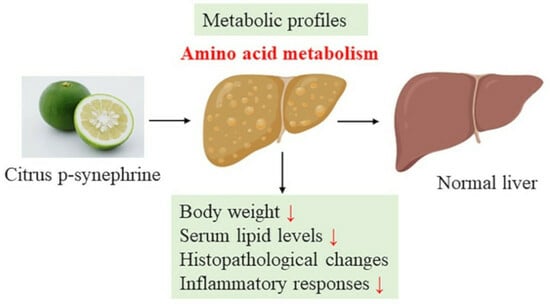
Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Experiments
2.2. Biochemical Analysis of Serum Lipid Level
2.3. Histology Examination of Liver and White Adipose Tissue
2.4. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Analysis of Inflammatory Cytokines
2.5. Non-Targeted Metabolome Analysis of Liver Tissue and Serum
2.6. Statistical Analysis
3. Results
3.1. Citrus p-Synephrine Reduces Body Weight and Fat Content in HFD-Induced Mice
3.2. Citrus p-Synephrine Reduces Serum Lipid Levels in HFD-Induced Mice
3.3. Citrus p-Synephrine Inhibits Energy Abnormalities-Related Symptoms in HFD Mice
3.4. Citrus p-Synephrine Inhibits HFD-Induced Inflammatory Responses
3.5. Citrus p-Synephrine Alters Liver Metabolic Profiles in HFD Mice
3.6. Citrus p-Synephrine Alters Serum Metabolic Profiles in HFD Mice
3.7. Inhibition of Lipogenesis Is Associated with the Regulation of Metabolites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Jauregibeitia, I.; Portune, K.; Rica, I.; Tueros, I.; Velasco, O.; Grau, G.; Trebolazabala, N.; Castaño, L.; Larocca, A.V.; Ferreri, C.; et al. Fatty Acid Profile of Mature Red Blood Cell Membranes and Dietary Intake as a New Approach to Characterize Children with Overweight and Obesity. Nutrients 2020, 12, 3446. [Google Scholar] [CrossRef] [PubMed]
- Howes, M.-J.R.; Perry, N.S.L.; Vásquez-Londoño, C.; Perry, E.K. Role of Phytochemicals as Nutraceuticals for Cognitive Functions Affected in Ageing. Br. J. Pharmacol. 2020, 177, 1294–1315. [Google Scholar] [CrossRef]
- Kumar, V.; Kaur, R.; Aggarwal, P.; Singh, G. Underutilized Citrus Species: An Insight of Their Nutraceutical Potential and Importance for the Development of Functional Food. Sci. Hortic. 2022, 296, 110909. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C.; Shi, H.; Liao, Y.; Xu, F.; Du, H.; Xiao, H.; Zheng, J. Nutrients and Bioactives in Citrus Fruits: Different Citrus Varieties, Fruit Parts, and Growth Stages. Crit. Rev. Food Sci. Nutr. 2023, 63, 2018–2041. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, C.; Del Coso, J.; Giráldez-Costas, V.; González-García, J.; Gutiérrez-Hellín, J. Effects of P-Synephrine during Exercise: A Brief Narrative Review. Nutrients 2021, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Karn, A.; Zhao, C.; Yang, F.; Cui, J.; Gao, Z.; Wang, M.; Wang, F.; Xiao, H.; Zheng, J. In-Vivo Biotransformation of Citrus Functional Components and Their Effects on Health. Crit. Rev. Food Sci. Nutr. 2021, 61, 756–776. [Google Scholar] [CrossRef]
- da Silva-Pereira, J.F.; Bubna, G.A.; de Almeida Gonçalves, G.; Bracht, F.; Peralta, R.M.; Bracht, A. Fast Hepatic Biotransformation of P-Synephrine and p-Octopamine and Implications for Their Oral Intake. Food Funct. 2016, 7, 1483–1491. [Google Scholar] [CrossRef]
- Xiong, J.; Liu, T.; Mi, L.; Kuang, H.; Xiong, X.; Chen, Z.; Li, S.; Lin, J.D. hnRNPU/TrkB Defines a Chromatin Accessibility Checkpoint for Liver Injury and NASH Pathogenesis. Hepatology 2020, 71, 1228–1246. [Google Scholar] [CrossRef]
- Arbo, M.D.; Schmitt, G.C.; Limberger, M.F.; Charão, M.F.; Moro, Â.M.; Ribeiro, G.L.; Dallegrave, E.; Garcia, S.C.; Leal, M.B.; Limberger, R.P. Subchronic Toxicity of Citrus Aurantium L. (Rutaceae) Extract and p-Synephrine in Mice. Regul. Toxicol. Pharmacol. 2009, 54, 114–117. [Google Scholar] [CrossRef]
- Koncz, D.; Tóth, B.; Bahar, M.A.; Roza, O.; Csupor, D. The Safety and Efficacy of Citrus aurantium (Bitter Orange) Extracts and p-Synephrine: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 4019. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, Y.; Stanton, C.; Paul Ross, R.; Zhao, J.; Zhang, H.; Yang, B.; Chen, W. Alleviation Effects of Bifidobacterium breve on DSS-Induced Colitis Depends on Intestinal Tract Barrier Maintenance and Gut Microbiota Modulation. Eur. J. Nutr. 2021, 60, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhao, Y.; Liu, J.; Ge, W.; Xu, X.; Wang, S.; Zhang, J. Dietary Succinoglycan Riclin Improves Glycemia Control in Mice with Type 2 Diabetes. J. Agric. Food Chem. 2022, 70, 1819–1829. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Zhao, J.; AL-Ansi, W.; Wang, J.; Xue, L.; Liu, J.; Wang, Y.; Fan, M.; Qian, H.; Li, Y.; et al. Oat β-Glucan Alleviates DSS-Induced Colitis via Regulating Gut Microbiota Metabolism in Mice. Food Funct. 2021, 12, 8976–8993. [Google Scholar] [CrossRef]
- Xu, L.; Guo, L.; Wang, Z.; Xu, X.; Zhang, S.; Wu, X.; Kuang, H.; Xu, C. Profiling and Identification of Biocatalyzed Transformation of Sulfoxaflor In Vivo. Angew. Chem. Int. Ed. 2020, 71, 16218–16224. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Wang, B.; Tan, X.; Huang, L.; Xiong, S. Regulatory Effect of Lactulose on Intestinal Flora and Serum Metabolites in Colitis Mice: In Vitro and in Vivo Evaluation. Food Chem. X 2023, 19, 100821. [Google Scholar] [CrossRef]
- Gutiérrez-Hellín, J.; Ruiz-Moreno, C.; Del Coso, J. Acute P-Synephrine Ingestion Increases Whole-Body Fat Oxidation during 1-h of Cycling at Fatmax. Eur. J. Nutr. 2020, 59, 3341–3345. [Google Scholar] [CrossRef]
- Takagi, M.; Kimura, K.; Nakashima, K.-I.; Hirai, T.; Inoue, M. Induction of Beige Adipocytes by Naturally Occurring Β3-Adrenoceptor Agonist p-Synephrine. Eur. J. Pharmacol. 2018, 836, 67–74. [Google Scholar] [CrossRef]
- Aljazzaf, B.; Regeai, S.; Elghmasi, S.; Alghazir, N.; Balgasim, A.; Hdud Ismail, I.M.; Eskandrani, A.A.; Shamlan, G.; Alansari, W.S.; AL-Farga, A.; et al. Evaluation of Antidiabetic Effect of Combined Leaf and Seed Extracts of Moringa oleifera (Moringaceae) on Alloxan-Induced Diabetes in Mice: A Biochemical and Histological Study. Oxid. Med. Cell. Longev. 2023, 2023, 9136217. [Google Scholar] [CrossRef]
- Yoo, J.S.; Han, K.-T.; Chung, S.H.; Park, E.-C. Association between Awareness of Nutrition Labeling and High-Density Lipoprotein Cholesterol Concentration in Cancer Survivors and the General Population: The Korean National Health and Nutrition Examination Survey (KNHANES) 2010-2016. BMC Cancer 2019, 19, 16. [Google Scholar] [CrossRef]
- Grepper, D.; Tabasso, C.; Aguettaz, A.K.F.; Martinotti, A.; Ebrahimi, A.; Lagarrigue, S.; Amati, F. Methodological Advancements in Organ-Specific Ectopic Lipid Quantitative Characterization: Effects of High Fat Diet on Muscle and Liver Intracellular Lipids. Mol. Metab. 2023, 68, 101669. [Google Scholar] [CrossRef] [PubMed]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The Gut Microbiota Regulates White Adipose Tissue Inflammation and Obesity via a Family of microRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef]
- Zhang, H.; Hu, H.; Greeley, N.; Jin, J.; Matthews, A.J.; Ohashi, E.; Caetano, M.S.; Li, H.S.; Wu, X.; Mandal, P.K.; et al. STAT3 Restrains RANK- and TLR4-Mediated Signaling by Suppressing Expression of the E2 Ubiquitin Ligase Ubc13. Nat. Commun. 2014, 5, 5798. [Google Scholar] [CrossRef] [PubMed]
- Peng, B.; Li, H.; Peng, X.-X. Functional Metabolomics: From Biomarker Discovery to Metabolome Reprogramming. Protein Cell 2015, 6, 628–637. [Google Scholar] [CrossRef]
- Xie, D.; Zhang, Y.; Guo, Y.; Xue, X.; Zhao, S.; Geng, C.; Li, Y.; Yang, R.; Gan, Y.; Li, H.; et al. The Impact of High-Glucose or High-Fat Diets on the Metabolomic Profiling of Mice. Front. Nutr. 2023, 10, 1171806. [Google Scholar] [CrossRef] [PubMed]
- Yida, Z.; Imam, M.U.; Ismail, M.; Ismail, N.; Ideris, A.; Abdullah, M.A. High Fat Diet-Induced Inflammation and Oxidative Stress Are Attenuated by N-Acetylneuraminic Acid in Rats. J. Biomed. Sci. 2015, 22, 96. [Google Scholar] [CrossRef]
- Yida, Z.; Imam, M.U.; Ismail, M.; Wong, W.; Abdullah, M.A.; Ideris, A.; Ismail, N. N-Acetylneuraminic Acid Attenuates Hypercoagulation on High Fat Diet-Induced Hyperlipidemic Rats. Food Nutr. Res. 2015, 59, 29046. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xiang, Q.; Tan, D.; Shi, L.; Liu, X.; Wu, Y.; Yu, R. Zuogui-Jiangtang-Qinggan-Fang Alleviates High-Fat Diet-Induced Type 2 Diabetes Mellitus with Non-Alcoholic Fatty Liver Disease by Modulating Gut Microbiome-Metabolites-Short Chain Fatty Acid Composition. Biomed. Pharmacother. 2023, 157, 114002. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, M.; Liu, X.; Zheng, M.; Xu, X.; Liu, X.; Gong, J.; Liu, H.; Liu, J. Metagenomic and Untargeted Metabolomic Analysis of the Effect of Sporisorium reilianum Polysaccharide on Improving Obesity. Foods 2023, 12, 1578. [Google Scholar] [CrossRef]
- Suzuki, R.; Sato, Y.; Fukaya, M.; Suzuki, D.; Yoshizawa, F.; Sato, Y. Energy Metabolism Profile of the Effects of Amino Acid Treatment on Hepatocytes: Phenylalanine and Phenylpyruvate Inhibit Glycolysis of Hepatocytes. Nutrition 2021, 82, 111042. [Google Scholar] [CrossRef]
- Suzuki, R.; Sato, Y.; Obeng, K.A.; Suzuki, D.; Komiya, Y.; Adachi, S.I.; Yoshizawa, F.; Sato, Y. Energy Metabolism Profile of the Effects of Amino Acid Treatment on Skeletal Muscle Cells: Leucine Inhibits Glycolysis of Myotubes. Nutrition 2020, 77, 110794. [Google Scholar] [CrossRef] [PubMed]
- Shimomura, Y.; Kitaura, Y. Physiological and Pathological Roles of Branched-Chain Amino Acids in the Regulation of Protein and Energy Metabolism and Neurological Functions. Pharmacol. Res. 2018, 133, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Kujawska-Łuczak, M.; Suliburska, J.; Markuszewski, L.; Pupek-Musialik, D.; Jabłecka, A.; Bogdański, P. The Effect of L-Arginine and Ascorbic Acid on the Visceral Fat and the Concentrations of Metalloproteinases 2 and 9 in High-Fat-Diet Rats. Endokrynol. Pol. 2015, 66, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Yasoshima, Y.; Matsui, S.; Yokota-Hashimoto, H.; Kobayashi, M.; Kitamura, T. Intraperitoneal Injection of D-Serine Inhibits High-Fat Diet Intake and Preference in Male Mice. Appetite 2017, 118, 120–128. [Google Scholar] [CrossRef]








| Gene | Sense Sequence (5′→3′) | Antisense Sequence (5′→3′) |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG | CCAGTTGGTAACAATGCCATGT |
| IL-6 | CCACTTCACAAGTCGGAGGCTTA | GCAAGTGCATCATCGTTGTTCATAC |
| IL-10 | GCTCTTACTGACTGGCATGAG | CGCAGCTCTAGGAGCATGTG |
| TNF-α | ACACCGAGATTTCCTTCAAACTG | CCATCTAGGGTTATGATGCTCTTCA |
| IL-1β | GGGCCTCAAAGGAAAGAATC | TACCAGTTGGGGAACTCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Tan, X.; Tang, S.; Liu, X.; Shao, L.; Wang, C.; Huang, L. Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice. Nutrients 2024, 16, 248. https://doi.org/10.3390/nu16020248
Bai J, Tan X, Tang S, Liu X, Shao L, Wang C, Huang L. Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice. Nutrients. 2024; 16(2):248. https://doi.org/10.3390/nu16020248
Chicago/Turabian StyleBai, Junying, Xiang Tan, Sheng Tang, Xin Liu, Linzi Shao, Chen Wang, and Linhua Huang. 2024. "Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice" Nutrients 16, no. 2: 248. https://doi.org/10.3390/nu16020248
APA StyleBai, J., Tan, X., Tang, S., Liu, X., Shao, L., Wang, C., & Huang, L. (2024). Citrus p-Synephrine Improves Energy Homeostasis by Regulating Amino Acid Metabolism in HFD-Induced Mice. Nutrients, 16(2), 248. https://doi.org/10.3390/nu16020248