.98) | 0.1246 | 0.0001 |
| Multivariable model 1 | 1 (ref) | 0.66 (0.55–0.78) | 0.56 (0.47–0.68) | 0.72 (0.59–0.87) | 0.0581 | <0.0001 | 1 (ref) | 0.77 (0.67–0.90) | 0.76 (0.65–0.89) | 0.74 (0.63–0.87) | 0.0054 | 0.0061 |
| Multivariable model 2 | 1 (ref) | 0.66 (0.55–0.79) | 0.57 (0.47–0.69) | 0.72 (0.59–0.88) | 0.0605 | <0.0001 | 1 (ref) | 0.78 (0.67–0.90) | 0.76 (0.66–0.89) | 0.75 (0.63–0.88) | 0.0064 | 0.0069 |
| Flavan-3ols | ||||||||||||
| Median (min, max) | 3.26 (0, 6.35) | 10.4 (6.36, 17.4) | 30.7 (17.4, 52.7) | 132 (52.8, 1755) | 3.61 (0, 7.05) | 11.5 (7.06, 18.5) | 31.2 (18.5, 52.6) | 135 (52.6, 1808) | ||||
| Age-adjusted model | 1 (ref) | 0.65 (0.54–0.78) | 0.72 (0.60–0.86) | 0.83 (0.69–0.99) | 0.7922 | <0.0001 | 1 (ref) | 0.82 (0.71–0.95) | 0.92 (0.79–1.07) | 0.89 (0.75–1.04) | 0.6569 | 0.0001 |
| Multivariable model 1 | 1 (ref) | 0.64 (0.53–0.77) | 0.70 (0.58–0.85) | 0.76 (0.63–0.92) | 0.6804 | <0.0001 | 1 (ref) | 0.82 (0.70–0.95) | 0.88 (0.75–1.03) | 0.84 (0.72–0.99) | 0.3449 | 0.0300 |
| Multivariable model 2 | 1 (ref) | 0.64 (0.53–0.77) | 0.71 (0.59–0.86) | 0.76 (0.63–0.92) | 0.6851 | <0.0001 | 1 (ref) | 0.82 (0.71–0.95) | 0.88 (0.75–1.03) | 0.85 (0.72–1.00) | 0.3745 | 0.0300 |
| Anthocyanins | ||||||||||||
| Median (min, max) | 0.86 (0, 1.9) | 3.07 (1.90, 4.60) | 6.38 (4.6, 9.01) | 14.7 (9.01, 150) | 1.25 (0, 2.63) | 4.20 (2.63, 6.19) | 8.57 (6.19, 12.7) | 19.8 (12.7, 225) | ||||
| Age-adjusted model | 1 (ref) | 0.69 (0.58–0.82) | 0.62 (0.52–0.75) | 0.70 (0.58–0.84) | 0.0112 | <0.0001 | 1 (ref) | 0.76 (0.65–0.88) | 0.71 (0.61–0.83) | 0.82 (0.70–0.95) | 0.1371 | <0.0001 |
| Multivariable model 1 | 1 (ref) | 0.69 (0.58–0.82) | 0.61 (0.50–0.73) | 0.70 (0.57–0.85) | 0.0271 | <0.0001 | 1 (ref) | 0.76 (0.66–0.88) | 0.70 (0.60–0.82) | 0.73 (0.62–0.86) | 0.0047 | 0.0003 |
| Multivariable model 2 | 1 (ref) | 0.69 (0.58–0.82) | 0.61 (0.51–0.74) | 0.70 (0.57–0.86) | 0.0279 | <0.0001 | 1 (ref) | 0.76 (0.66–0.89) | 0.70 (0.60–0.82) | 0.73 (0.62–0.86) | 0.0053 | 0.0003 |
| Isoflavones | ||||||||||||
| Median (min, max) | 5.86 (0.25, 8.56) | 11.7 (8.58, 15.1) | 19.8 (15.1, 25.9) | 36.8 (26.0, 157) | 5.59 (0, 8.3) | 11.1 (8.31, 14.2) | 18.7 (14.2, 25.0) | 35.5 (25.0, 191) | ||||
| Age-adjusted model | 1 (ref) | 0.74 (0.62–0.89) | 0.62 (0.52–0.74) | 0.80 (0.67–0.96) | 0.1167 | <0.0001 | 1 (ref) | 0.76 (0.66–0.89) | 0.71 (0.61–0.82) | 0.90 (0.78–1.03) | 0.6540 | <0.0001 |
| Multivariable model 1 | 1 (ref) | 0.76 (0.63–0.92) | 0.61 (0.51–0.74) | 0.77 (0.64–0.94) | 0.0807 | <0.0001 | 1 (ref) | 0.75 (0.64–0.87) | 0.67 (0.58–0.79) | 0.80 (0.68–0.94) | 0.1141 | <0.0001 |
| Multivariable model 2 | 1 (ref) | 0.77 (0.63–0.92) | 0.62 (0.51–0.75) | 0.78 (0.64–0.95) | 0.1328 | <0.0001 | 1 (ref) | 0.75 (0.64–0.87) | 0.68 (0.58–0.79) | 0.80 (0.68–0.94) | 0.1390 | <0.0001 |
| Proanthocyanidins | ||||||||||||
| Median (min, max) | 11.5 (0, 19.7) | 27.4 (19.7, 36.5) | 48.7 (36.6, 64.2) | 95.4 (64.3, 641) | 14.8 (0, 24.3) | 34.4 (24.3, 46.3) | 62.8 (46.4, 85.6) | 126 (85.8, 836) | ||||
| Age-adjusted model | 1 (ref) | 0.85 (0.71–1.01) | 0.78 (0.65–0.93) | 0.79 (0.66–0.96) | 0.0400 | 0.0750 | 1 (ref) | 0.78 (0.67–0.91) | 0.77 (0.66–0.89) | 0.80 (0.68–0.94) | 0.0493 | <0.0001 |
| Multivariable model 1 | 1 (ref) | 0.79 (0.66–0.94) | 0.73 (0.61–0.89) | 0.74 (0.60–0.92) | 0.0342 | 0.0270 | 1 (ref) | 0.78 (0.67–0.90) | 0.69 (0.59–0.81) | 0.70 (0.59–0.84) | 0.0012 | 0.0012 |
| Multivariable model 2 | 1 (ref) | 0.80 (0.66–0.96) | 0.74 (0.61–0.90) | 0.76 (0.61–0.94) | 0.0531 | 0.0380 | 1 (ref) | 0.78 (0.67–0.91) | 0.70 (0.60–0.82) | 0.71 (0.59–0.85) | 0.0020 | 0.0014 |
| Food Item | Contribution to Intake (%) b | Percentage of the Variation c | Men (n = 3766) | Plineard | Pnon-lineare | Contribution to Intake (%) | Percentage of the Variation | Women (n = 6559) | Plinear | Pnon-linear | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q2 | Q3 | Q4 | Q2 | Q3 | Q4 | |||||||||
| Total flavonoids from | ||||||||||||||
| Green tea | 19.8 | 68.6 | 0.59 (0.48–0.73) | 0.67 (0.56–0.79) | 0.75 (0.63–0.89) | 0.2261 | <0.0001 | 17.7 | 67.1 | 0.66 (0.55–0.79) | 0.87 (0.76–0.99) | 0.80 (0.69–0.92) | 0.0723 | <0.0001 |
| Apple/apple juice | 11.5 | 17.0 | 0.72 (0.60–0.86) | 0.66 (0.55–0.80) | 0.63 (0.52–0.76) | 0.0005 | 0.0004 | 14.2 | 19.9 | 0.82 (0.71–0.94) | 0.62 (0.53–0.72) | 0.72 (0.62–0.85) | 0.0055 | <0.0001 |
| Grapes/grape juice | 11.1 | 7.45 | 0.63 (0.53–0.75) | 0.60 (0.50–0.72) | 0.64 (0.52–0.77) | 0.0087 | <0.0001 | 13.3 | 7.89 | 0.81 (0.70–0.94) | 0.69 (0.59–0.80) | 0.67 (0.57–0.78) | <0.0001 | 0.0038 |
| Flavonols from | ||||||||||||||
| Lettuce | 17.2 | 13.2 | 0.90 (0.75–1.08) | 0.65 (0.53–0.79) | 0.78 (0.64–0.94) | 0.0145 | 0.0011 | 18.2 | 21.1 | 0.82 (0.71–0.95) | 0.67 (0.57–0.78) | 0.79 (0.68–0.92) | 0.0215 | <0.0001 |
| Radish Kimchi | 11.4 | 5.32 | 1.01 (0.84–1.22) | 0.80 (0.67–0.97) | 0.83 (0.68–1.00) | 0.0192 | 0.1190 | 7.88 | 3.82 | 0.75 (0.64–0.87) | 0.69 (0.59–0.80) | 0.76 (0.65–0.88) | 0.0667 | <0.0001 |
| Other green vegetables f | 9.73 | 49.6 | 0.51 (0.43–0.60) | 0.53 (0.44–0.64) | 0.68 (0.57–0.82) | 0.2638 | <0.0001 | 12.0 | 44.5 | 0.76 (0.66–0.88) | 0.59 (0.51–0.69) | 0.71 (0.61–0.83) | 0.0119 | <0.0001 |
| Green tea | 8.07 | 9.29 | 0.59 (0.48–0.73) | 0.67 (0.56–0.79) | 0.75 (0.63–0.89) | 0.2261 | <0.0001 | 8.06 | 12.9 | 0.66 (0.55–0.79) | 0.87 (0.76–0.99) | 0.80 (0.69–0.92) | 0.0723 | <0.0001 |
| Flavones from | ||||||||||||||
| Green pepper | 18.9 | 38.1 | 0.79 (0.66–0.95) | 0.76 (0.63–0.91) | 0.83 (0.68–1.01) | 0.3294 | 0.0113 | 18.9 | 39.3 | 0.77 (0.67–0.89) | 0.54 (0.46–0.63) | 0.84 (0.73–0.97) | 0.2518 | <0.0001 |
| Baechu-kimchi | 16.8 | 0.97 | 1.03 (0.86–1.22) | 0.45 (0.36–0.57) | 1.16 (0.99–1.37) | 0.8829 | <0.0001 | 14.4 | 0.71 | 0.93 (0.81–1.08) | 0.54 (0.47–0.64) | 1.25 (1.08–1.45) | 0.9591 | <0.0001 |
| Tangerine | 13.8 | 11.9 | 0.71 (0.59–0.85) | 0.56 (0.47–0.67) | 0.63 (0.53–0.76) | <0.0001 | <0.0001 | 18.2 | 25.9 | 0.68 (0.59–0.79) | 0.67 (0.57–0.77) | 0.79 (0.68–0.93) | 0.0900 | <0.0001 |
| Orange/orange juice | 6.12 | 25.2 | 0.56 (0.41–0.75) | 0.68 (0.57–0.80) | 0.70 (0.58–0.83) | 0.0053 | <0.0001 | 7.80 | 15.5 | 0.63 (0.52–0.75) | 0.77 (0.67–0.88) | 0.73 (0.63–0.85) | 0.0084 | <0.0001 |
| Flavanones from | ||||||||||||||
| Tangerine | 48.3 | 25.8 | 0.71 (0.59–0.85) | 0.56 (0.47–0.67) | 0.63 (0.53–0.76) | <0.0001 | <0.0001 | 50.6 | 34.2 | 0.68 (0.59–0.79) | 0.67 (0.58–0.78) | 0.79 (0.67–0.92) | 0.0705 | <0.0001 |
| Grapes/grape juice | 26.7 | 9.94 | 0.63 (0.53–0.75) | 0.60 (0.50–0.72) | 0.64 (0.52–0.77) | 0.0087 | <0.0001 | 26.0 | 9.25 | 0.81 (0.70–0.94) | 0.69 (0.59–0.80) | 0.67 (0.58–0.78) | <0.0001 | 0.0039 |
| Orange/orange juice | 18.6 | 64.3 | 0.56 (0.41–0.75) | 0.68 (0.57–0.80) | 0.70 (0.58–0.83) | 0.0053 | <0.0001 | 19.2 | 56.5 | 0.62 (0.52–0.75) | 0.77 (0.67–0.88) | 0.73 (0.63–0.85) | 0.0088 | <0.0001 |
| Flavan-3-ols from | ||||||||||||||
| Green tea | 46.7 | 99.6 | 0.59 (0.48–0.73) | 0.67 (0.56–0.79) | 0.75 (0.63–0.89) | 0.2261 | <0.0001 | 44.4 | 99.5 | 0.66 (0.55–0.79) | 0.87 (0.76–0.99) | 0.80 (0.69–0.92) | 0.0725 | <0.0001 |
| Grapes/grape juice | 11.7 | 0.24 | 0.63 (0.53–0.75) | 0.60 (0.50–0.72) | 0.64 (0.52–0.77) | 0.0087 | <0.0001 | 14.2 | 0.30 | 0.83 (0.72–0.96) | 0.70 (0.60–0.81) | 0.68 (0.58–0.79) | <0.0001 | 0.0065 |
| Anthocyanins from | ||||||||||||||
| Grapes/grape juice | 48.1 | 87.7 | 0.63 (0.53–0.75) | 0.60 (0.50–0.72) | 0.64 (0.52–0.77) | 0.0087 | <0.0001 | 51.7 | 88.7 | 0.81 (0.70–0.94) | 0.69 (0.59–0.80) | 0.67 (0.57–0.78) | <0.0001 | 0.0038 |
| Strawberries | 25.3 | 10.3 | 0.79 (0.66–0.94) | 0.48 (0.39–0.59) | 0.79 (0.65–0.96) | 0.1853 | <0.0001 | 24.0 | 9.40 | 0.83 (0.72–0.95) | 0.69 (0.59–0.81) | 0.66 (0.56–0.77) | <0.0001 | 0.0053 |
| Isoflavones from | ||||||||||||||
| Tofu | 25.8 | 28.6 | 0.84 (0.70–1.02) | 1.07 (0.89–1.29) | 0.87 (0.71–1.07) | 0.4473 | 0.0184 | 25.9 | 30.9 | 0.75 (0.64–0.87) | 1.02 (0.88–1.19) | 0.82 (0.70–0.97) | 0.2771 | <0.0001 |
| Soybean paste g | 19.1 | 3.60 | 0.82 (0.68–0.98) | 0.76 (0.63–0.91) | 0.85 (0.70–1.03) | 0.3299 | 0.0193 | 18.9 | 3.73 | 0.82 (0.70–0.96) | 0.79 (0.68–0.92) | 0.79 (0.68–0.92) | 0.0218 | 0.0288 |
| Cooked rice with beans | 10.2 | 40.0 | 0.74 (0.62–0.89) | NA | NA | 10.9 | 34.1 | 0.77 (0.67–0.89) | NA | NA | ||||
| Multi–grain rice | 9.72 | 0.75 | 0.51 (0.43–0.62) | 0.83 (0.71–0.97) | 0.0017 | <0.0001 | 12.7 | 0.68 | 0.37 (0.30–0.45) | 0.52 (0.45–0.6) | 0.82 (0.72–0.94) | 0.002 | <0.0001 | |
| Soybean/soybean cooked in soy sauce | 8.61 | 15.0 | 0.35 (0.26–0.49) | 0.81 (0.69–0.94) | 0.65 (0.55–0.77) | 0.0008 | <0.0001 | 8.79 | 19.7 | 0.39 (0.31–0.49) | 0.80 (0.70–0.92) | 0.69 (0.60–0.80) | 0.0013 | <0.0001 |
| Proanthocyanindins | ||||||||||||||
| Apple/apple juice | 27.0 | 75.0 | 0.72 (0.60–0.86) | 0.66 (0.55–0.80) | 0.63 (0.52–0.76) | 0.0005 | 0.0004 | 29.7 | 78.8 | 0.82 (0.71–0.94) | 0.62 (0.53–0.72) | 0.72 (0.62–0.85) | 0.0055 | <0.0001 |
| Grapes/grape juice | 17.1 | 14.9 | 0.63 (0.53–0.75) | 0.60 (0.50–0.72) | 0.63 (0.52–0.77) | 0.0074 | <0.0001 | 18.7 | 13.9 | 0.81 (0.70–0.94) | 0.69 (0.59–0.80) | 0.67 (0.58–0.78) | <0.0001 | 0.0039 |
| Strawberries | 13.3 | 5.52 | 0.79 (0.66–0.93) | 0.48 (0.39–0.59) | 0.79 (0.66–0.96) | 0.1941 | <0.0001 | 13.1 | 4.39 | 0.82 (0.71–0.94) | 0.69 (0.59–0.81) | 0.65 (0.55–0.76) | <0.0001 | 0.0041 |
| Multi-grain rice | 12.8 | 1.23 | 0.53 (0.44–0.64) | 0.80 (0.68–0.93) | 0.0006 | <0.0001 | 13.5 | 0.64 | 0.36 (0.30–0.45) | 0.51 (0.44–0.58) | 0.86 (0.75–0.98) | 0.0058 | <0.0001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, J.-S.; Kim, Y.-M.; Woo, H.-W.; Shin, M.-H.; Koh, S.-B.; Kim, H.-C.; Shin, J.-H.; Kim, M.-K. Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS). Nutrients 2023, 15, 1186. https://doi.org/10.3390/nu15051186
Kong J-S, Kim Y-M, Woo H-W, Shin M-H, Koh S-B, Kim H-C, Shin J-H, Kim M-K. Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS). Nutrients. 2023; 15(5):1186. https://doi.org/10.3390/nu15051186
Chicago/Turabian StyleKong, Ji-Sook, Yu-Mi Kim, Hye-Won Woo, Min-Ho Shin, Sang-Baek Koh, Hyeon-Chang Kim, Jin-Ho Shin, and Mi-Kyung Kim. 2023. "Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS)" Nutrients 15, no. 5: 1186. https://doi.org/10.3390/nu15051186
APA StyleKong, J. -S., Kim, Y. -M., Woo, H. -W., Shin, M. -H., Koh, S. -B., Kim, H. -C., Shin, J. -H., & Kim, M. -K. (2023). Prospective Associations between Cumulative Average Intake of Flavonoids and Hypertension Risk in the CArdioVascular Disease Association Study (CAVAS). Nutrients, 15(5), 1186. https://doi.org/10.3390/nu15051186