Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Data Collection
2.3.1. Dietary Acrylamide
2.3.2. Smoking Acrylamide
2.3.3. Measurement of Clinical Characteristics
2.3.4. Sociodemographic Data and Personal History
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koszucka, A.; Nowak, A.; Nowak, I.; Motyl, I. Acrylamide in Human Diet, Its Metabolism, Toxicity, Inactivation and the Associated European Union Legal Regulations in Food Industry. Crit. Rev. Food Sci. Nutr. 2019, 60, 1677–1692. [Google Scholar] [CrossRef] [PubMed]
- Semla, M.; Goc, Z.; Martiniaková, M.; Omelka, R.; Formicki, G. Acrylamide: A Common Food Toxin Related to Physiological Functions and Health. Physiol. Res 2017, 66, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Kenwood, B.M.; Zhu, W.; Zhang, L.; Bhandari, D.; Blount, B.C. Cigarette Smoking Is Associated with Acrylamide Exposure among the U.S. Population: NHANES 2011–2016. Environ. Res. 2022, 209, 112774. [Google Scholar] [CrossRef] [PubMed]
- Mojska, H.; Gielecińska, I.; Cendrowski, A. Acrylamide Content in Cigarette Mainstream Smoke and Estimation of Exposure to Acrylamide from Tobacco Smoke in Poland. Ann. Agric. Environ. Med. 2016, 23, 456–461. [Google Scholar] [CrossRef]
- Tareke, E.; Rydberg, P.; Karlsson, P.; Eriksson, S.; Törnqvist, M. Analysis of Acrylamide, a Carcinogen Formed in Heated Foodstuffs. J. Agric. Food Chem. 2002, 50, 4998–5006. [Google Scholar] [CrossRef]
- Tareke, E.; Lyn-Cook, B.; Robinson, B.; Ali, S.F. Acrylamide: A Dietary Carcinogen Formed in Vivo? J. Agric. Food Chem. 2008, 56, 6020–6023. [Google Scholar] [CrossRef]
- Lindeman, B.; Johansson, Y.; Andreassen, M.; Husøy, T.; Dirven, H.; Hofer, T.; Knutsen, H.K.; Caspersen, I.H.; Vejrup, K.; Paulsen, R.E.; et al. Does the Food Processing Contaminant Acrylamide Cause Developmental Neurotoxicity? A Review and Identification of Knowledge Gaps. Reprod. Toxicol. 2021, 101, 93–114. [Google Scholar] [CrossRef]
- Wang, F.; Fan, B.; Chen, C.; Zhang, W. Acrylamide Causes Neurotoxicity by Inhibiting Glycolysis and Causing the Accumulation of Carbonyl Compounds in BV2 Microglial Cells. Food Chem. Toxicol. 2022, 163, 112982. [Google Scholar] [CrossRef]
- Duan, X.; Wang, Q.C.; Chen, K.L.; Zhu, C.C.; Liu, J.; Sun, S.C. Acrylamide Toxic Effects on Mouse Oocyte Quality and Fertility in Vivo. Sci. Rep. 2015, 5, 11562. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific Opinion on Acrylamide in Food. EFSA J. 2015, 13, 4104. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer; World Health Organization. Some Industrial Chemicals; International Agency for Research on Cancer: Lyon, France, 1994; ISBN 978-92-832-1260-7. [Google Scholar]
- Poteser, M.; Laguzzi, F.; Schettgen, T.; Vogel, N.; Weber, T.; Zimmermann, P.; Hahn, D.; Kolossa-Gehring, M.; Namorado, S.; van Nieuwenhuyse, A.; et al. Time Trends of Acrylamide Exposure in Europe: Combined Analysis of Published Reports and Current HBM4EU Studies. Toxics 2022, 10, 481. [Google Scholar] [CrossRef]
- Larsson, S.C.; Åkesson, A.; Bergkvist, L.; Wolk, A. Dietary Acrylamide Intake and Risk of Colorectal Cancer in a Prospective Cohort of Men. Eur. J. Cancer 2009, 45, 513–516. [Google Scholar] [CrossRef]
- Liu, R.; Sobue, T.; Kitamura, T.; Kitamura, Y.; Ishihara, J.; Kotemori, A.; Zha, L.; Ikeda, S.; Sawada, N.; Iwasaki, M.; et al. Dietary Acrylamide Intake and Risk of Esophageal, Gastric, and Colorectal Cancer: The Japan Public Health Center-Based Prospective Study. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1461–1468. [Google Scholar] [CrossRef] [PubMed]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary Acrylamide Intake and Risk of Breast Cancer: The Japan Public Health Center-Based Prospective Study. Cancer Sci. 2018, 109, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Bellicha, A.; Wendeu-Foyet, G.; Coumoul, X.; Koual, M.; Pierre, F.; Gueraud, F.; Zelek, L.; Debras, C.; Srour, B.; Sellem, L.; et al. Dietary Exposure to Acrylamide and Breast Cancer Risk: Results from the NutriNet-Santé Cohort. Am. J. Clin. Nutr. 2022, 116, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Kotemori, A.; Ishihara, J.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary Acrylamide Intake and the Risk of Endometrial or Ovarian Cancers in Japanese Women. Cancer Sci. 2018, 109, 3316–3325. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, J.G.F.; Schouten, L.J. Dietary Acrylamide and Human Cancer; Even after 20 Years of Research an Open Question. Am. J. Clin. Nutr. 2022, 116, 846–847. [Google Scholar] [CrossRef] [PubMed]
- Hogervorst, J.G.; Schouten, L.J.; Konings, E.J.; Goldbohm, A.; van den Brandt, P.A. Dietary Acrylamide Intake and the Risk of Renal Cell, Bladder, and Prostate Cancer. Am. J. Clin. Nutr. 2008, 87, 1428–1438. [Google Scholar] [CrossRef]
- Ikeda, S.; Sobue, T.; Kitamura, T.; Ishihara, J.; Kotemori, A.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Tsugane, S. Dietary Acrylamide Intake and the Risks of Renal Cell, Prostate, and Bladder Cancers: A Japan Public Health Center-Based Prospective Study. Nutrients 2021, 13, 780. [Google Scholar] [CrossRef]
- Hirvonen, T.; Kontto, J.; Jestoi, M.; Valsta, L.; Peltonen, K.; Pietinen, P.; Virtanen, S.M.; Sinkko, H.; Kronberg-Kippilä, C.; Albanes, D.; et al. Dietary Acrylamide Intake and the Risk of Cancer among Finnish Male Smokers. Cancer Causes Control 2010, 21, 2223–2229. [Google Scholar] [CrossRef]
- Pelucchi, C.; Galeone, C.; Levi, F.; Negri, E.; Franceschi, S.; Talamini, R.; Bosetti, C.; Giacosa, A.; la Vecchia, C. Dietary Acrylamide and Human Cancer. Int. J. Cancer 2006, 118, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Bälter, K.; Adami, H.O.; Grönberg, H.; Vikström, A.C.; Paulsson, B.; Törnqvist, M.; Mucci, L.A. Acrylamide Exposure Measured by Food Frequency Questionnaire and Hemoglobin Adduct Levels and Prostate Cancer Risk in the Cancer of the Prostate in Sweden Study. Int. J. Cancer 2009, 124, 2384–2390. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.M.; Giovannucci, E.; Stampfer, M.J.; Mucci, L.A. Dietary Acrylamide and Risk of Prostate Cancer. Int. J. Cancer 2012, 131, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Åkesson, A.; Wolk, A. Dietary Acrylamide Intake and Prostate Cancer Risk in a Prospective Cohort of Swedish Men. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1939–1941. [Google Scholar] [CrossRef]
- Filippini, T.; Halldorsson, T.I.; Capitão, C.; Martins, R.; Giannakou, K.; Hogervorst, J.; Vinceti, M.; Åkesson, A.; Leander, K.; Katsonouri, A.; et al. Dietary Acrylamide Exposure and Risk of Site-Specific Cancer: A Systematic Review and Dose-Response Meta-Analysis of Epidemiological Studies. Front. Nutr. 2022, 9, 875607. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Bosetti, C.; Galeone, C.; La Vecchia, C. Dietary Acrylamide and Cancer Risk: An Updated Meta-Analysis. Int. J. Cancer 2015, 136, 2912–2922. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Alva, A.; Hussain, M. The Changing Natural History of Metastatic Prostate Cancer. Cancer J. 2013, 19, 19–24. [Google Scholar] [CrossRef]
- Lozano-Lorca, M.; Olmedo-Requena, R.; Vega-Galindo, M.V.; Vázquez-Alonso, F.; Jiménez-Pacheco, A.; Salcedo-Bellido, I.; Sánchez, M.J.; Jiménez-Moleón, J.J. Night Shift Work, Chronotype, Sleep Duration, and Prostate Cancer Risk: Caplife Study. Int. J. Environ. Res. Public Health 2020, 17, 6300. [Google Scholar] [CrossRef]
- Olmedo-Requena, R.; Lozano-Lorca, M.; Salcedo-Bellido, I.; Jiménez-Pacheco, A.; Vázquez-Alonso, F.; García-Caballos, M.; Sánchez, M.J.; Jiménez-Moleón, J.J.J. Compliance with the 2018 World Cancer Research Fund/American Institute for Cancer Research Cancer Prevention Recommendations and Prostate Cancer. Nutrients 2020, 12, 768. [Google Scholar] [CrossRef]
- Lozano-Lorca, M.; Olmedo-Requena, R.; Barrios-Rodríguez, R.; Jiménez-Pacheco, A.; Vázquez-Alonso, F.; Castillo-Bueno, H.M.; Rodríguez-Barranco, M.; Jiménez-Moleón, J.J. Ejaculation Frequency and Prostate Cancer: CAPLIFE Study. World J. Men’s Health 2023, 41, 724–733. [Google Scholar] [CrossRef]
- ICD-10 Version:2016. Available online: https://icd.who.int/browse10/2016/en (accessed on 20 January 2020).
- Fernández-Ballart, J.D.; Piñol, J.L.; Zazpe, I.; Corella, D.; Carrasco, P.; Toledo, E.; Perez-Bauer, M.; Martínez-González, M.Á.; Salas-Salvadó, J.; Martn-Moreno, J.M. Relative Validity of a Semi-Quantitative Food-Frequency Questionnaire in an Elderly Mediterranean Population of Spain. Br. J. Nutr. 2010, 103, 1808–1816. [Google Scholar] [CrossRef]
- Martin-Moreno, J.M.; Boyle, P.; Gorgojo, L.; Maisonneuve, P.; Fernandez-Rodriguez, J.C.; Salvini, S.; Willett, W.C. Development and Validation of a Food Frequency Questionnaire in Spain. Int. J. Epidemiol. 1993, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Mesias, M.; Nouali, A.; Delgado-Andrade, C.; Morales, F.J. How Far Is the Spanish Snack Sector from Meeting the Acrylamide Regulation 2017/2158? Foods 2020, 9, 247. [Google Scholar] [CrossRef]
- González-Mulero, L.; Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Acrylamide Exposure from Common Culinary Preparations in Spain, in Household, Catering and Industrial Settings. Foods 2021, 10, 2008. [Google Scholar] [CrossRef]
- Mesías, M.; Morales, F.J.; Delgado-Andrade, C. Acrylamide in Biscuits Commercialised in Spain: A View of the Spanish Market from 2007 to 2019. Food Funct. 2019, 10, 6624–6632. [Google Scholar] [CrossRef]
- Mesías, M.; Sáez-Escudero, L.; Morales, F.J.; Delgado-Andrade, C. Reassessment of Acrylamide Content in Breakfast Cereals. Evolution of the Spanish Market from 2006 to 2018. Food Control 2019, 105, 94–101. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Morales, F.J.; Seiquer, I.; Pilar Navarro, M. Maillard Reaction Products Profile and Intake from Spanish Typical Dishes. Food Res. Int. 2010, 43, 1304–1311. [Google Scholar] [CrossRef]
- Bermudo, E.; Moyano, E.; Puignou, L.; Galceran, M.T. Liquid Chromatography Coupled to Tandem Mass Spectrometry for the Analysis of Acrylamide in Typical Spanish Products. Talanta 2008, 76, 389–394. [Google Scholar] [CrossRef]
- Morales, F.J.; Mesias, M. Analysis of Acrylamide in Coffee. In Coffee in Health and Disease Prevention; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 1013–1021. ISBN 9780124167162. [Google Scholar]
- Mesias, M.; Delgado-Andrade, C.; Morales, F.J. Process Contaminants in Battered and Breaded Foods Prepared at Public Food Service Establishments. Food Control 2020, 114, 107217. [Google Scholar] [CrossRef]
- Willett, W. Nutritional Epidemiology; Oxford University Press: Oxford, UK, 2013; ISBN 9780199979448. [Google Scholar]
- Esposito, F.; Squillante, J.; Nolasco, A.; Montuori, P.; Macrì, P.G.; Cirillo, T. Acrylamide Levels in Smoke from Conventional Cigarettes and Heated Tobacco Products and Exposure Assessment in Habitual Smokers. Environ. Res. 2022, 208, 112659. [Google Scholar] [CrossRef]
- Epstein, J.I.; Egevad, L.; Amin, M.B.; Delahunt, B.; Srigley, J.R.; Humphrey, P.A.; Grading Committee. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am. J. Surg. Pathol. 2015, 40, 244–252. [Google Scholar] [CrossRef]
- Neuzillet, Y.; Raynaud, J.-P.; Dreyfus, J.-F.J.-F.; Radulescu, C.C.; Rouanne, M.; Schneider, M.; Krish, S.; Rouprêt, M.; Drouin, S.J.; Comperat, E.; et al. Aggressiveness of Localized Prostate Cancer: The Key Value of Testosterone Deficiency Evaluated by Both Total and Bioavailable Testosterone: AndroCan Study Results. Horm. Cancer 2019, 10, 36–44. [Google Scholar] [CrossRef]
- Mottet, N.; van den Bergh, R.C.N.; Vice-chair, P.C.; de Santis, M.; Gillessen, S.; Govorov, A.; Grummet, J.; Henry, A.M.; Lam, T.B.; Mason, M.D.; et al. EAU—ESTRO—ESUR—SIOG Guidelines on Prostate Cancer-2020 Update. Part 1: Screening, Diagnosis, and Local Treatment with Curative Intent. Eur. Urol. 2021, 79, 243–262. [Google Scholar] [CrossRef]
- Román Viñas, B.; Ribas Barba, L.; Ngo, J.; Serra Majem, L. Validación En Población Catalana Del Cuestionario Internacional de Actividad Física. Gac. Sanit. 2013, 27, 254–257. [Google Scholar] [CrossRef]
- Benisi-Kohansal, S.; Salari-Moghaddam, A.; Seyed Rohani, Z.; Esmaillzadeh, A. Dietary Acrylamide Intake and Risk of Women’s Cancers: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Br. J. Nutr. 2021, 126, 1355–1363. [Google Scholar] [CrossRef]
- Adani, G.; Filippini, T.; Wise, L.A.; Halldorsson, T.I.; Blaha, L.; Vinceti, M. Dietary Intake of Acrylamide and Risk of Breast, Endometrial, and Ovarian Cancers: A Systematic Review and Dose⇓response Meta-Analysis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1095–1106. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. DNA Adduction and Mutagenic Properties of Acrylamide. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 580, 31–40. [Google Scholar] [CrossRef]
- Besaratinia, A.; Pfeifer, G.P. Genotoxicity of Acrylamide and Glycidamide. J. Natl. Cancer Inst. 2004, 96, 1023–1029. [Google Scholar] [CrossRef]
- Chu, P.L.; Liu, H.S.; Wang, C.; Lin, C.Y. Association between Acrylamide Exposure and Sex Hormones in Males: NHANES, 2003–2004. PLoS ONE 2020, 15, e0234622. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative Stress, Inflammation, and Cancer: How Are They Linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Baum, M.; Fauth, E.; Fritzen, S.; Herrmann, A.; Mertes, P.; Rudolphi, M.; Spormann, T.; Zankl, H.; Eisenbrand, G.; Bertow, D. Acrylamide and Glycidamide: Approach towards Risk Assessment Based on Biomarker Guided Dosimetry of Genotoxic/Mutagenic Effects in Human Blood. Adv. Exp. Med. Biol. 2005, 561, 77–88. [Google Scholar] [CrossRef]
- Heudorf, U.; Hartmann, E.; Angerer, J. Acrylamide in Children—Exposure Assessment via Urinary Acrylamide Metabolites as Biomarkers. Int. J. Hyg. Environ. Health 2009, 212, 135–141. [Google Scholar] [CrossRef]
- Cade, J.; Thompson, R.; Burley, V.; Warm, D. Development, Validation and Utilisation of Food-Frequency Questionnaires—A Review. Public Health Nutr. 2002, 5, 567–587. [Google Scholar] [CrossRef]
- Schwedler, G.; Murawski, A.; Schmied-Tobies, M.I.H.; Rucic, E.; Scherer, M.; Pluym, N.; Scherer, G.; Bethke, R.; Kolossa-Gehring, M. Benzene Metabolite SPMA and Acrylamide Metabolites AAMA and GAMA in Urine of Children and Adolescents in Germany—Human Biomonitoring Results of the German Environmental Survey 2014–2017 (GerES V). Environ. Res. 2021, 192, 110295. [Google Scholar] [CrossRef]
- Kito, K.; Ishihara, J.; Kotemori, A.; Zha, L.; Liu, R.; Sawada, N.; Iwasaki, M.; Sobue, T.; Tsugane, S. Dietary Acrylamide Intake and the Risk of Pancreatic Cancer: The Japan Public Health Center-Based Prospective Study. Nutrients 2020, 12, 3584. [Google Scholar] [CrossRef]
- Jensen, O.M.; Wahrendorf, J.; Rosenqvist, A.; Geser, A. The Reliability of Questionnaire-Derived Historical Dietary Information and Temporal Stability of Food Habits in Individuals. Am. J. Epidemiol. 1984, 120, 281–290. [Google Scholar] [CrossRef]




| Controls n = 393 | PCa Cases n = 428 | p-Value | |
|---|---|---|---|
| Dietary acrylamide intake (µg/day), median (IQR) | 19.2 (13.5–29.5) | 18.9 (13.3–31.1) | 0.717 |
| Acrylamide from cigarette smoking a (µg/day), median (IQR) | 8.5 (0.0–14.2) | 8.5 (0.0–11.6) | 0.638 |
| Age (years), median (IQR) | 66.5 (61.3–72.3) | 68.5 (62.8–73.9) | 0.005 |
| Education level, n (%) | 0.377 | ||
| Primary | 109 (27.8) | 124 (28.9) | |
| Secondary | 199 (50.6) | 228 (53.3) | |
| University | 85 (21.6) | 76 (17.8) | |
| Marital status, n (%) | 0.760 | ||
| Married | 330 (83.9) | 356 (83.2) | |
| Not married | 63 (16.1) | 72 (16.8) | |
| Smoking status, n (%) | 0.794 | ||
| Never smoker | 103 (26.2) | 112 (26.2) | |
| Former smoker | 219 (55.7) | 231 (53.9) | |
| Current smoker | 71 (18.1) | 85 (19.9) | |
| Years of smoking, median (IQR) | 35.6 (22.0–45.0) | 37.0 (27.0–45.0) | 0.478 |
| Number of cigarettes per day, median (IQR) a | 20.0 (10.0–30.0) | 20.0 (10.0–30.0) | 0.564 |
| Body mass index, n (%) | 0.758 | ||
| Normal weight | 72 (18.4) | 87 (20.4) | |
| Overweight | 207 (52.6) | 219 (51.3) | |
| Obesity | 114 (29.0) | 121 (28.3) | |
| Missing | – | 1 | |
| Physical activity, n (%) | 0.158 | ||
| Low | 133 (33.8) | 166 (38.7) | |
| Moderate | 201 (51.2) | 214 (50.0) | |
| High | 59 (15.0) | 48 (11.3) | |
| Sedentary behavior (h/day), median (IQR) | 7.0 (5.0–9.0) | 7.0 (5.0–10.0) | 0.268 |
| Energy intake (Kcal/day), median (IQR) | 2290.3 (1943.1–2809.4) | 2428.9 (2057.5–2918.9) | 0.021 |
| Alcohol consumption (g/day), median (IQR) | 7.3 (1.4–15.9) | 8.0 (1.5–18.5) | 0.349 |
| Diabetes mellitus, n (%) | 0.364 | ||
| No | 299 (76.3) | 337 (78.9) | |
| Yes | 93 (23.7) | 90 (21.1) | |
| Missing | 1 | 1 | |
| First-degree family history of PCa, n (%) | <0.001 | ||
| No | 350 (89.1) | 333 (77.9) | |
| Yes | 43 (10.9) | 94 (22.1) | |
| Missing | – | 1 | |
| ISUP grade b, n (%) | – | ||
| 1–2 | – | 321 (75.2) | |
| 3–5 | – | 106 (24.8) | |
| Staging of PCa, n (%) | |||
| Localized | – | 369 (86.2) | |
| Locally advanced–metastatic | – | 59 (13.8) |
| Tertiles of Dietary Acrylamide Intake a | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | p-Trend | ||
| Overall | Controls/PCa cases | 131/138 | 131/137 | 131/153 | |
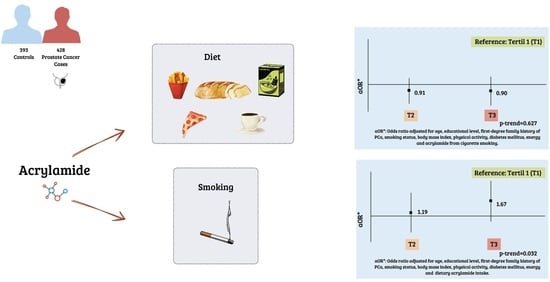
| aOR (95% CI) b | Reference | 0.89 (0.62, 1.29) | 0.89 (0.59, 1.36) | 0.599 | |
| aOR (95% CI) c | Reference | 0.91 (0.63, 1.32) | 0.90 (0.59, 1.37) | 0.627 | |
| ISUP grade | |||||
| 1–2 | Controls/PCa cases | 131/103 | 131/103 | 131/115 | |
| aOR (95% CI) b | Reference | 0.92 (0.62, 1.36) | 0.98 (0.62, 1.55) | 0.939 | |
| aOR (95% CI) c | Reference | 0.93 (0.63, 1.39) | 0.99 (0.63, 1.57) | 0.972 | |
| 3–5 | Controls/PCa cases | 131/34 | 131/34 | 131/38 | |
| aOR (95% CI) b | Reference | 0.78 (0.43, 1.43) | 0.63 (0.31, 1.27) | 0.198 | |
| aOR (95% CI) c | Reference | 0.80 (0.44, 1.47) | 0.63 (0.31, 1.27) | 0.195 | |
| Stage of tumor | |||||
| Localized | Controls/PCa cases | 131/121 | 131/110 | 131/138 | |
| aOR (95% CI) b | Reference | 0.81 (0.55, 1.19) | 0.94 (0.61, 1.44) | 0.762 | |
| aOR (95% CI) c | Reference | 0.82 (0.56, 1.21) | 0.94 (0.61, 1.45) | 0.771 | |
| Locally advanced–metastatic | Controls/PCa cases | 131/17 | 131/27 | 131/15 | |
| aOR (95% CI) b | Reference | 1.49 (0.72, 3.09) | 0.58 (0.22, 1.53) | 0.329 | |
| aOR (95% CI) c | Reference | 1.59 (0.76, 3.32) | 0.62 (0.23, 1.66) | 0.400 | |
| Tertiles of Acrylamide from Cigarette Smoking a | |||||
|---|---|---|---|---|---|
| T1 | T2 | T3 | p-Trend | ||
| Overall | Controls/PCa cases | 133/140 | 150/150 | 105/135 | |
| aOR (95% CI) b | Reference | 1.19 (0.66, 2.13) | 1.67 (0.92, 3.04) | 0.031 | |
| aOR (95% CI) c | Reference | 1.19 (0.66, 2.13) | 1.67 (0.92, 3.04) | 0.032 | |
| ISUP grade | |||||
| 1–2 | Controls/PCa cases | 133/99 | 150/114 | 105/105 | |
| aOR (95% CI) b | Reference | 1.09 (0.58, 2.02) | 1.61 (0.85, 3.04) | 0.039 | |
| aOR (95% CI) c | Reference | 1.08 (0.58, 2.02) | 1.61 (0.85, 3.03) | 0.041 | |
| 3–5 | Controls/PCa cases | 133/41 | 150/35 | 105/30 | |
| aOR (95% CI) b | Reference | 1.47 (0.53, 4.05) | 1.92 (0.69, 5.37) | 0.177 | |
| aOR (95% CI) c | Reference | 1.48 (0.54, 4.07) | 1.93 (0.69, 5.39) | 0.177 | |
| Stage of PCa | |||||
| Localized | Controls/PCa cases | 133/114 | 150/133 | 105/120 | |
| aOR (95% CI) b | Reference | 1.22 (0.66, 2.23) | 1.75 (0.94, 3.26) | 0.024 | |
| aOR (95% CI) c | Reference | 1.22 (0.66, 2.23) | 1.75 (0.94, 3.26) | 0.025 | |
| Locally advanced–metastatic | Controls/PCa cases | 133/26 | 150/17 | 105/15 | |
| aOR (95% CI) b | Reference | 0.98 (0.29, 3.32) | 1.30 (0.38, 4.43) | 0.528 | |
| aOR (95% CI) c | Reference | 0.98 (0.29, 3.32) | 1.30 (0.38, 4.45) | 0.523 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Lorca, M.; Muñoz-Bravo, C.; Barrios-Rodríguez, R.; Castillo-Hermoso, M.Á.; Kouiti, M.; González-Palacios Torres, C.; Jiménez-Moleón, J.-J.; Olmedo-Requena, R. Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study. Nutrients 2024, 16, 836. https://doi.org/10.3390/nu16060836
Lozano-Lorca M, Muñoz-Bravo C, Barrios-Rodríguez R, Castillo-Hermoso MÁ, Kouiti M, González-Palacios Torres C, Jiménez-Moleón J-J, Olmedo-Requena R. Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study. Nutrients. 2024; 16(6):836. https://doi.org/10.3390/nu16060836
Chicago/Turabian StyleLozano-Lorca, Macarena, Carlos Muñoz-Bravo, Rocío Barrios-Rodríguez, María Ángeles Castillo-Hermoso, Malak Kouiti, Carla González-Palacios Torres, José-Juan Jiménez-Moleón, and Rocío Olmedo-Requena. 2024. "Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study" Nutrients 16, no. 6: 836. https://doi.org/10.3390/nu16060836
APA StyleLozano-Lorca, M., Muñoz-Bravo, C., Barrios-Rodríguez, R., Castillo-Hermoso, M. Á., Kouiti, M., González-Palacios Torres, C., Jiménez-Moleón, J. -J., & Olmedo-Requena, R. (2024). Dietary and Smoking Acrylamide and Prostate Cancer Risk: CAPLIFE Study. Nutrients, 16(6), 836. https://doi.org/10.3390/nu16060836