Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy
Abstract
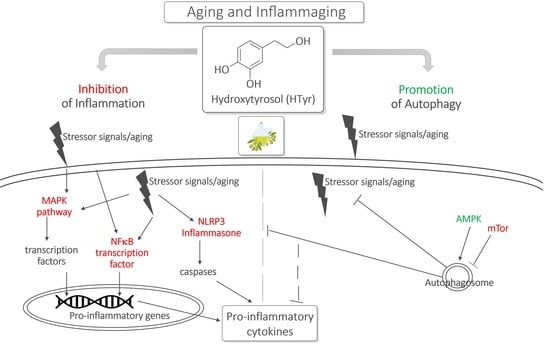
:1. Introduction
2. Hydroxytyrosol (HTyr)
3. Inflammation, Inflammaging and HTyr
3.1. Inflammation and Inflammaging
3.2. HTyr as Modulatory Agent of Inflammation
3.2.1. Evidence In Vitro
| Treatment and Dose | Model | Effects | Ref. |
|---|---|---|---|
| Pre-treatment 25, 50, 100 μM HTyr | Human Macrophages: THP-1 + LPS | ↓ TNF-α | [117] |
| Pre-treatment 0.25, 0.5, 1 μM HTyr | Human Mononuclear Cells: PBMCs + oxysterol mixture | ↓ IL-1β, MIF, RANTES ↓ p38MAPK and JNK phosphorylation | [118] |
| Co-treatment 10, 20, 40, 80 μM HTyr | Murine Macrophages: RAW 264.7 + LPS | ↓ IL-1β, TNF-α ↓ NF-κB phosphorylation | [119] |
| Pre-treatment 50 μM HTyr 12.5, 25, 50 μM Per-HTyr | Murine Macrophages: ex vivo peritoneal macrophages + LPS | ↓ IL-1β, IL-6, TNF-α, IL-17, IFN-γ ↓ STAT3 activation ↓ IL-18 via non-canonical NLRP3 inflammasome | [72] |
| Pre-treatment 50, 100 μM HTyr | Murine Macrophages: RAW 264.7 + LPS | ↓ M1, ↑ M2 macrophages ↓ IL-1β, IL-6, TNF-α ↑ IL-10, IL-4 ↓ ERK1/2 phosphorylation | [121] |
| Co-treatment 5, 10 μM HTyr | Human Peripheral Blood Monocytes: ex vivo monocytes + LPS | No change in IL-1β, TNF-α | [122] |
| Co-treatment 50, 100 μM HTyr | Murine Macrophages: RAW 264.7 + LPS | ↑ TNF-α | [123] |
| Co-treatment 80 μM HTyr | Murine Macrophages: ex vivo peritoneal macrophages + LPS | ↑ TNF-α, IL-10 no change in NF-κB expression and phosphorylation | [124] |
| Co-treatment 1, 10, 25, 50 μM HTyr | Murine Microglia: BV2 + LPS | ↓ IL-1β, TNF-α, IL-6, CXCL10 ↓ JNK1/2 and p38MAPK phosphorylation ↓ NF-κBp65 translocation to the nucleus ↓ NLRP3 inflammasome | [125] |
| Pre-treatment 25, 50, 100 μM HTyr | Murine Microglia: BV2 + LPS primary microglia + LPS | ↓ M1 ↑ M2 ↓ IL-1 β, TNF-α, IL-6 ↓ TLR-4 ↓ NF-κBp65 phosphorylation ↓ ERK1/2 phosphorylation | [126] |
| Co-treatment 20, 100 μM HTyr | Human Nucleus Pulposus Cells and Rat Microglia: primary HNPC + TNF-α microglia + LPS | ↓ IL-1β, TNF-α, IL-6 ↓ NLRP3 inflammasome ↓ NF-κB activation ↓ ERK phosphorylation | [127] |
| Pre-treatment 1 μM HTyr for 4 weeks | Human Pre-senescent and Senescent Fibroblasts: MRC5 NHDF NHDF + TNF-α | ↓ IL-6 ↓ NF-κB activation | [128] |
| Pre-treatment 12.5–100 μM HTyr 12.5–100 μM HTyr-Ac | Human Keratinocytes: primary keratinocytes + IL-1β primary keratinocytes + Poly I:C | ↓ TNF-α, IL-6, IL-8 ↓ NF-κB activation and translocation to binding site in the IL-8 promoter | [129] |
| Pre-treatment 25, 50, 100 μM HTyr | Human Psoriatic Keratinocytes: HaCaT + M5 cytokine cocktail | ↓ IL-6, IL-8, TNF-α | [130] |
| Pre-treatment 1 μM HTyr | Chemical Carcinogenesis in Human Primary Colonic Epithelial Cells: HCoEpC + B[a]P | ↓ IL-6, IL-8, VEGF, CXCL13 ↓ ERK1/2 phosphorylation | [131] |
3.2.2. Evidence In Vivo
| HTyr Treatment and Dose | Model | Effects | Ref. |
|---|---|---|---|
| 100 mg/kg diet: 4 g/day orally for 6 months | Murine pristane-induced Systemic Lupus Erythematosus: ex vivo splenocytes+LPS ex vivo macrophages+LPS renal tissue | ↓ IL-1β, IL-6 ↓ IκB degradation, p65NF-kB nuclear translocation ↓ MAPK phosphorylation | [132] |
| 100 mg/kg/day orally for 2 days | Murine LPS-induced Acute Liver Injury: liver tissue liver macrophages serum | ↓ M1 ↑ M2 macrophages ↓ IL-1 β, TNF-α, IL-6 ↓ IL-10, IL-4 | [121] |
| 10 mg/kg/day orally for 16 weeks | ApoE−/− Mice Atherosclerosis: blood heart tissue liver tissue | ↓ IL-1β, TNF-α, IL-6, CRP ↑ IL-10 ↓ NF-kB activation ↓ p38MAPK phosphorylation | [133] |
| 10 mg/kg/day orally for 5 weeks | Rats with Nonalcoholic Fatty Liver Disease: liver tissue | ↓ TNF-α, IL-6 | [134] |
| 80 mg/kg/daily for 2 or 5 days | Murine LPS-induced Systemic Inflammation: plasma | ↓ TNF-α | [135] |
| 100 mg/kg/day orally for 2 days | Murine LPS-induced Brain Inflammation: brain tissue | ↓ IL-6, IL-1 β, and TNF-α | [126] |
| 2 μL of 100 μM injected intrathecally | Rat Chronic Compression of Dorsal Root Ganglion-induced Neuropathic Pain: spinal dorsal horn | ↓ IL-1β, TNF-α, IL-6 ↓ ERK phosphorylation | [127] |
| 10 and 50 mg/kg/day orally | Murine DSS-induced Colitis: colon tissue fecal samples | ↓ IL-6, IL-1β, and TNF-α ↑ IL-10 ↓ NF-κB activation ↓ Inflammation-related microbes of gut microbiota | [137] |
| 40 mg/kg/day orally for 14 days | Murine DSS-induced Colitis: colon tissue fecal samples | ↓ IL-18 and IL-1β via ↓ NLRP3 inflammasome activation ↓ inflammation-related microbes of gut microbiota | [138] |
4. Autophagy, Inflammaging and HTyr
4.1. Autophagy and Inflammaging
4.2. HTyr as a Modulatory Agent of Autophagy
4.2.1. Evidence In Vitro
4.2.2. Evidence In Vivo
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflammaging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef]
- Cevenini, E.; Caruso, C.; Candore, G.; Capri, M.; Nuzzo, D.; Duro, G.; Rizzo, C.; Colonna-Romano, G.; Lio, D.; Di Carlo, D.; et al. Age-related inflammation: The contribution of different organs, tissues and systems. How to face it for therapeutic approaches. Curr. Pharm. Des. 2010, 16, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Vitale, G.; Capri, M.; Salvioli, S. Inflammaging and Garbaging. Trends Endocrinol. Metab. 2017, 28, 199–212. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Rasa, S.M.M.; Annunziata, F.; Krepelova, A.; Nunna, S.; Omrani, O.; Gebert, N.; Adam, L.; Kappel, S.; Höhn, S.; Donati, G.; et al. Inflammaging is driven by upregulation of innate immune receptors and systemic interferon signaling and is ameliorated by dietary restriction. Cell Rep. 2022, 39, 111017. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Regulation of the aging process by autophagy. Trends Mol. Med. 2009, 15, 217–224. [Google Scholar] [CrossRef]
- Rajawat, Y.S.; Hilioti, Z.; Bossis, I. Aging: Central role for autophagy and the lysosomal degradative system. Ageing Res. Rev. 2009, 8, 199–213. [Google Scholar] [CrossRef]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Deretic, V. Autophagy in inflammation, infection, and immunometabolism. Immunity 2021, 54, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Zinecker, H.; Simon, A.K. Autophagy takes it all-autophagy inducers target immune aging. Dis. Model Mech. 2022, 15, dmm049345. [Google Scholar] [CrossRef]
- Krabbe, K.S.; Pedersen, M.; Bruunsgaard, H. Inflammatory mediators in the elderly. Exp. Gerontol. 2004, 39, 687–699. [Google Scholar] [CrossRef]
- Wei, J.; Xu, H.; Davies, J.L.; Hemmings, G.P. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992, 51, 1953–1956. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Newman, A.B. Inflammatory markers in population studies of aging. Ageing Res. Rev. 2010, 10, 319–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koelman, L.; Pivovarova-Ramich, O.; Pfeiffer, A.F.H.; Grune, T.; Aleksandrova, K. Cytokines for evaluation of chronic inflammatory status in ageing research: Reliability and phenotypic characterisation. Immun. Ageing 2019, 16, 11. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Hartmann, C.; Secci, R.; Hermann, A.; Fuellen, G.; Walter, M. Ranking biomarkers of aging by citation profiling and effort scoring. Front. Genet. 2021, 12, 686320. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef] [Green Version]
- Sethi, G.; Sung, B.; Aggarwal, B.B. TNF: A master switch for inflammation to cancer. Front. Biosci. 2008, 13, 5094–5107. [Google Scholar] [CrossRef] [Green Version]
- Webster, J.D.; Vucic, D. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Minciullo, P.L.; Catalano, A.; Mandraffino, G.; Casciaro, M.; Crucitti, A.; Maltese, G.; Morabito, N.; Lasco, A.; Gangemi, S.; Basile, G. Inflammaging and anti-Inflammaging: The role of cytokines in extreme longevity. Arch. Immunol. Ther. Exp. (Warsz) 2016, 64, 111–126. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and age-related diseases: Role of inflammation triggers and cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef]
- Boka, G.; Anglade, P.; Wallach, D.; Javoy-Agid, F.; Agid, Y.; Hirsch, E.C. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 1994, 172, 151–154. [Google Scholar] [CrossRef]
- Mogi, M.; Harada, M.; Kondo, T.; Riederer, P.; Inagaki, H.; Minami, M.; Nagatsu, T. Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growthfactor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 1994, 180, 147–150. [Google Scholar] [CrossRef]
- Diaz, K.; Kohut, M.L.; Russell, D.W.; Stegemoller, E.L. Peripheral inflammatory cytokines and motor symptoms in persons with Parkinson’s disease. Brain Behav. Immun. Health 2022, 21, 100442. [Google Scholar] [CrossRef] [PubMed]
- Gerard, C.; Rollins, B.J. Chemokines and disease. Nat. Immunol. 2001, 2, 108–115. [Google Scholar] [CrossRef]
- Hickman, S.E.; El Khoury, J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Altara, R.; Manca, M.; Hessel, M.H.; Gu, Y.; van Vark, L.C.; Akkerhuis, K.M.; Staessen, J.A.; Struyker Boudier, H.; Booz, G.W.; Blankesteijn, M. CXCL10 is a circulating inflammatory marker in patients with advanced heart failure: A pilot study. J. Cardiovasc. Transl. Res. 2016, 9, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Corsini, E.; Vismara, L.; Lucchi, L.; Viviani, B.; Govoni, S.; Galli, C.L.; Marinovich, M.; Racchi, M. High interleukin-10 production is associated with low antibody response to influenza vaccination in the elderly. J. Leukoc. Biol. 2006, 80, 376–382. [Google Scholar] [CrossRef] [Green Version]
- Almanan, M.; Raynor, J.; Ogunsulire, I.; Malyshkina, A.; Mukherjee, S.; Hummel, S.A.; Ingram, J.T.; Saini, A.; Xie, M.M.; Alenghat, T.; et al. IL-10–producing Tfh cells accumulate with age and link inflammation with age-related immune suppression. Sci. Adv. 2020, 6, eabb0806. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Witkowski, J.M. Human inflammaging. Gerontology 2019, 65, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 9, 505–522. [Google Scholar] [CrossRef] [PubMed]
- Barcena, M.L.; Aslam, M.; Pozdniakova, S.; Norman, K.; Ladilov, Y. Cardiovascular inflammaging: Mechanisms and translational aspects. Cells 2022, 11, 1010. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, Y.; Martin-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds in fruits and beverages consumed as part of the Mediterranean diet: Their role in prevention of chronic diseases. Phytochem. Rev. 2016, 15, 405–423. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C. Olive oil phenols and their potential effects on human health. J. Agric. Food Chem. 1998, 46, 4292–4296. [Google Scholar] [CrossRef]
- Owen, R.W.; Haubner, R.; Wurtele, G.; Hull, E.; Spiegelhalder, B.; Bartsch, H. Olives and olive oil in cancer prevention. Eur. J. Cancer Prev. 2004, 13, 319–326. [Google Scholar] [CrossRef]
- Servili, M.; Esposto, S.; Fabiani, R.; Urbani, S.; Taticchi, A.; Mariucci, F.; Selvaggini, R.; Montedoro, G.F. Phenolic compounds in olive oil: Antioxidant, health and organoleptic activities according to their chemical structure. Inflammopharmacology 2009, 17, 76–84. [Google Scholar] [CrossRef]
- Romani, A.; Ieri, F.; Urciuoli, S.; Noce, A.; Marrone, G.; Nediani, C.; Bernini, R. Health effects of phenolic compounds found in extra-virgin olive oil, by-products, and leaf of Olea europaea L. Nutrients 2019, 11, 1776. [Google Scholar] [CrossRef] [Green Version]
- EFSA. Scientific Opinion. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/2033 (accessed on 3 March 2023).
- Lucarini, M.; Durazzo, A.; Bernini, R.; Campo, M.; Vita, C.; Souto, E.B.; Lombardi-Boccia, G.; Ramadan, M.F.; Santini, A.; Romani, A. Fruit wastes as valuable source of value-added compounds: A collaborative perspective. Molecules 2021, 26, 6338. [Google Scholar] [CrossRef]
- Pineiro, Z.; Cantos-Villar, E.; Palma, M.; Puertas, B. Direct liquid chromatography method for the simultaneous quantification of hydroxytyrosol and tyrosol in red wines. J. Agric. Food Chem. 2011, 59, 11683–11689. [Google Scholar] [CrossRef]
- Bernini, R.; Merendino, N.; Romani, A.; Velotti, F. Naturally occurring hydroxytyrosol: Synthesis and anticancer potential. Curr. Med. Chem. 2013, 20, 655–670. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; He, X.W.; Jiang, J.G.; Xu, X.L. Hydroxytyrosol and its potential therapeutic effects. J. Agric. Food Chem. 2014, 62, 1449–1455. [Google Scholar] [CrossRef]
- Robles-Almazan, M.; Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Rofriguez-Garcia, C.; Quiles, J.L.; Ramirez-Tortosa, M.C. Hydroxytyrosol: Bioavailability, toxicity and clinical applications. Food Res. Int. 2018, 105, 654–667. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Ceccarelli, M.; Bernini, R.; Clemente, M.; Santi, L.; Caruso, C.; Micheli, L.; Tirone, F. Hydroxytyrosol stimulates neurogenesis in aged dentate gyrus by enhancing stem and progenitor cell proliferation and neuron survival. FASEB J. 2020, 34, 4512–4526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romanucci, V.; Garcia-Vinuales, S.; Tempra, S.; Bernini, R.; Zarrelli, A.; Lolicato, F.; Minardi, D.; Di Fabio, G. Modulating Aβ aggregation by tyrosol-based ligands: The crucial role of the catechol moiety. Biophys. Chem. 2020, 265, 106434. [Google Scholar] [CrossRef]
- Laghezza Masci, V.; Bernini, R.; Villanova, N.; Clemente, M.; Cicaloni, V.; Tinti, L.; Salvini, L.; Taddei, A.R.; Tiezzi, A.; Ovidi, E. In vitro anti-proliferative and apoptotic effects of hydroxytyrosyl oleate on SH-SY5Y human neuroblastoma cells. Int. J. Mol. Sci. 2022, 23, 12348. [Google Scholar] [CrossRef]
- Lan, X.; Chang, K.; Zeng, L.; Liu, X.; Qiu, F.; Zheng, W.; Quan, H.; Liao, Z.; Chen, M.; Huang, W.; et al. Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PLoS ONE 2013, 8, e75459. [Google Scholar] [CrossRef] [PubMed]
- Gambacorta, A.; Tofani, D.; Bernini, R.; Migliorini, A. High yielding preparation of a stable precursor of hydroxytyrosol by total synthesis and from the natural glucoside oleuropein. J. Agric. Food Chem. 2007, 55, 3386–3391. [Google Scholar] [CrossRef]
- Vissers, M.N.; Zock, P.L.; Roodenburg, A.J.C.; Leenen, R.; Katan, M.B. Olive oil phenols are absorbed in humans. J. Nutr. 2002, 132, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Mosele, J.I.; Martin-Pelaez, S.; Macia, A.; Farras, M.; Valls, R.-M.; Catalan, U.; Motilva, M.-J. Faecal microbial metabolism of olive oil phenolic compounds: In vitro and in vivo approaches. Mol. Nutr. Food Res. 2014, 58, 1809–1819. [Google Scholar] [CrossRef]
- Romani, A.; Pinelli, P.F.; Ieri Bernini, R. Sustainability, innovation and green chemistry in the production and valorization of phenolic extracts from Olea europaea L. Sustainability 2016, 8, 1002. [Google Scholar] [CrossRef] [Green Version]
- Bernini, R.; Carastro, I.; Palmini, G.; Tanini, A.; Zonefrati, R.; Pinelli, P.; Brandi, M.L.; Romani, A. Lipophilization of Hydroxytyrosol-enriched fractions from Olea europaea L. by-products and evaluation of the in vitro effects on a model of colorectal cancer cells. J. Agric. Food Chem. 2017, 65, 6506–6512. [Google Scholar] [CrossRef] [PubMed]
- Luzi, F.; Pannucci, E.; Clemente, M.; Grande, E.; Urciuoli, S.; Romani, A.; Torre, L.; Puglia, D.; Bernini, R.; Santi, L. Hydroxytyrosol and oleuropein-enriched extracts obtained from olive oil wastes and by-products as active antioxidant ingredients for poly(vinyl alcohol)-based films. Molecules 2021, 26, 2104. [Google Scholar] [CrossRef]
- Meschini, R.; D’Eliseo, D.; Filippi, S.; Bertini, L.; Bizzarri, B.M.; Botta, L.; Saladino, R.; Velotti, F. Tyrosinase-treated hydroxytyrosol-enriched olive vegetation waste with increased antioxidant activity promotes autophagy and inhibits the inflammatory response in human THP-1 monocytes. J. Agric. Food Chem. 2018, 66, 12274–12284. [Google Scholar] [CrossRef]
- Dickinson, J.R.; Salgado, L.E.J.; Hewlins, M.J.E. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J. Biol. Chem. 2003, 278, 8028–8034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuck, K.L.; Freeman, M.P.; Hayball, P.J.; Stretch, G.L.; Stupans, I. The in vivo fate of hydroxytyrosol and tyrosol, antioxidant phenolic constituents of olive oil, after intravenous and oral dosing of labeled compounds to rats. J. Nutr. 2001, 131, 1993–1996. [Google Scholar] [CrossRef] [Green Version]
- Dominguez-Perles, R.; Aunon, D.; Ferreres, F.; Gil-Izquierdo, A. Gender differences in plasma and urine metabolites from Sprague-Dawley rats after oral administration of normal and high doses of hydroxytyrosol, hydroxytyrosol acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef]
- Serra, A.; Rubio, L.; Borras, X.; Macia, A.; Romero, M.-P.; Motilva, M.J. Distribution of olive oil phenolic compounds in rat tissues after administration of a phenolic extract from olive cake. Mol. Nutr. Food Res. 2012, 56, 486–496. [Google Scholar] [CrossRef]
- Visioli, F.; Galli, C.; Grande, S.; Colonnelli, K.; Patelli, C.; Galli, G.; Caruso, D. Hydroxytyrosol excretion differs between rats and humans and depends on the vehicle of administration. J. Nutr. 2003, 133, 2612–2615. [Google Scholar] [CrossRef] [Green Version]
- Aunon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef]
- Aunon-Calles, D.; Canut, L.; Visioli, F. Hydroxytyrosol is not genotoxic in vitro. Pharm. Res. 2013, 74, 87–93. [Google Scholar] [CrossRef]
- Siracusa, R.; Scuto, M.; Fusco, R.; Trovato, A.; Ontario, M.L.; Crea, R.; Di Paola, R.; Cuzzocrea, S.; Calabrese, V. Anti-inflammatory and antioxidant activity of Hidrox® in rotenone-induced Parkinson’s disease in mice. Antioxidants 2020, 9, 824. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef]
- Bernini, R.; Fabrizi, G.; Pouysegu, L.; Deffieux, D.; Quideau, S. Synthesis of biologically active catecholic compounds via ortho-selective oxygenation of phenolic compounds using hypervalent iodine(V) reagents. Curr. Org. Synth. 2012, 9, 650–669. [Google Scholar] [CrossRef]
- Bozzini, T.; Botta, G.; Delfino, M.; Onofri, S.; Saladino, R.; Amatore, D.; Sgarbanti, R.; Nencioni, L.; Palamara, A.T. Tyrosinase and Layer-by-Layer supported tyrosinases in the synthesis of lipophilic catechols with antiinfluenza activity. Bioorg. Med. Chem. 2013, 21, 7699–7708. [Google Scholar] [CrossRef]
- Bernini, R.; Crisante, F.; Merendino, N.; Molinari, R.; Soldatelli, M.C.; Velotti, F. Synthesis of a novel ester of hydroxytyrosol and α-lipoic acid exhibiting an antiproliferative effect on human colon cancer HT-29 cells. Eur. J. Med. Chem. 2011, 46, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Gilardini Montani, M.S.; Merendino, N.; Romani, A.; Velotti, F. Hydroxytyrosol-derived compounds: A basis for the creation of new pharmacological agents for cancer prevention and therapy. J. Med. Chem. 2015, 58, 9089–9107. [Google Scholar] [CrossRef] [PubMed]
- Bernini, R.; Barontini, M.; Cis, V.; Carastro, I.; Tofani, D.; Chiodo, R.A.; Lupattelli, P.; Incerpi, S. Synthesis and evaluation of the antioxidant activity of lipophilic phenethyl trifluoroacetate esters by in vitro ABTS, DPPH and in cell-culture DCF assays. Molecules 2018, 23, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernini, R.; Carastro, I.; Santoni, F.; Clemente, M. Synthesis of lipophilic esters of tyrosol, homovanillyl alcohol and hydroxytyrosol. Antioxidants 2019, 8, 174. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, N.; Mateos, R.; Collantes de Teran, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar] [CrossRef]
- Montoya, T.; Aparicio-Soto, M.; Castejón, M.L.; Rosillo, M.A.; Sánchez-Hidalgo, M.; Begines, P.; Fernández-Bolaños, J.G.; Alarcón-de-la-Lastra, C. Peracetylated hydroxytyrosol, a new hydroxytyrosol derivate, attenuates LPS-induced inflammatory response in murine peritoneal macrophages via regulation of non-canonical inflammasome, Nrf2/HO1 and JAK/STAT signaling pathways. J. Nutr. Biochem. 2018, 57, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Kunnumakkara, A.B.; Aggarwal, S.; Aggarwal, B.B. Inflammation, a double-edge sword for cancer and other age-related diseases. Front. Immunol. 2018, 9, 2160. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Capri, M.; Monti, D.; Giunta, S.; Olivieri, F.; Sevini, F.; Panourgia, M.P.; Invidia, L.; Celani, L.; Scurti, M.; et al. Inflammaging and anti-inflammaging: A systemic perspective on aging and longevity emerged from studies in humans. Mech. Ageing Dev. 2007, 128, 92–105. [Google Scholar] [CrossRef]
- Kovacs, E.J.; Palmer, J.L.; Fortin, C.F.; Fulop, J.T.; Goldstein, D.R.; Linton, P.J. Aging and innate immunity in the mouse: Impact of intrinsic and extrinsic factors. Trends Immunol. 2009, 30, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, inflammaging, and frailty: Role of myeloid cells in age-related diseases. Clin. Rev. Allergy Immunol. 2022, 15, 1–22. [Google Scholar] [CrossRef]
- Desdin-Mico, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabande-Rodriguez, E.; Blanco, E.M.; Alfranca, A.; Cusso, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef]
- Ramos, G.C.; van den Berg, A.; Nunes-Silva, V.; Weirather, J.; Peters, L.; Burkard, M.; Friedrich, M.; Pinnecker, J.; Abesser, M.; Heinze, K.G.; et al. Myocardial aging as a T-cell-mediated phenomenon. Proc. Natl. Acad. Sci. USA 2017, 114, E2420–E2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delgobo, M.; Heinrichs, M.; Hapke, N.; Ashour, D.; Appel, M.; Srivastava, M.; Heckel, T.; Spyridopoulos, I.; Hofmann, U.; Frantz, S.; et al. Terminally differentiated CD4+ T cells promote myocardial inflammaging. Front. Immunol. 2021, 12, 584538. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. (Landmark Ed.) 2021, 26, 50–96. [Google Scholar] [CrossRef]
- Ronkina, N.; Gaestel, M. MAPK-activated protein kinases: Servant or partner? Annu. Rev. Biochem. 2022, 91, 505–540. [Google Scholar] [CrossRef]
- Salminen, A.; Huuskonen, J.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Activation of innate immunity system during aging: NF-κB signaling is the culprit of inflamm-aging. Ageing Res. Rev. 2008, 7, 83–105. [Google Scholar] [CrossRef]
- Tilstra, J.S.; Clauson, C.L.; Niedernhofer, L.J.; Robbins, P.D. NF-κB in Aging and Disease. Aging Dis. 2011, 2, 449–465. [Google Scholar] [PubMed]
- Osorio, F.; Soria-Valles, C.; Santiago-Fernández, O.; Freije, J.; López-Otín, C. NF-κB signaling as a driver of ageing. Int. Rev. Cell Mol. Biol. 2016, 326, 133–174. [Google Scholar] [PubMed]
- Tavenier, J.; Rasmussen, L.J.H.; Houlind, M.B.; Andersen, A.L.; Panum, I.; Andersen, O.; Petersen, J.; Langkilde, A.; Nehlin, J.O. Alterations of monocyte NF-kappaB p65/RelA signaling in a cohort of older medical patients, age-matched controls, and healthy young adults. Immun. Ageing 2020, 17, 25. [Google Scholar] [CrossRef]
- García-García, V.; Alameda, J.; Page, A.; Casanova, M. Role of NF-κB in Ageing and Age-Related Diseases: Lessons from Genetically Modified Mouse Models. Cells 2021, 10, 1906. [Google Scholar] [CrossRef]
- Martinon, F.; Mayor, A.; Tschopp, J. The inflammasomes: Guardians of the body. Annu. Rev. Immunol. 2009, 27, 229–265. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef] [PubMed]
- Youm, Y.H.; Grant, R.W.; McCabe, L.R.; Albarado, D.C.; Nguyen, K.Y.; Ravussin, A.; Pistell, P.; Newman, S.; Carter, R.; Laque, A.; et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013, 18, 519–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [Green Version]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nunez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef] [Green Version]
- Conway, J.A.; Duggal, N. Ageing of the gut microbiome: Potential influences on immune senescence and inflammageing. Ageing Res. Rev. 2021, 68, 101323. [Google Scholar] [CrossRef]
- Santoro, A.; Zhao, J.; Wu, L.; Carru, C.; Biagi, E.; Franceschi, C. Microbiomes other than the gut: Inflammaging and age-related diseases. Semin. Immunopathol. 2020, 42, 589–605. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serreli, G.; Deiana, M. Extra virgin olive oil polyphenols: Modulation of cellular pathways related to oxidant species and inflammation in aging. Cells 2020, 9, 478. [Google Scholar] [CrossRef] [Green Version]
- Pojero, F.; Aiello, A.; Gervasi, F.; Caruso, C.; Ligotti, M.E.; Calabrò, A.; Procopio, A.; Candore, G.; Accardi, G.; Allegra, M. Effects of oleuropein and hydroxytyrosol on inflammatory mediators: Consequences on inflammaging. Int. J. Mol. Sci. 2022, 24, 380. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.Y.; Pang, K.L. Therapeutic effects of olive and its derivatives on osteoarthritis: From bench to bedside. Nutrients 2017, 9, 1060. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; de Pablos, R.M.; Krisa, S.; Richard, T.; Garcia-Parrilla, M.C.; Troncoso, A.M. Phenolic compounds characteristic of the Mediterranean diet in mitigating microglia-mediated neuroinflammation. Front. Cell. Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Ontario, M.L.; Siracusa, R.; Modafferi, S.; Scuto, M.; Sciuto, S.; Greco, V.; Bertuccio, M.P.; Trovato Salinaro, A.; Crea, R.; Calabrese, E.J.; et al. Potential prevention and treatment of neurodegenerative disorders by olive polyphenols and Hidrox. Mech. Ageing Dev. 2022, 203, 111637. [Google Scholar] [CrossRef]
- Bitler, C.M.; Viale, T.M.; Damaj, B.; Crea, R. Hydrolyzed olive vegetation water in mice has anti-inflammatory activity. J. Nutr. 2005, 135, 1475–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, N.; Arnold, S.; Hoeller, U.; Kilpert, C.; Wertz, K.; Schwager, J. Hydroxytyrosol is the major anti-inflammatory compound in aqueous olive extracts and impairs cytokine and chemokine production in macrophages. Planta Med. 2011, 77, 1890–1897. [Google Scholar] [CrossRef] [Green Version]
- Kondreddy, V.K.R.; Naidu, K.A. Oleic acid, hydroxytyrosol and n-3 fatty acids collectively modulate colitis through reduction of oxidative stress and IL-8 synthesis; in vitro and in vivo studies. Int. Immunopharmacol. 2016, 35, 29–42. [Google Scholar]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Martin, M.E.; Millan-Linares, M.C.; Naranjo, M.C.; Toscano, R.; Abia, R.; Muriana, F.J.G.; Bermudez, B.; Montserrat-de la Paz, S. Minor compounds from virgin olive oil attenuate LPS-induced inflammation via visfatin-related gene modulation on primary human monocytes. J. Food Biochem. 2019, 43, e12941. [Google Scholar] [CrossRef]
- Rosillo, M.A.; Alarcon-de-la-Lastra, C.; Castejon, M.L.; Montoya, T.; Cejudo-Guillen, M.; Sanchez-Hidalgo, M. Polyphenolic extract from extra virgin olive oil inhibits the inflammatory response in IL-1β-activated synovial fibroblasts. Br. J. Nutr. 2019, 121, 55–62. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. BioMed Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef] [Green Version]
- Hioki, T.; Tokuda, H.; Kuroyanagi, G.; Kim, W.; Tachi, J.; Matsushima-Nishiwaki, R.; Iida, H.; Kozawa, O. Olive polyphenols attenuate TNF-α-stimulated M-CSF and IL-6 synthesis in osteoblasts: Suppression of Akt and p44/p42 MAP kinase signaling pathways. Biomed. Pharmacother. 2021, 141, 111816. [Google Scholar] [CrossRef] [PubMed]
- Leo, M.; Muccillo, L.; Dugo, L.; Bernini, R.; Santi, L.; Sabatino, L. Polyphenols extracts from oil production waste products (OPWPs) reduce cell viability and exert anti-inflammatory activity via PPAR induction in colorectal cancer cells. Antioxidants 2022, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Manna, C.; Della Ragione, F.; Cucciolla, V.; Borriello, A.; D’Angelo, S.; Galletti, P.; Zappia, V. Biological effects of hydroxytyrosol, a polyphenol from olive oil endowed with antioxidant activity. Adv. Exp. Med. Biol. 1999, 472, 115–130. [Google Scholar] [PubMed]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.R.; Resende, D.; Monteiro, M.; Coimbra, M.A.; Silva, A.M.S.; Cardoso, S.M. Application of hydroxytyrosol in the functional foods field: From ingredient to dietary supplements. Antioxidants 2020, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, C.; Franceschelli, S.; Quiles, J.L.; Speranza, L. Wide biological role of hydroxytyrosol: Possible therapeutic and preventive properties in cardiovascular diseases. Cells 2020, 9, 1932. [Google Scholar] [CrossRef] [PubMed]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive polyphenols: Antioxidant and anti-inflammatory properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Petrella, C.; Francati, S.; Lucarelli, M.; Barbato, C.; Minni, A.; Ralli, M.; Greco, A.; Tarani, L.; Fiore, M.; et al. Antioxidant intervention to improve cognition in the aging brain: The example of hydroxytyrosol and resveratrol. Int. J. Mol. Sci. 2022, 23, 15674. [Google Scholar] [CrossRef]
- Rufino-Palomares, E.E.; Perez-Jimenez, A.; Garcia-Salguero, L.; Mokhtari, K.; Reyes-Zurita, F.J.; Peragon-Sanchez, J.; Lupianez, J.A. Nutraceutical role of polyphenols and triterpenes present in the extracts of fruits and leaves of Olea europaea as antioxidants, anti-infectives and anticancer agents on healthy growth. Molecules 2022, 27, 2341. [Google Scholar] [CrossRef]
- Zhang, X.; Cao, J.; Zhong, L. Hydroxytyrosol inhibits pro-inflammatory cytokines, iNOS, and COX-2 expression in human monocytic cells. Naunyn-Schmiedebergs Arch. Exp. Pathol. Pharmakol. 2009, 379, 581–586. [Google Scholar] [CrossRef]
- Serra, G.; Deiana, M.; Spencer, J.P.E.; Corona, G. Olive oil phenolics prevent oxysterol-induced proinflammatory cytokine secretion and reactive oxygen species production in human peripheral blood mononuclear cells, through modulation of p38 and JNK pathways. Mol. Nutr. Food Res. 2017, 61, 1700283. [Google Scholar] [CrossRef] [Green Version]
- Yonezawa, Y.; Miyashita, T.; Nejishima, H.; Takeda, Y.; Imai, K.; Ogawa, H. Anti-inflammatory effects of olive-derived hydroxytyrosol on lipopolysaccharide-induced inflammation in RAW264.7 cells. J. Vet. Med. Sci. 2018, 80, 1801–1807. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Insulin/IGF-1 signaling promotes immunosuppression via the STAT3 pathway: Impact on the aging process and age-related diseases. Inflamm. Res. 2021, 70, 1043–1061. [Google Scholar] [CrossRef]
- Yu, Y.B.; Zhuang, H.Z.; Ji, X.J.; Dong, L.; Duan, M.L. Hydroxytyrosol suppresses LPS-induced intrahepatic inflammatory responses via inhibition of ERK signaling pathway activation in acute liver injury. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 6455–6462. [Google Scholar] [PubMed]
- Bigagli, E.; Cinci, L.; Paccosi, S.; Parenti, A.; D’Ambrosio, M.; Luceri, C. Nutritionally relevant concentrations of resveratrol and hydroxytyrosol mitigate oxidative burst of human granulocytes and monocytes and the production of pro-inflammatory mediators in LPS-stimulated RAW 264.7 macrophages. Int. Immunopharmacol. 2017, 43, 147–155. [Google Scholar] [CrossRef]
- Rosignoli, P.; Fuccelli, R.; Fabiani, R.; Servili, M.; Morozzi, G. Effect of olive oil phenols on the production of inflammatory mediators in freshly isolated human monocytes. J. Nutr. Biochem. 2013, 24, 1513–1519. [Google Scholar] [CrossRef]
- Takeda, Y.; Bui, V.N.; Iwasaki, K.; Kobayashi, T.; Ogawa, H.; Imai, K. Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependent inflammatory response of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 2014, 446, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Fernández, M.; Hornedo-Ortega, R.; Alonso-Bellido, I.M.; Rodriguez-Gomez, J.A.; Troncoso, A.M.; Garcia-Parrilla, M.C.; Venero, J.L.; Espinosa-Oliva, A.M.; Pablos, R.M. Hydroxytyrosol decreases LPS- and α-synuclein-induced microglial activation in vitro. Antioxidants 2019, 9, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, J.; Jiang, X.; Yang, L.; Zhang, Q.; Wang, B.; Cui, L.; Wang, X. Hydroxytyrosol inhibits LPS-induced neuroinflammatory responses via suppression of TLR-4-mediated NF-κB P65 activation and ERK aignaling pathway. Neuroscience 2020, 426, 189–200. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.; Wei, F.; Hou, G.; You, Y.; Wang, X.; Ca, S.; Yang, X.; Liu, W.; Zhang, S.; et al. Hydroxytyrosol ameliorates intervertebral disc degeneration and neuropathic pain by reducing oxidative stress and inflammation. Oxid. Med. Cell Longev. 2022, 2022, 2240894. [Google Scholar] [CrossRef]
- Menicacci, B.; Cipriani, C.; Margheri, F.; Mocali, A.; Giovannelli, L. Modulation of the senescence-associated inflammatory phenotype in human fibroblasts by olive phenols. Int. J. Mol. Sci. 2017, 18, 2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Soto, M.; Redhu, D.; Sanchez-Hidalgo, M.; Fernandez-Bolanos, J.G.; Alarcon-de-la-Lastra, C.; Worm, M.; Babina, M. Olive-oil-derived polyphenols effectively attenuate inflammatory responses of human keratinocytes by interfering with the NF-κB pathway. Mol. Nutr. Food Res. 2019, 63, e1900019. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Chen, L.; Zhou, J.; Cai, R.; Ye, Z.; Zhang, D. Anti-psoriasis activities of hydroxytyrosol on HaCaT cells under psoriatic inflammation in vitro. Immunopharmacol. Immunotoxicol. 2022, 10, 1–6. [Google Scholar] [CrossRef]
- Santarelli, R.; Pompili, C.; Gilardini Montani, M.S.; Evangelista, L.; Gonnella, R.; Cirone, M. 3,4-Dihydroxyphenylethanol (DPE or hydroxytyrosol) counteracts ERK1/2 and mTOR activation, pro-inflammatory cytokine release, autophagy and mitophagy reduction mediated by benzo[a]pyrene in primary human colonic epithelial cells. Pharmaceutics 2022, 14, 663. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Sanchez-Hidalgo, M.; Cardeno, A.; Gonzalez-Benjumea, A.; Fernandez-Bolanos, J.G.; Alarcon-De-La-Lastra, C. Dietary hydroxytyrosol and hydroxytyrosyl acetate supplementation prevent pristane-induced systemic lupus erythematous in mice. J. Funct. Foods 2017, 29, 84–92. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, Y.; Wan, X.; Liu, H.; Iv, C.; Ruan, W.; Lu, L.; He, L.; Guo, X. Hydroxytyrosol plays antiatherosclerotic effects through regulating lipid metabolism via inhibiting the p38 signal pathway. BioMed Res. Int. 2020, 2020, 5036572. [Google Scholar] [CrossRef]
- Pirozzi, C.; Lama, A.; Simeoli, R.; Paciello, O.; Pagano, T.B.; Mollica, M.P.; Di Guida, F.; Russo, R.; Magliocca, S.; Canani, R.B.; et al. Hydroxytyrosol prevents metabolic impairment reducing hepatic inflammation and restoring duodenal integrity in a rat model of NAFLD. J. Nutr. Biochem. 2016, 30, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Fuccelli, R.; Fabiani, R.; Rosignoli, P. Hydroxytyrosol exerts anti-inflammatory and antioxidant activities in a mouse model of systemic inflammation. Molecules 2018, 23, 3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez de las Hazas, M.-C.; Godinho-Pereira, J.; Macia, A.; Filipa Almeida, A.; Ventura, M.R.; Motilva, M.-J.; Santos, C.N. Brain uptake of hydroxytyrosol and its main circulating metabolites: Protective potential in neuronal cells. J. Funct. Foods 2018, 46, 110–117. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Abdullah, T.W.; Qiu, Z.; Song, M.; Cao, Y.; Xiao, J. Hydroxytyrosol alleviates dextran sulfate sodium-induced colitis by modulating inflammatory responses, intestinal barrier, and microbiome. J. Agric. Food Chem. 2022, 70, 2241–2252. [Google Scholar] [CrossRef]
- Miao, F. Hydroxytyrosol alleviates dextran sodium sulfate-induced colitis by inhibiting NLRP3 inflammasome activation and modulating gut microbiota in vivo. Nutrition 2022, 97, 111579. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Klionsky, D.J. Regulation mechanisms and signaling pathways of autophagy. Annu. Rev. Genet. 2009, 43, 67–93. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Cuervo, A.M. Autophagy in the cellular energetic balance. Cell Metab. 2011, 13, 495–504. [Google Scholar] [CrossRef] [Green Version]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Petroni, G.; Amaravadi, R.K.; Baehrecke, E.H.; Ballabio, A.; Boya, P.; Bravo-San Pedro, J.M.; Cadwell, K.; Cecconi, F.; Choi, A.M.K.; et al. Autophagy in major human diseases. EMBO J. 2021, 40, e108863. [Google Scholar] [CrossRef]
- Dikic, I.; Elazar, Z. Mechanism and medical implications of mammalian autophagy. Nat. Rev. Mol. Cell Biol. 2018, 19, 349–364. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Zhou, M.; Chen, C.; Wu, X.; Wang, X. Role of AMPK mediated pathways in autophagy and aging. Biochimie 2022, 195, 100–113. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Yuan, M.; Fan, H.; Cai, Z. Role of AMPK in autophagy. Front. Physiol. 2022, 13, 1015500. [Google Scholar] [CrossRef]
- Lee, I.H. Mechanisms and disease implications of sirtuin-mediated autophagic regulation. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, S.; Armstrong, C.M.; Kaeberlein, M.; Guarente, L. Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 2000, 403, 795–800. [Google Scholar] [CrossRef]
- Kawahara, T.L.; Michishita, E.; Adler, A.S.; Damian, M.; Berber, E.; Lin, M.; McCord, R.A.; Ongaigui, K.C.; Boxer, L.D.; Chang, H.Y.; et al. SIRT6 links histone H3 lysine 9 deacetylation to NF-kappa B-dependent gene expression and organismal life span. Cell 2009, 136, 62–74. [Google Scholar] [CrossRef] [Green Version]
- Sadria, M.; Layton, A.T. Interactions among mTORC, AMPK and SIRT: A computational model for cell energy balance and metabolism. Cell Commun. Signal. 2021, 19, 57. [Google Scholar] [CrossRef] [PubMed]
- Wakui, H.; Yoshii, Y.; Yumino, Y.; Fujii, S. Autophagy Induction by SIRT6 through attenuation of insulin-like growth factor signaling is involved in the regulation of human bronchial epithelial cell senescence. J. Immunol. 2014, 192, 958–968. [Google Scholar]
- Klionsky, D.J. Guidelines for the use and interpretation of assays for monitoring autophagy. Autophahy 2016, 12, 1–222. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Otin, C.; Kroemer, G. Decelerating ageing and biological clocks by autophagy. Nat. Rev. Mol. Cell Biol. 2019, 20, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V. Multiple regulatory and effector roles of autophagy in immunity. Curr. Opin. Immunol. 2009, 21, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Germic, N.; Frangez, Z.; Yousefi, S.; Simon, H.-U. Regulation of the innate immune system by autophagy: Monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019, 26, 715–727. [Google Scholar] [CrossRef] [PubMed]
- Metur, S.P.; Klionsky, D.J. Adaptive immunity at the crossroads of autophagy and metabolism. Cell. Mol. Immunol. 2021, 18, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Deretic, V.; Levine, B. Autophagy balances inflammation in innate immunity. Autophagy 2018, 14, 243–251. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Hyttinen, J.M.; Kaarniranta, K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J. Mol. Med. 2011, 89, 667–676. [Google Scholar] [CrossRef] [Green Version]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Inflammaging: Disturbed interplay between autophagy and inflammasome. Aging 2022, 4, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Lu, R.; Zhang, L.; Yang, X. Interaction between autophagy and the NLRP3 inflammasome in Alzheimer’s and Parkinson’s disease. Front. Aging Neurosci. 2022, 14, 1018848. [Google Scholar] [CrossRef]
- Jeong, J.; Choi, Y.J.; Lee, H.K. The role of autophagy in the function of CD4+ T cells and the development of chronic inflammatory diseases. Front. Pharmacol. 2022, 13, 860146. [Google Scholar] [CrossRef]
- Choi, A.J.S.; Ryter, S.W. Autophagy in inflammatory diseases. Int. J. Cell Biol. 2011, 2011, 732798. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013, 12, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Sureda, A.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Devi, K.P.; Ahmed, T.; Ishaq, N.; Hashim, R.; Sobarzo-Sanchez, E.; et al. Regulation of autophagy by polyphenols: Paving the road for treatment of neurodegeneration. Biotechnol. Adv. 2018, 36, 1768–1778. [Google Scholar] [CrossRef]
- Leri, M.; Scuto, M.; Ontario, M.L.; Calabrese, V.; Calabrese, E.J.; Bucciantini, M.; Stefani, M. Healthy effects of plant polyphenols: Molecular mechanisms. Int. J. Mol. Sci. 2020, 21, 1250. [Google Scholar] [CrossRef] [Green Version]
- Tian, Z.; Zhang, X.; Sun, M. Plant phytochemicals mediate autophagy against osteoarthritis by maintaining cartilage homeostasis. Front. Pharmacol. 2021, 12, 795058. [Google Scholar] [CrossRef] [PubMed]
- Brimson, J.M.; Prasanth, M.I.; Malar, D.S.; Thitilertdecha, P.; Kabra, A.; Tencomnao, T.; Prasansuklab, A. Polyphenols for aging health: Implication from their autophagy modulating properties in age-associated diseases. Pharmaceuticals 2021, 14, 982. [Google Scholar] [CrossRef] [PubMed]
- García-Aguilar, A.; Palomino, O.; Benito, M.; Guillén, C. Dietary polyphenols in metabolic and neurodegenerative diseases: Molecular targets in autophagy and biological effects. Antioxidants 2021, 10, 142. [Google Scholar] [CrossRef]
- Wu, M.; Luo, Q.; Nie, R.; Yang, X.; Tang, Z.; Chen, H. Potential implications of polyphenols on aging considering oxidative stress, inflammation, autophagy, and gut microbiota. Crit. Rev. Food Sci. Nutr. 2021, 61, 2175–2193. [Google Scholar] [CrossRef]
- Blanco-Benitez, M.; Calderon-Fernandez, A.; Canales-Cortes, S.; Alegre-Cortes, E.; Uribe-Carretero, E.; Paredes-Barquero, M.; Gimenez-Bejarano, A.; Duque Gonzalez, G.; Gomez-Suaga, P.; Ortega-Vidal, J.; et al. Biological effects of olive oil phenolic compounds on mitochondria. Mol. Cell Oncol. 2022, 9, 2044263. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Luo, Y.; Yin, J.; Huang, M.; Luo, F. Targeting AMPK signaling by polyphenols: A novel strategy for tackling aging. Food Funct. 2023, 14, 56–73. [Google Scholar] [CrossRef]
- de Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Leri, M.; Bertolini, A.; Stefani, M.; Bucciantini, M. EVOO polyphenols relieve synergistically autophagy dysregulation in a cellular model of Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 7225. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.Q.; Yao, S.X.; Liu, H.L.; Li, M.; Duan, N.; Ma, J.B. Hydroxytyrosol inhibits the inflammatory response of osteoarthritis chondrocytes via SIRT6-mediated autophagy. Mol. Med. Rep. 2018, 17, 4035–4042. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Chen, Q.; Zhu, S.Y.; Wu, Q.; Liao, C.R.; Wang, Z.; Wu, X.H.; Wu, H.T.; Chen, J.T. Hydroxytyrosol promotes autophagy by regulating SIRT1 against advanced oxidation protein product induced NADPH oxidase and inflammatory response. Int. J. Mol. Med. 2019, 44, 1531–1540. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Sun, T.; Wang, J.; Jia, J.; Yi, Y.H.; Chen, Y.X.; Miao, Y.; Hu, Z.Q. Hydroxytyrosol prevents dermal papilla cells inflammation under oxidative stress by inducing autophagy. J. Biochem. Mol. Toxicol. 2019, 33, e22377. [Google Scholar] [CrossRef]
- Yang, X.; Jing, T.; Li, Y.; He, Y.; Zhang, W.; Wang, B.; Xiao, Y.; Wang, W.; Zhang, J.; Wei, J.; et al. Hydroxytyrosol attenuates LPS-induced acute lung injury in mice by regulating autophagy and sirtuin sxpression. Curr. Mol. Med. 2017, 17, 149–159. [Google Scholar] [CrossRef]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet supplementation with hydroxytyrosol ameliorates brain pathology and restores cognitive functions in a mouse model of amyloid-β deposition. J. Alzheimer Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Mosca, A.; Crudele, A.; Smeriglio, A.; Braghini, M.R.; Panera, N.; Comparcola, D.; Alterio, A.; Sartorelli, M.R.; Tozzi, G.; Raponi, M.; et al. Antioxidant activity of Hydroxytyrosol and Vitamin E reduces systemic inflammation in children with paediatric NAFDL. Dig. Liver Dis. 2021, 53, 1154–1158. [Google Scholar] [CrossRef]




| HTyr Treatment and Dose | Model | Effects | Ref. |
|---|---|---|---|
| Pre-treatment 25, 50, 100 μM | Rat Arthritis: ex vivo primary chondrocytes + TNF-α | ↓ IL-1β, IL-6 through ↑ autophagy via SIRT6 | [174] |
| Pre-treatment 75 μM | Rat Arthritis: ex vivo primary condrocytes + AOPPs | ↓ IL-6, TNF-α through ↑ autophagy via SIRT1 | [175] |
| Pre-treatment 25, 50, 100 μM | Rat Vasculitis: ex vivo primary vascular adventitial fibroblasts + TNF-α | ↓ IL-1 β, IL-6, MCP-1 through ↑ autophagy via SIRT1-mediated ↓ AKT/mTOR | [176] |
| Pre-treatment 1 μM | Chemical Carcinogenesis in Human Primary Colonic Epithelial Cells: HCoEpC + B[a]P | ↓ IL-6, Il-8, VEGF, CXCL13 ↑ autophagy via ↓ 4EBP1 phosphorylation (↓ mTOR pathway) | [131] |
| Pre-treatment 75 μM | Rat Alopecia: primary dermal papilla cells + H2O2 | ↓ IL-6, TNF-α via ↑ autophagy ↑ FGF, PDGF | [177] |
| HTyr Treatment and Dose | Model | Effects | Ref. |
|---|---|---|---|
| 100 mg/kg intragastrically | Murine Acute Lung Injury intranasal LPS-induced: lung tissue bronchoalveolar lavage fluid | ↓ TNF-α, IL-1β, IL-6, MCP-1, IL-10 ↑ autophagy via SIRT-1 ↓ MAPK phosphorylation ↓ neutrophil and lymphocyte infiltration | [178] |
| 50 mg/kg of diet for 8 weeks | Murine Alzheimer’s disease in TgCRND8 mice: brain tissues | ↓ TNF-α ↓ amyloid protein load ↑ autophagy ↑ cognitive function: memory | [179] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velotti, F.; Bernini, R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients 2023, 15, 1774. https://doi.org/10.3390/nu15071774
Velotti F, Bernini R. Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients. 2023; 15(7):1774. https://doi.org/10.3390/nu15071774
Chicago/Turabian StyleVelotti, Francesca, and Roberta Bernini. 2023. "Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy" Nutrients 15, no. 7: 1774. https://doi.org/10.3390/nu15071774
APA StyleVelotti, F., & Bernini, R. (2023). Hydroxytyrosol Interference with Inflammaging via Modulation of Inflammation and Autophagy. Nutrients, 15(7), 1774. https://doi.org/10.3390/nu15071774