Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential
Abstract
:1. Introduction
2. Materials and Methods
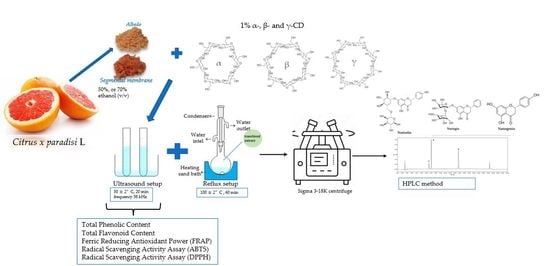
2.1. Material
2.2. Methods
2.2.1. Extracts’ Preparation
2.2.2. HPLC–PDA Conditions
2.3. Statistical Data Analysis
2.4. Preparation of Extracts for Total Phenolic, Flavonoid, and Antioxidant Activity
2.4.1. Analysis of Total Phenolic Content
2.4.2. Total Flavonoid Content Evaluation
- TFC–total flavonoid content; mg RE/g DW;
- C–concentration of standards used mg/L;
- Ve–the volume of solvent used;
- F–dilution coefficient of the sample; m–a mass of the sample, g.
2.5. Antioxidant Activity
2.5.1. Radical-Scavenging Assay (ABTS)
2.5.2. Radical Scavenging Assay (DPPH)
2.5.3. Ferric Reducing Antioxidant Power (FRAP)
3. Results and Discussion
3.1. The Quantity of Flavanones Using the Additional Substances α-, β-, γ-Cyclodextrins
3.2. Total Phenolic and Flavonoid Content Determination
3.3. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zheng, W.; Cheng, L.; Li, M.; Huang, J.; Bao, S.; Xu, Q.; Ma, Z. Citrus fruits are rich in flavonoids for immunoregulation and potential targeting ACE2. Nat. Prod. Bioprospect. 2022, 12, 14. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Lin, Y.; Dong, L.; Jia, X.; Shen, Y.; Liu, L.; Chi, J.; Huang, F.; Zhang, M.; Zhang, R. Physicochemical and functional properties of dietary fiber from pummelo (Citrus grandis L. Osbeck) and grapefruit (Citrus paradisi Mcfad) cultivars. Food Biosci. 2021, 40, 100890. [Google Scholar] [CrossRef]
- Mohd Zaid, N.A.; Sekar, M.; Bonam, S.R.; Gan, S.H.; Lum, P.T.; Begum, M.Y.; Mat Rani, N.N.; Vaijanathappa, J.; Wu, Y.S.; Subramaniyan, V.; et al. Promising Natural Products in New Drug Design, Development, and Therapy for Skin Disorders: An Overview of Scientific Evidence and Understanding Their Mechanism of Action. Drug Des. Dev. Ther. 2022, ume 16, 23–66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, D.; Tan, C.; Hu, Y.; Sundararajan, B.; Zhou, Z. Profiling of Flavonoid and Antioxidant Activity of Fruit Tissues from 27 Chinese Local Citrus Cultivars. Plants 2020, 9, 196. [Google Scholar] [CrossRef] [Green Version]
- Qurtam, A.A.; Mechchate, H.; Es-Safi, I.; Al-Zharani, M.; Nasr, F.A.; Noman, O.M.; Aleissa, M.; Imtara, H.; Aleissa, A.M.; Bouhrim, M.; et al. Citrus Flavanone Narirutin, In Vitro and In Silico Mechanistic Antidiabetic Potential. Pharmaceutics 2021, 13, 1818. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Bautista, R.J.H.; Sandhu, M.A.; Hussein, O.E. Beneficial Effects of Citrus Flavonoids on Cardiovascular and Metabolic Health. Oxidative Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Memariani, Z.; Abbas, S.Q.; ul Hassan, S.S.; Ahmadi, A.; Chabra, A. Naringin and naringenin as anticancer agents and adjuvants in cancer combination therapy: Efficacy and molecular mechanisms of action, a comprehensive narrative review. Pharmacol. Res. 2021, 171, 105264. [Google Scholar] [CrossRef]
- Peterson, J.J.; Beecher, G.R.; Bhagwat, S.A.; Dwyer, J.T.; Gebhardt, S.E.; Haytowitz, D.B.; Holden, J.M. Flavanones in grapefruit, lemons, and limes: A compilation and review of the data from the analytical literature. J. Food Compos. Anal. 2006, 19, S74–S80. [Google Scholar] [CrossRef]
- Di Majo, D.; Giammanco, M.; La Guardia, M.; Tripoli, E.; Giammanco, S.; Finotti, E. Flavanones in Citrus fruit: Structure–antioxidant activity relationships. Food Res. Int. 2005, 38, 1161–1166. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Stecko, J.; Sobieraj, J.; Szymańska, N.; Kozłowska, J. Naringenin and Its Derivatives—Health-Promoting Phytobiotic against Resistant Bacteria and Fungi in Humans. Antibiotics 2022, 11, 1628. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, M.U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4′,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The Therapeutic Potential of Naringenin: A Review of Clinical Trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stabrauskiene, J.; Kopustinskiene, D.M.; Lazauskas, R.; Bernatoniene, J. Naringin and Naringenin: Their Mechanisms of Action and the Potential Anticancer Activities. Biomedicines 2022, 10, 1686. [Google Scholar] [CrossRef]
- Zhao, Z.; Jin, G.; Ge, Y.; Guo, Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology 2019, 27, 1021–1036. [Google Scholar] [CrossRef]
- TuTunchi, H.; Naeini, F.; Ostadrahimi, A.; Hosseinzadeh-Attar, M.J. Naringenin, a flavanone with antiviral and anti-inflammatory effects: A promising treatment strategy against COVID -19. Phytother. Res. 2020, 34, 3137–3147. [Google Scholar] [CrossRef]
- Ginwala, R.; Bhavsar, R.; Chigbu, D.G.I.; Jain, P.; Khan, Z.K. Potential Role of Flavonoids in Treating Chronic Inflammatory Diseases with a Special Focus on the Anti-Inflammatory Activity of Apigenin. Antioxidants 2019, 8, 35. [Google Scholar] [CrossRef] [Green Version]
- Hartogh, D.J.D.; Tsiani, E. Antidiabetic Properties of Naringenin: A Citrus Fruit Polyphenol. Biomolecules 2019, 9, 99. [Google Scholar] [CrossRef] [Green Version]
- Manchope, M.F.; Calixto-Campos, C.; Coelho-Silva, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; Verri, W.A., Jr. Naringenin Inhibits Superoxide Anion-Induced Inflammatory Pain: Role of Oxidative Stress, Cytokines, Nrf-2 and the NO−cGMP−PKG−KATPChannel Signaling Pathway. PLoS ONE 2016, 11, e0153015. [Google Scholar] [CrossRef] [Green Version]
- Eom, S.; Lee, B.-B.; Lee, S.; Park, Y.; Yeom, H.D.; Kim, T.-H.; Nam, S.-H.; Lee, J.H. Antioxidative and Analgesic Effects of Naringin through Selective Inhibition of Transient Receptor Potential Vanilloid Member 1. Antioxidants 2021, 11, 64. [Google Scholar] [CrossRef]
- Baba, S.A.; Malik, S.A. Determination of total phenolic and flavonoid content, antimicrobial and antioxidant activity of a root extract of Arisaema jacquemontii Blume. J. Taibah Univ. Sci. 2015, 9, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Min, K.Y.; Kim, H.J.; Lee, K.A.; Kim, K.-T.; Paik, H.-D. Antimicrobial activity of acid-hydrolyzed Citrus unshiu peel extract in milk. J. Dairy Sci. 2014, 97, 1955–1960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomar, A.; Broor, S.; Kaushik, S.; Bharara, T.; Arya, D.S. Synergistic effect of naringenin with conventional antibiotics against methicillin resistant staphylococcus aureus. Clin. Med. 2021, 8, 15. [Google Scholar]
- Rebello, C.J.; Beyl, R.A.; Lertora, J.J.L.; Greenway, F.L.; Ravussin, E.; Ribnicky, D.M.; Poulev, A.; Kennedy, B.J.; Castro, H.F.; Campagna, S.R.; et al. Safety and pharmacokinetics of naringenin: A randomized, controlled, single-ascending-dose clinical trial. Diabetes Obes. Metab. 2020, 22, 91–98. [Google Scholar] [CrossRef]
- Rotimi, S.O.; Adelani, I.B.; Bankole, G.E.; Rotimi, O.A. Naringin enhances reverse cholesterol transport in high fat/low streptozocin induced diabetic rats. Biomed. Pharmacother. 2018, 101, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Mulvihill, E.E.; Allister, E.M.; Sutherland, B.G.; Telford, D.E.; Sawyez, C.G.; Edwards, J.Y.; Markle, J.M.; Hegele, R.A.; Huff, M.W. Naringenin Prevents Dyslipidemia, Apolipoprotein B Overproduction, and Hyperinsulinemia in LDL Receptor–Null Mice With Diet-Induced Insulin Resistance. Diabetes 2009, 58, 2198–2210. [Google Scholar] [CrossRef] [Green Version]
- Fu, L.; Xu, B.T.; Xu, X.R.; Gan, R.Y.; Zhang, Y.; Xia, E.Q.; Li, H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef]
- Latos-Brozio, M.; Masek, A.; Piotrowska, M. Polymeric Forms of Plant Flavonoids Obtained by Enzymatic Reactions. Molecules 2022, 27, 3702. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.S.; Ho, W.Y.; Yeap, S.K.; Bin Sharifudin, S.A. Phytochemical composition and in vitro antioxidant activities of Citrus sinensis peel extracts. PeerJ 2018, 6, e5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-Y.; Cheng, C.-W.; Liang, J.-Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Zhang, H.; Wei, X.; Ye, X.; Tian, J. Phytochemicals and Antioxidant Capacities of Young Citrus Fruits Cultivated in China. Molecules 2022, 27, 5185. [Google Scholar] [CrossRef]
- Su, D.; Liu, H.; Zeng, Q.; Qi, X.; Yao, X.; Zhang, J. Changes in the phenolic contents and antioxidant activities of citrus peels from different cultivars after in vitro digestion. Int. J. Food Sci. Technol. 2017, 52, 2471–2478. [Google Scholar] [CrossRef]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2014, 23, 729–747. [Google Scholar] [CrossRef]
- Cai, R.; Yuan, Y.; Cui, L.; Wang, Z.; Yue, T. Cyclodextrin-assisted extraction of phenolic compounds: Current research and future prospects. Trends Food Sci. Technol. 2018, 79, 19–27. [Google Scholar] [CrossRef]
- Guan, M.; Shi, R.; Zheng, Y.; Zeng, X.; Fan, W.; Wang, Y.; Su, W. Characterization, in Vitro and in Vivo Evaluation of Naringenin-Hydroxypropyl-β-Cyclodextrin Inclusion for Pulmonary Delivery. Molecules 2020, 25, 554. [Google Scholar] [CrossRef]
- Lu, W.J.; Ferlito, V.; Xu, C.; Flockhart, D.A.; Caccamese, S. Enantiomers of naringenin as pleiotropic, stereoselective inhibitors of cytochrome P450 isoforms. Chirality 2011, 23, 891–896. [Google Scholar] [CrossRef] [Green Version]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Kazlauskaite, J.A.; Ivanauskas, L.; Marksa, M.; Bernatoniene, J. The Effect of Traditional and Cyclodextrin-Assisted Extraction Methods on Trifolium pratense L. (Red Clover) Extracts Antioxidant Potential. Antioxidants 2022, 11, 435. [Google Scholar] [CrossRef]
- Shulman, M.; Cohen, M.; Soto-Gutierrez, A.; Yagi, H.; Wang, H.; Goldwasser, J.; Lee-Parsons, C.W.; Benny-Ratsaby, O.; Yarmush, M.L.; Nahmias, Y. Enhancement of Naringenin Bioavailability by Complexation with Hydroxypropoyl-β-Cyclodextrin. PLoS ONE 2011, 6, e18033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Blaskovich, M.A.; Falconer, J.R. Understanding the relationship between solubility and permeability of γ-cyclodextrin-based systems embedded with poorly aqueous soluble benznidazole. Int. J. Pharm. 2022, 616, 121487. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Carpena, M.; Oliveira, P.G.; Mejuto, J.; Prieto, M.; Gandara, J.S. Main Applications of Cyclodextrins in the Food Industry as the Compounds of Choice to Form Host–Guest Complexes. Int. J. Mol. Sci. 2021, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Bernatoniene, J. Optimization of Naringin and Naringenin Extraction from Citrus × paradisi L. Using Hydrolysis and Excipients as Adsorbent. Pharmaceutics 2022, 14, 890. [Google Scholar] [CrossRef] [PubMed]
- Wolfender, J.L. HPLC in natural product analysis: The detection issue. Planta Med. 2009, 75, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Chandra, S.; Khan, S.; Avula, B.; Lata, H.; Yang, M.H.; ElSohly, M.A.; Khan, I.A. Assessment of Total Phenolic and Flavonoid Content, Antioxidant Properties, and Yield of Aeroponically and Conventionally Grown Leafy Vegetables and Fruit Crops: A Comparative Study. Evid.-Based Complement. Altern. Med. 2014, 2014, 253875. [Google Scholar] [CrossRef] [Green Version]
- Ghafar, M.F.; Prasad, K.N.; Weng, K.K.; Ismail, A. Flavonoid, hesperidine, total phenolic contents and antioxidant activities from Citrus species. Afr. J. Biotechnol. 2010, 9, 6. [Google Scholar]
- Pudziuvelyte, L.; Liaudanskas, M.; Jekabsone, A.; Sadauskiene, I.; Bernatoniene, J. Elsholtzia ciliata (Thunb.) Hyl. Extracts from Different Plant Parts: Phenolic Composition, Antioxidant, and Anti-Inflammatory Activities. Molecules 2020, 25, 1153. [Google Scholar] [CrossRef] [Green Version]
- Yim, S.-H.; Nam, S.-H. Physiochemical, nutritional and functional characterization of 10 different pear cultivars (Pyrus spp.). J. Appl. Bot. Food Qual. 2016, 89, 7381. [Google Scholar] [CrossRef]
- Vijayalakshmi, M.; Ruckmani, K. Ferric reducing anti-oxidant power assay in plant extract. Bangladesh J. Pharmacol. 2016, 11, 570–572. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; Da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.F.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-De-Peredo, A.V.; Barbero, G.F.; et al. Extraction of Flavonoids From Natural Sources Using Modern Techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Chen, M.; Li, R.; Gao, Y.; Zheng, Y.; Liao, L.; Cao, Y.; Li, J.; Zhou, W. Encapsulation of Hydrophobic and Low-Soluble Polyphenols into Nanoliposomes by pH-Driven Method: Naringenin and Naringin as Model Compounds. Foods 2021, 10, 963. [Google Scholar] [CrossRef]
- Slámová, K.; Kapešová, J.; Valentová, K. “Sweet Flavonoids”: Glycosidase-Catalyzed Modifications. Int. J. Mol. Sci. 2018, 19, 2126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorinstein, S.; Martin-Belloso, O.; Park, Y.-S.; Haruenkit, R.; Lojek, A.; Ĉíž, M.; Caspi, A.; Libman, I.; Trakhtenberg, S. Comparison of some biochemical characteristics of different citrus fruits. Food Chem. 2001, 74, 309–315. [Google Scholar] [CrossRef]
- Xi, W.; Lu, J.; Qun, J.; Jiao, B. Characterization of phenolic profile and antioxidant capacity of different fruit part from lemon (Citrus limon Burm.) cultivars. J. Food Sci. Technol. 2017, 54, 1108–1118. [Google Scholar] [CrossRef] [Green Version]
- Nurcholis, W.; Alfadzrin, R.; Izzati, N.; Arianti, R.; Vinnai, B.; Sabri, F.; Kristóf, E.; Artika, I.M. Effects of Methods and Durations of Extraction on Total Flavonoid and Phenolic Contents and Antioxidant Activity of Java Cardamom (Amomum compactum Soland Ex Maton) Fruit. Plants 2022, 11, 2221. [Google Scholar] [CrossRef]
- Gupta, A.K.; Dhua, S.; Sahu, P.P.; Abate, G.; Mishra, P.; Mastinu, A. Variation in Phytochemical, Antioxidant and Volatile Composition of Pomelo Fruit (Citrus grandis (L.) Osbeck) during Seasonal Growth and Development. Plants 2021, 10, 1941. [Google Scholar] [CrossRef]
- Rajurkar, N.; Hande, S. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J. Pharm. Sci. 2011, 73, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Castro-Vazquez, L.; Alañón, M.E.; Rodríguez-Robledo, V.; Pérez-Coello, M.S.; Hermosín-Gutierrez, I.; Díaz-Maroto, M.C.; Jordán, J.; Galindo, M.F.; Arroyo-Jiménez, M.D.M. Bioactive Flavonoids, Antioxidant Behaviour, and Cytoprotective Effects of Dried Grapefruit Peels (Citrus paradisi Macf.). Oxidative Med. Cell. Longev. 2016, 2016, 8915729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakholiya, K.; Kaneria, M.; Nagani, K.; Patel, A.; Chanda, S. Comparative analysis and simultaneous quantification of antioxidant capacity of four terminalia species using various photometric assays. World J. Pharm. Res. 2015, 4, 1280–1296. [Google Scholar]
- Benslama, A.; Harrar, A. Free radicals scavenging activity and reducing power of two Algerian Sahara medicinal plants extracts. Int. J. Herb. Med. 2016, 4, 158–161. [Google Scholar] [CrossRef] [Green Version]
















| Extract ID * | Extraction Method | Solvent (Ethanol v/v) | Excipient (Cyclodextrins CD) |
|---|---|---|---|
| AT1 (control sample) | UAE combined with thermal hydrolysis | 50% | |
| AT2 (control sample) | 70% | ||
| ST1 (control sample) | 50% | ||
| ST2 (control sample) | 70% | ||
| AA1 | 50% | α | |
| AA2 | 70% | α | |
| SA1 | 50% | α | |
| SA2 | 70% | α | |
| AB1 | 50% | β | |
| AB2 | 70% | β | |
| SB1 | 50% | β | |
| SB2 | 70% | β | |
| AG1 | 50% | γ | |
| AG2 | 70% | γ | |
| SG1 | 50% | γ | |
| SG2 | 70% | γ |
| Component | Calibration Equation | Coefficient of Determination R2 | Correlation Coefficient | LOD µg/mL | LOQ µg/mL |
|---|---|---|---|---|---|
| Naringin | Y = 25.50x + 6720 | 0.99992 | 0.99996 | 0.146 | 0.583 |
| Naringenin | ±Y = 33.30x + 3570 | 0.99992 | 0.99996 | 0.118 | 0.430 |
| Narirutin | Y = 18.60x + 8100 | 0.99999 | 0.99999 | 0.281 | 0.5032 |
| Extract ID | Naringin mg/g | Narirutin mg/g | Naringenin μg/g |
|---|---|---|---|
| AT1 * | 13.97 ± 0.698 | 2.24 ± 0.12 | 23.58 ± 1.17 |
| AT2 * | 14.07 ± 0.70 | 2.36 ± 0.18 | 25.06 ± 1.25 |
| ST1 * | 10.53 ± 0.526 | 2.34 ± 0.17 | 65.84 ± 3.29 |
| ST2 * | 7.8 ± 0.39 | 1.95 ± 0.09 | 67.59 ± 3.37 |
| Extract ID * | Naringin mg/g | Narirutin mg/g | Naringenin µg/g |
|---|---|---|---|
| AT1 | 13.99 | 2.24 | 23.58 |
| ST1 | 10.53 | 2.34 | 65.84 |
| AA1 | 18.87 | 3.33 | 43.44 |
| SA1 | 46.53 | 0.70 | 91.19 |
| AB1 | 6.87 | 1.74 | 16.21 |
| SB1 | 51.11 | 0.81 | 0.45 |
| AG1 | 5.99 | 1.79 | 0.84 |
| SG1 | 7.52 | 2.29 | 0 |
| AT2 | 14.07 | 2.36 | 24.06 |
| ST2 | 7.80 | 1.95 | 67.59 |
| AA2 | 11.58 | 2.25 | 28.77 |
| SA2 | 49.72 | 0.80 | 86.69 |
| AB2 | 8.75 | 1.74 | 23.80 |
| SB2 | 58.08 | 0.98 | 0.20 |
| AG2 | 10.59 | 2.13 | 0 |
| SG2 | 6.76 | 2.15 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stabrauskiene, J.; Marksa, M.; Ivanauskas, L.; Viskelis, P.; Viskelis, J.; Bernatoniene, J. Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients 2023, 15, 1276. https://doi.org/10.3390/nu15051276
Stabrauskiene J, Marksa M, Ivanauskas L, Viskelis P, Viskelis J, Bernatoniene J. Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients. 2023; 15(5):1276. https://doi.org/10.3390/nu15051276
Chicago/Turabian StyleStabrauskiene, Jolita, Mindaugas Marksa, Liudas Ivanauskas, Pranas Viskelis, Jonas Viskelis, and Jurga Bernatoniene. 2023. "Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential" Nutrients 15, no. 5: 1276. https://doi.org/10.3390/nu15051276
APA StyleStabrauskiene, J., Marksa, M., Ivanauskas, L., Viskelis, P., Viskelis, J., & Bernatoniene, J. (2023). Citrus × paradisi L. Fruit Waste: The Impact of Eco-Friendly Extraction Techniques on the Phytochemical and Antioxidant Potential. Nutrients, 15(5), 1276. https://doi.org/10.3390/nu15051276