Dietary Strategies for the Treatment of Cadmium and Lead Toxicity
Abstract
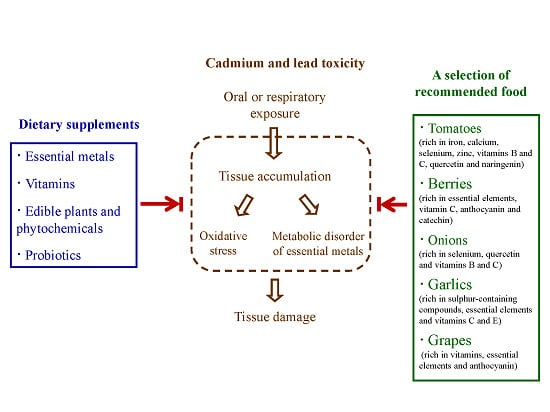
:1. Introduction
2. Essential Metals
| Essential Metal | Administered Form | Duration | AnimalModel | TargetSites | ProtectiveEffects | Ref. |
|---|---|---|---|---|---|---|
| Zinc | 40 mg/L ZnCl2 in drinking water | 30 days | Male rats exposed to 40 mg/L CdCl2 in drinking water | Testes | Zinc restored the activity of GPx and SOD in the testes and attenuated DNA oxidation in the gonads. | [40] |
| 0.02% Zn2+ in drinking water | PND 1 to PND 21, stop at weaning | Pregnant mice exposed to 0.2% Pb-acetate in drinking water | Brain | Zinc restored the activity of SOD, XO and CAT, and decreased the LP levels in the pups’ brains. | [41] | |
| Selenium | 20 μmol/kg b.w. (PhSe)2 by oral treatment | 4 weeks | Male rats exposed to 10 μmol/kg b.w. CdCl2 (s.c.) | Brain and lungs | (PhSe)2 restored the activity of SOD and CAT, increased the vitamin C content and decreased the level of LP in the brain. It also decreased the Cd level in the lungs. | [42] |
| 0.2 mg/L Na2SeO3 in drinking water | 21 days | Lactating rats exposed to 100 mg/L Pb-acetate in drinking water | Brain and nervous system | Na2SeO3 improved the spatial memory and the level of LTP and decreased neuron apoptosis in the pups. | [43] | |
| Iron | 120 mg/kg b.w. Fe in diet | 4 or 8 weeks | Male rats exposed to 100 μg/kg b.w. CdCl2 by oral gavage | Kidney, liver and intestinal tract | An iron-sufficient diet decreased the Cd burden in the tissue and regulated intestinal Cd absorption through the iron transporters. | [45] |
| Calcium | 0.02% Ca2+ in drinking water | GD 6 to PND 21 | Pregnant mice exposed to 0.2% Pb-acetate in drinking water | Brain and nervous system | Calcium decreased the synaptosomal AChE and mitochondrial MAO activity and improved the pups’ total locomotor activity and exploratory behaviour. | [48] |
| Magnesium | 20 mg/kg b.w. Mg orally | 1 or 2 weeks | Male mice exposed to 10 mg/kg b.w. Cd | Testes and kidneys | Mg pre-treatment was efficient in restoring the renal and testis GSH levels. | [49] |
3. Vitamins
4. Edible Plants and Dietary Phytochemicals
| Edible Plant | Administered Form | Duration | Animal Model | Target Sites | Protective Effects | Ref. |
|---|---|---|---|---|---|---|
| Soybean | Diet containing soybean as a protein source | 60 days | Male rats exposed to 100 mg/L CdCl2 in drinking water | Heart and aorta | A soybean-based diet ameliorated cardiac and aorta oxidative stress and recovered morphological alterations in the aorta. | [71,72] |
| Garlic (Allium sativum) | 250 or 500 mg/kg b.w. garlic extract orally | 30 days | Male mice exposed to 50 mg/kg b.w. Pb-nitrate orally | Blood, kidneys and brain | Garlic decreased the Pb burden and recovered immunological parameters in the blood and tissues. | [73] |
| Ginger (Zingiber officinale) | 150 mg/kg b.w. ginger extract by oral gavage | 1 or 3 weeks | Male rats exposed to 300 mg/kg b.w. Pb-nitrate by oral gavage | Kidneys | Ginger recovered the GSH level and the activity of antioxidant enzymes and alleviated renal histological changes. | [77] |
| Onion (Allium cepa) | 5 mL/kg b.w. onion extract by oral gavage | 4 weeks | Male rats exposed to 15 mg/kg b.w. Cd | Testis | Onion reduced testicular oxidative damage and alleviated spermiotoxicity. | [78] |
| Green tea | 1.5% w/v green tea extract in drinking water | 8 weeks | Male rats exposed to 0.4% Pb-acetate in drinking water | Liver | Green tea recovered hepatic function and alleviated histological changes in the liver. | [89] |
| Curry leaf (Murraya koenigii) | 100 mg/kg b.w. curry leaf extract orally | 15 days | Male rats exposed to 0.44 mg/kg b.w. CdCl2 s.c. | Heart | Curry leaf increased the activity of cardiac antioxidant enzymes and decreased the cardiac LP and Cd levels. | [82] |
| Grape | 1.18 or 2.36 g/kg b.w. grape juice concentrate orally | 56 days | Male rats exposed to 1.2 mg/kg b.w. CdCl2 i.p. | Testis | Grape improved serum testosterone levels, the relative weight of the epididymis and the percentage of normal sperm. | [83] |
| Tomato | 1.5 mL tomato paste orally | 8 weeks | Male rats exposed to 1% Pb-acetate in drinking water | Kidney | Tomato intake recovered renal function and prevented the alterations of antioxidant enzymes activities in blood plasma. | [85] |
| Phytochemical | Toxic Metal | Protective Mechanisms | Ref. | Food Sources |
|---|---|---|---|---|
| Quercetin | Cd | Quercetin induces eNOS, iNOS, COX-2 and MT expression. | [90,91] | Onion, tomato, capers and radish |
| Pb | Quercetin modulates the MAPKs and NF-κB signalling pathway and forms excretable complex with Pb. | [92,93,94] | ||
| Catechin | Cd | Catechin inhibits Cd absorption and normalises bone metabolic disorders through the bone mineral density, bone mineral content and bone calcium content. | [95] | Tea, cocoa, peach and berries. |
| Pb | Catechin protects hepatic cell membrane fluidity, increases cell viability and modulates oxidative stress. | [96] | ||
| Anthocyanin | Cd | Anthocyanin protects against Cd-induced oxidative stress. | [97] | Cherry, grape and berries. |
| Pb | Anthocyanin appears to effectively diminish oxidative stress. | [98,99] | ||
| Curcumin | Cd | Curcumin protects against Cd-induced lipid peroxidation. | [100,101] | Turmeric |
| Pb | Curcumin binds Pb to form an excretable complex, reducing neurotoxicity. | [102] | ||
| Naringenin | Cd | Naringenin quenches free radicals, recovers antioxidant enzyme activity and chelates Cd. | [103] | Orange, grapefruit and tomato |
| γ-Oryzanol | Cd | γ-Oryzanol reduces the testicular Cd concentration, improves ALAD activity and prevents lipid peroxidation. | [104] | Rice |
| Puerarin | Pb | Puerarin modulates the PI3K/Akt/eNOS pathway, reduces reactive oxygen species and protects against DNA damage and apoptosis. | [105,106] | Pueraria |
5. Probiotics as Functional Food Supplements
6. Other Dietary Supplements
7. Conclusions and Perspectives

Acknowledgments
Author Contributions
Abbreviations
| AChE | acetylcholinesterase |
| Akt | protein kinase B |
| ALAD | δ-aminolevulinic acid dehydratase |
| BLL | blood lead level |
| b.w. | body weight |
| CAT | catalase |
| Cd | cadmium |
| COX-2 | cyclooxygenase-2 |
| DMSA | meso-2,3-dimercaptosuccinic acid |
| DMT1 | divalent metal transporter-1 |
| eNOS | endothelial nitric oxide synthase |
| GD | gestational day |
| GPx | glutathione peroxidase |
| GSH | glutathione |
| iNOS | inducible nitric oxide synthase |
| i.p. | intraperitoneally |
| LP | lipid peroxidation |
| LTP | hippocampal long-term potentiation |
| MAO | monoamine oxidase; |
| MT | metallothionein; |
| MTP1 | metal transporter protein 1 |
| MAPKs | mitogen-activated protein kinases |
| NF-κB | nuclear factor kappa B |
| Pb | lead |
| PI3K | phosphoinositide-3-kinase |
| PND | postnatal day |
| s.c. | subcutaneously |
| SOD | superoxide dismutase |
| XO | xanthine oxidase |
Conflicts of Interest
References
- Nordberg, G.F.; Nogawa, K.; Nordberg, M.; Friberg, L.T. Foreword: Metals—A new old environmental problem and Chapter 23: Cadmium. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Academic Press: Burlington, MA, USA, 2011; pp. vii, 446–451, 463–470, 600–609. [Google Scholar]
- Goyer, R.A.; Clarkson, T.W. Toxic effects of metals. In Casarett and Doull’s Toxicology: The Basic Science of Poisons, 6th ed.; Klaassen, C., Ed.; McGraw-Hill Health Professions Division: New York, NY, USA, 2001; pp. 822–826. [Google Scholar]
- Hassanien, M.A.; Elshahawy, A.M. Environmental heavy metals and mental disorders of children in developing countries. In Environmental Heavy Metal Pollution and Effects on Child Mental Development: Risk Assessment and Prevention Strategies, 1st ed.; Simeonov, L.I., Kochubovski, M.V., Simeonova, B.G., Eds.; Springer: Dordrecht, The Netherlands, 2010; p. 13. [Google Scholar]
- Zhang, S.M.; Dai, Y.H.; Xie, X.H.; Fan, Z.Y.; Zhang, Y.F. Surveillance of childhood blood lead levels in 14 cities of China in 2004–2006. Biomed. Environ. Sci. 2009, 22, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, G.; El-Shahawi, H.; Mokhtar, A. Blood lead levels in Egyptian children from high and low lead-polluted areas: Impact on cognitive function. Acta Neurol. Scand. 2009, 120, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hegde, S.; Sridhar, M.; Bolar, D.R.; Arehalli, S.; Sanghavi, M.B. Relating tooth-and blood-lead levels in children residing near a zinc-lead smelter in India. Int. J. Paediatr. Dent. 2010, 20, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.L.; Du, Y.; Zhai, M.-M.; Shang, Q. Cadmium exposure and its health effects: A 19-year follow-up study of a polluted area in China. Sci. Total Environ. 2014, 470, 224–228. [Google Scholar] [CrossRef] [PubMed]
- Minh, N.D.; Hough, R.L.; Thuy, L.T.; Nyberg, Y.; Mai, L.B.; Vinh, N.C.; Khai, N.M.; Öborn, I. Assessing dietary exposure to cadmium in a metal recycling community in Vietnam: Age and gender aspects. Sci. Total Environ. 2012, 416, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Lauwerys, R.R.; Buchet, J.P.; Roels, H.A.; Brouwers, J.; Stanescu, D. Epidemiological survey of workers exposed to cadmium. Arch. Environ. Health. 1974, 28, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Hong, F.; Jin, T.; Zhang, A. Risk assessment on renal dysfunction caused by co-exposure to arsenic and cadmium using benchmark dose calculation in a Chinese population. Biometals 2004, 17, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Koyu, A.; Gokcimen, A.; Ozguner, F.; Bayram, D.S.; Kocak, A. Evaluation of the effects of cadmium on rat liver. Mol. Cell. Biochem. 2006, 284, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Murata, I.; Hirono, T.; Saeki, Y.; Nakagawa, S. Cadmium enteropathy, renal osteomalacia (“Itai Itai” disease in Japan). Bull. Soc. Int. Chir. 1970, 29, 34–42. [Google Scholar] [PubMed]
- Rehm, S.; Waalkes, M.P. Cadmium-induced ovarian toxicity in hamsters, mice, and rats. Fundam. Appl. Toxicol. 1988, 10, 635–647. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Plaza, M.; Navas-Acien, A.; Crainiceanu, C.M.; Guallar, E. Cadmium exposure and hypertension in the 1999–2004 National Health and Nutrition Examination Survey (NHANES). Environ. Health Perspect. 2008, 116, 51–56. [Google Scholar] [CrossRef] [PubMed]
- IARC. Lyon IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 1993; pp. 148–161, 206–210. [Google Scholar]
- Lidsky, T.I.; Schneider, J.S. Lead neurotoxicity in children: Basic mechanisms and clinical correlates. Brain 2003, 126, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Bergdahl, I.A.; Sheveleva, M.; Schütz, A.; Artamonova, V.G.; Skerfving, S. Plasma and blood lead in humans: Capacity-limited binding to δ-aminolevulinic acid dehydratase and other lead-binding components. Toxicol. Sci. 1998, 46, 247–253. [Google Scholar] [PubMed]
- Sandhir, R.; Gill, K. Effect of lead on lipid peroxidation in liver of rats. Biol. Trace Elem. Res. 1995, 48, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B.A.; Kimmel, C.A.; Woods, J.S.; McConnell, E.E.; Grant, L.D. Chronic low-level lead toxicity in the rat: III. An integrated assessment of long-term toxicity with special reference to the kidney. Toxicol. Appl. Pharmacol. 1980, 56, 59–77. [Google Scholar] [CrossRef] [PubMed]
- Ronis, M.J.; Badger, T.M.; Shema, S.J.; Roberson, P.K.; Shaikh, F. Reproductive toxicity and growth effects in rats exposed to lead at different periods during development. Toxicol. Appl. Pharmacol. 1996, 136, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, E.E.; Edwards, B.B.; Jensen, R.L.; Mahaffey, K.R.; Fomon, S.J. Absorption and retention of lead by infants. Pediatr. Res. 1978, 12, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Siddiqui, M.K.J. Environmental lead toxicity and nutritional factors. Clin. Nutr. 2007, 26, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Vesey, D.A. Transport pathways for cadmium in the intestine and kidney proximal tubule: Focus on the interaction with essential metals. Toxicol. Lett. 2010, 198, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Farmand, F.; Ehdaie, A.; Roberts, C.K.; Sindhu, R.K. Lead-induced dysregulation of superoxide dismutases, catalase, glutathione peroxidase, and guanylate cyclase. Environ. Res. 2005, 98, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qu, W.; Kadiiska, M.B. Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol. Appl. Pharmacol. 2009, 238, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Olin, K.L.; Fraga, C.G.; Keen, C.L. Zinc deficiency causes oxidative damage to proteins, lipids and DNA in rat testes. J. Nutr. 1995, 125, 823–829. [Google Scholar] [PubMed]
- Brenneisen, P.; Steinbrenner, H.; Sies, H. Selenium, oxidative stress, and health aspects. Mol. Asp. Med. 2005, 26, 256–267. [Google Scholar] [CrossRef]
- McCarty, M.F. Zinc and multi-mineral supplementation should mitigate the pathogenic impact of cadmium exposure. Med. Hypotheses 2012, 79, 642–648. [Google Scholar] [CrossRef]
- Porru, S.; Alessio, L. The use of chelating agents in occupational lead poisoning. Occup. Med. 1996, 46, 41–48. [Google Scholar] [CrossRef]
- Aposhian, H.V.; Maiorino, R.M.; Gonzalez-Ramirez, D.; Zuniga-Charles, M.; Xu, Z.; Hurlbut, K.M.; Junco-Munoz, P.; Dart, R.C.; Aposhian, M.M. Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 1995, 97, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Liebelt, E.L.; Shannon, M.W. Oral chelators for childhood lead poisoning. Pediatr. Ann. 1994, 23, 616–626. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.; Travers, J. Succimer, an oral lead chelator. Clin. Pharm. 1991, 10, 914–922. [Google Scholar] [PubMed]
- Reeves, P.G.; Chaney, R.L. Marginal nutritional status of zinc, iron, and calcium increases cadmium retention in the duodenum and other organs of rats fed rice-based diets. Environ. Res. 2004, 96, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.E.; Piscator, M. Effect of cadmium on skeletal tissue in normal and calcium-deficient rats. Isr. J. Med. Sci. 1971, 7, 495–498. [Google Scholar] [PubMed]
- Hammad, T.A.; Sexton, M.; Langenberg, P. Relationship between blood lead and dietary iron intake in preschool children: A cross-sectional study. Ann. Epidemiol. 1996, 6, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Bridges, C.C.; Zalups, R.K. Molecular and ionic mimicry and the transport of toxic metals. Toxicol. Appl. Pharmacol. 2005, 204, 274–308. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, C.A.; Ohta, H.; Albores, A.; Koropatnick, J.; Cherian, M.G. Induction of metallothionein synthesis by zinc in cadmium pretreated rats. Toxicology 1990, 63, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, M.; Nordberg, G. Toxicological aspects of metallothionein. Cell. Mol. Biol. 2000, 46, 451–463. [Google Scholar] [PubMed]
- Flora, S.; Tandon, S. Beneficial effects of zinc supplementation during chelation treatment of lead intoxication in rats. Toxicology 1990, 64, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Abdelmelek, H.; Garrel, C.; Guiraud, P.; Douki, T.; Ravanat, J.L.; Favier, A.; Sakly, M.; Ben, R.K. Preventive effect of zinc against cadmium-induced oxidative stress in the rat testis. J. Reprod. Dev. 2008, 54, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Prasanthi, R.; Devi, C.B.; Basha, D.C.; Reddy, N.S.; Reddy, G.R. Calcium and zinc supplementation protects lead (Pb)-induced perturbations in antioxidant enzymes and lipid peroxidation in developing mouse brain. Int. J. Dev. Neurosci. 2010, 28, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.; Brandão, R.; de Oliveira, R.; Nogueira, C.W.; Santos, F.W. Efficacy of diphenyl diselenide against cerebral and pulmonary damage induced by cadmium in mice. Toxicol. Lett. 2007, 173, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.C.; Xu, Y.; Chen, Y.M.; Li, J.; Zhao, F.; Zheng, G.; Jing, J.F.; Ke, T.; Chen, J.Y.; Luo, W.J. The effect of sodium selenite on lead induced cognitive dysfunction. Neurotoxicology 2013, 36, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Whanger, P. Selenium in the treatment of heavy metal poisoning and chemical carcinogenesis. J. Trace Elem. Electrolytes. Health. Dis. 1992, 6, 209–221. [Google Scholar] [PubMed]
- Ryu, D.Y.; Lee, S.J.; Park, D.W.; Choi, B.S.; Klaassen, C.D.; Park, J.D. Dietary iron regulates intestinal cadmium absorption through iron transporters in rats. Toxicol. Lett. 2004, 152, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Ueda, H.; Kihara, T.; Tanaka, K. Increased hepatic accumulation of ingested Cd is associated with upregulation of several intestinal transporters in mice fed diets deficient in essential metals. Toxicol. Sci. 2008, 106, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Labbe, R. Lead poisoning mechanisms. Clin. Chem. 1990, 36, 1870. [Google Scholar] [PubMed]
- Basha, D.C.; Rani, M.U.; Devi, C.B.; Kumar, M.R.; Reddy, G.R. Perinatal lead exposure alters postnatal cholinergic and aminergic system in rat brain: Reversal effect of calcium co-administration. Int. J. Dev. Neurosci. 2012, 30, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Djukić-Ćosić, D.; Ninković, M.; Maličević, Z.; Matović, V.; Soldatović, D. Effect of magnesium pretreatment on reduced glutathione levels in tissues of mice exposed to acute and subacute cadmium intoxication: A time course study. Magnes. Res. 2007, 20, 177–186. [Google Scholar] [PubMed]
- Åkesson, A.; Berglund, M.; Schütz, A.; Bjellerup, P.; Bremme, K.; Vahter, M. Cadmium exposure in pregnancy and lactation in relation to iron status. Am. J. Public Health 2002, 92, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Nishijo, M.; Tawara, K.; Honda, R.; Kuriwaki, J.; Nakagawa, H.; Tanebe, K.; Saito, S. Cadmium and nutritional intake in pregnant Japanese women. Toxicol. Lett. 2004, 148, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.B.; Laden, F.; Guller, U.; Shankar, A.; Kazani, S.; Garshick, E. Relation between blood lead levels and childhood anemia in India. Am. J. Epidemiol. 2005, 161, 968–973. [Google Scholar] [CrossRef] [PubMed]
- Schutte, R.; Nawrot, T.S.; Richart, T.; Thijs, L.; Vanderschueren, D.; Kuznetsova, T.; Van, H.E.; Roels, H.A.; Staessen, J.A. Bone resorption and environmental exposure to cadmium in women: A population study. Environ. Health. Perspect. 2008, 116, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, M. Nutritional influences on metal toxicity: Cadmium as a model toxic element. Environ. Health Perspect. 1979, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.A.; Hudes, E.S. Relationship of ascorbic acid to blood lead levels. JAMA 1999, 281, 2289–2293. [Google Scholar] [CrossRef] [PubMed]
- El-Sokkary, G.H.; Awadalla, E.A. The protective role of vitamin C against cerebral and pulmonary damage induced by cadmium chloride in male adult albino rat. Open Neuroendocrinol. J. 2011, 4, 1–8. [Google Scholar] [CrossRef]
- Shaban El-Neweshy, M.; Said El-Sayed, Y. Influence of vitamin C supplementation on lead-induced histopathological alterations in male rats. Exp. Toxicol. Pathol. 2011, 63, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Goyer, R.A.; Cherian, M.G. Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci. 1979, 24, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Dawson, E.B.; Evans, D.R.; Harris, W.A.; Teter, M.C.; McGanity, W.J. The effect of ascorbic acid supplementation on the blood lead levels of smokers. J. Am. Coll. Nutr. 1999, 18, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Stoddard, A.; Leonard, D.A.; Dinardi, S.R. The effects of vitamin C supplementation on blood and hair levels of cadmium, lead, and mercury. Ann. N.Y. Acad. Sci. 1987, 498, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Ognjanović, B.; Pavlović, S.; Maletić, S.; Zikić, R.; Stajn, A.S.; Radojicić, R.M.; Saicić, Z.S.; Petrović, V.M. Protective influence of vitamin E on antioxidant defense system in the blood of rats treated with cadmium. Physiol. Res. 2003, 52, 563–570. [Google Scholar] [PubMed]
- Nemmiche, S.; Chabane-Sari, D.; Guiraud, P. Role of α-tocopherol in cadmium-induced oxidative stress in Wistar rat’s blood, liver and brain. Chem. Biol. Interact. 2007, 170, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Acharya, U.R.; Mishra, M.; Patro, J.; Panda, M.K. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod. Toxicol. 2008, 25, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Gupta, E.S.; Dhakal, B.K.; Thakur, A.R.; Ahnn, J. Vitamin C and vitamin Е protect the rat testes from cadmium-induced reactive oxygen species. Mol. Cells 2004, 17, 132–139. [Google Scholar] [PubMed]
- Rendón-Ramírez, A.-L.; Maldonado-Vega, M.; Quintanar-Escorza, M.-A.; Hernández, G.; Arévalo-Rivas, B.-I.; Zentella-Dehesa, A.; Calderón-Salinas, J.-V. Effect of vitamin E and C supplementation on oxidative damage and total antioxidant capacity in lead-exposed workers. Environ. Toxicol. Pharmacol. 2014, 37, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Bratton, G.R.; Żmudzki, J.; Bell, M.C.; Warnock, L.G. Thiamin (vitamin B1) effects on lead intoxication and deposition of lead in tissues: Therapeutic potential. Toxicol. Appl. Pharmacol. 1981, 59, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Flora, S.; Sharma, R. Influence of dietary supplementation with thiamine on lead intoxication in rats. Biol. Trace Elem. Res. 1986, 10, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.Y.; Pullakhandam, R.; Kumar, B.D. Thiamine reduces tissue lead levels in rats: Mechanism of interaction. Biometals 2010, 23, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Sasser, L.B.; Hall, G.G.; Bratton, G.R.; Zmudzki, J. Absorption and tissue distribution of lead in thiamin-replete and thiamin-deficient rats. J. Nutr. 1984, 114, 1816–1825. [Google Scholar] [PubMed]
- Tandon, S.K.; Flora, S.; Singh, S. Influence of pyridoxine (vitamin B6) on lead intoxication in rats. Ind. Health 1987, 25, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Pérez Díaz, M.F.; Acosta, M.; Mohamed, F.H.; Ferramola, M.L.; Oliveros, L.B.; Gimenez, M.S. Protective effect of soybeans as protein source in the diet against cadmium-aorta redox and morphological alteration. Toxicol. Appl. Pharmacol. 2013, 272, 806–815. [Google Scholar] [CrossRef] [PubMed]
- Ferramola, M.L.; Pérez Díaz, M.F.; Honoré, S.M.; Sánchez, S.S.; Antón, R.I.; Anzulovich, A.C.; Giménez, M.S. Cadmium-induced oxidative stress and histological damage in the myocardium: Effects of a soy-based diet. Toxicol. Appl. Pharmacol. 2012, 265, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, A.; Kansal, L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem. Toxicol. 2010, 48, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Bideskan, A.E.; Alipour, F.; Fazel, A.; Haghir, H. The Effect of ascorbic acid and garlic administration on lead-induced neural damage in rat offspring’s hippocampus. Iran. J. Basic Med. Sci. 2013, 16, 157–164. [Google Scholar] [PubMed]
- Murugavel, P.; Pari, L.; Sitasawad, S.L.; Kumar, S.; Kumar, S. Cadmium induced mitochondrial injury and apoptosis in vero cells: Protective effect of diallyl tetrasufide from garlic. Int. J. Biochem. Cell Biol. 2007, 39, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Lawal, A.O.; Ellis, E.M. The chemopreventive effects of aged garlic extract against cadmium-induced toxicity. Environ. Toxicol. Pharmacol. 2011, 32, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.A.; Chalamaiah, M.; Ramesh, B.; Balaji, G.; Indira, P. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J. Food Sci. Technol. 2011, 1, 1–7. [Google Scholar]
- Ola-Mudathir, K.F.; Suru, S.M.; Fafunso, M.A.; Obioha, U.E.; Faremi, T.Y. Protective roles of onion and garlic extracts on cadmium-induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008, 46, 3604–3611. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.G.; Elhalwagy, M.E.; Farid, H.E. Effect of ginger supplementation on developmental toxicity induced by fenitrothion insecticide and/or lead in albino rats. Pestic. Biochem. Physiol. 2010, 97, 267–274. [Google Scholar] [CrossRef]
- Arulselvan, P.; Subramanian, S.P. Beneficial effects of Murraya koenigii leaves on antioxidant defense system and ultra structural changes of pancreatic β-cells in experimental diabetes in rats. Chem.-Biol. Interact. 2007, 165, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Sathaye, S.; Bagul, Y.; Gupta, S.; Kaur, H.; Redkar, R. Hepatoprotective effects of aqueous leaf extract and crude isolates of Murraya koenigii against in vitro ethanol-induced hepatotoxicity model. Exp. Toxicol. Pathol. 2011, 63, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Mitra, E.; Ghosh, A.K.; Ghosh, D.; Mukherjee, D.; Chattopadhyay, A.; Dutta, S.; Pattari, S.K.; Bandyopadhyay, D. Protective effect of aqueous Curry leaf (Murraya koenigii) extract against cadmium-induced oxidative stress in rat heart. Food Chem. Toxicol. 2012, 50, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Pires, V.C.; Gollücke, A.; Ribeiro, D.A.; Lungato, L.; D’Almeida, V.; Aguiar, O. Grape juice concentrate protects reproductive parameters of male rats against cadmium-induced damage: A chronic assay. Br. J. Nutr. 2013, 110, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Maguer, M.L. Lycopene in tomatoes: Chemical and physical properties affected by food processing. Crit. Rev. Food Sci. Nutr. 2000, 40, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Salawu, E.O.; Adeleke, A.A.; Oyewo, O.O.; Ashamu, E.A.; Ishola, O.O.; Afolabi, A.O.; Adesanya, T.A. Prevention of renal toxicity from lead exposure by oral administration of Lycopersicon esculentum. J. Toxicol. Environ. Health Sci. 2009, 1, 22–27. [Google Scholar]
- Tito, A.; Carola, A.; Bimonte, M.; Barbulova, A.; Arciello, S.; de Laurentiis, F.; Monoli, I.; Hill, J.; Gibertoni, S.; Colucci, G. A tomato stem cell extract, containing antioxidant compounds and metal chelating factors, protects skin cells from heavy metal-induced damages. Int. J. Cosmet. Sci. 2011, 33, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Steffens, J.; Hunt, D.; Williams, B. Accumulation of non-protein metal-binding polypeptides (gamma-glutamyl-cysteinyl) n-glycine in selected cadmium-resistant tomato cells. J. Biol. Chem. 1986, 261, 13879–13882. [Google Scholar] [PubMed]
- Nwokocha, C.R.; Nwokocha, M.I.; Aneto, I.; Obi, J.; Udekweleze, D.C.; Olatunde, B.; Owu, D.U.; Iwuala, M.O. Comparative analysis on the effect of Lycopersicon esculentum (tomato) in reducing cadmium, mercury and lead accumulation in liver. Food Chem. Toxicol. 2012, 50, 2070–2073. [Google Scholar] [CrossRef] [PubMed]
- Mehana, E.; Meki, A.R.; Fazili, K.M. Ameliorated effects of green tea extract on lead induced liver toxicity in rats. Exp. Toxicol. Pathol. 2012, 64, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Vicente-Sánchez, C.; Egido, J.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M.; Morales, A.I. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem. Toxicol. 2008, 46, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.I.; Vicente-Sánchez, C.; Jerkic, M.; Santiago, J.M.; Sánchez-González, P.D.; Pérez-Barriocanal, F.; López-Novoa, J.M. Effect of quercetin on metallothionein, nitric oxide synthases and cyclooxygenase-2 expression on experimental chronic cadmium nephrotoxicity in rats. Toxicol. Appl. Pharmacol. 2006, 210, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.; Ming, Q.; Sun, J.; Cheng, C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic. Res. 2013, 47, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Sun, Y.Z.; Sun, J.M.; Ma, J.Q.; Cheng, C. Protective role of quercetin against lead-induced inflammatory response in rat kidney through the ROS-mediated MAPKs and NF-κB pathway. Biochim. Biophys. Acta 2012, 1820, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Malešev, D.; Kuntić, V. Investigation of metal-flavonoid chelates and the determination of flavonoids via metal-flavonoid complexing reactions. J. Serbian Chem. Soc. 2007, 72, 921–939. [Google Scholar] [CrossRef]
- Choi, J.H.; Rhee, I.K.; Park, K.Y.; Park, K.Y.; Kim, J.K.; Rhee, S.J. Action of green tea catechin on bone metabolic disorder in chronic cadmium-poisoned rats. Life Sci. 2003, 73, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, X.; Jiao, H.; Zhao, B. Tea catechins protect against lead-induced cytotoxicity, lipid peroxidation, and membrane fluidity in HepG2 cells. Toxicol. Sci. 2002, 69, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Kopff, A.; Fijalkowski, P.; Kopff, M.; Niedworok, J.; Blaszczyk, J.; Kêdziora, J.; Tyslerowicz, P. Effect of anthocyanins on selected biochemical parameters in rats exposed to cadmium. Acta Biochim. Pol. 2003, 50, 543–548. [Google Scholar] [PubMed]
- El-Nekeety, A.A.; El-Kady, A.A.; Soliman, M.S.; Hassan, N.S.; Abdel-Wahhab, M.A. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem. Toxicol. 2009, 47, 2209–2215. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, E.; Jankowski, A.; Niedworok, J.; Smigielski, J.; Jankowska, B. The effect of anthocyanins from Aronii melanocarpa and acetylcysteine on selected after-effects of lead acetate poisoning. Pol. Merkur. Lekarski. 2002, 12, 221–223. [Google Scholar] [PubMed]
- Eybl, V.; Kotyzová, D.; Bludovská, M. The effect of curcumin on cadmium-induced oxidative damage and trace elements level in the liver of rats and mice. Toxicol. Lett. 2004, 151, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Eybl, V.; Kotyzova, D.; Koutensky, J. Comparative study of natural antioxidants-curcumin, resveratrol and melatonin-in cadmium-induced oxidative damage in mice. Toxicology 2006, 225, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Daniel, S.; Limson, J.L.; Dairam, A.; Watkins, G.M.; Daya, S. Through metal binding, curcumin protects against lead-and cadmium-induced lipid peroxidation in rat brain homogenates and against lead-induced tissue damage in rat brain. J. Inorg. Biochem. 2004, 98, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Renugadevi, J.; Prabu, S.M. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology 2009, 256, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Spiazzi, C.C.; Manfredini, V.; Barcellos da Silva, F.E.; Flores, É.M.; Izaguirry, A.P.; Vargas, L.M.; Soares, M.B.; Santos, F.W. γ-Oryzanol protects against acute cadmium-induced oxidative damage in mice testes. Food Chem. Toxicol. 2013, 55, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, Y.Z. Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2012, 258, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Ma, J.Q.; Sun, Y.Z. Puerarin protects the rat liver against oxidative stress-mediated DNA damage and apoptosis induced by lead. Exp. Toxicol. Pathol. 2012, 64, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Lee, J.R.; Jo, M.J.; Park, S.M.; Ku, S.K.; Kim, S.C. Protective effects of Korean red ginseng extract on cadmium-induced hepatic toxicity in rats. J. Ginseng. Res. 2013, 37, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.C.; Byun, S.H.; Yang, C.H.; Kim, C.Y.; Kim, J.W.; Kim, S.G. Cytoprotective effects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and cytochrome c-mediated PARP cleavage). Toxicology 2004, 197, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Jackie, T.; Chakravarthi, S.; Rao, M.; Pasupathi, T. Protective effects of Etlingera elatior extract on lead acetate-induced changes in oxidative biomarkers in bone marrow of rats. Food Chem. Toxicol. 2010, 48, 2688–2694. [Google Scholar] [CrossRef] [PubMed]
- Dewanjee, S.; Sahu, R.; Karmakar, S.; Gangopadhyay, M. Toxic effects of lead exposure in Wistar rats: Involvement of oxidative stress and the beneficial role of edible jute (Corchorus olitorius) leaves. Food Chem. Toxicol. 2013, 55, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Foligné, B.; Daniel, C.; Pot, B. Probiotics from research to market: The possibilities, risks and challenges. Curr. Opin. Microbiol. 2013, 16, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, I.; Sybesma, W.; Phothirath, P.; Ananta, E.; Mercenier, A. Application of probiotics in food products-challenges and new approaches. Curr. Opin. Biotechnol. 2010, 21, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Rijkers, G.T.; Bengmark, S.; Enck, P.; Haller, D.; Herz, U.; Kalliomaki, M.; Kudo, S.; Lenoir-Wijnkoop, I.; Mercenier, A.; Myllyluoma, E. Guidance for substantiating the evidence for beneficial effects of probiotics: Current status and recommendations for future research. J. Nutr. 2010, 140, 671S–676S. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, T.; Collado, M.; El-Nezami, H.; Meriluoto, J.; Salminen, S. Combining strains of lactic acid bacteria may reduce their toxin and heavy metal removal efficiency from aqueous solution. Lett. Appl. Microbiol. 2008, 46, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Halttunen, T.; Salminen, S.; Tahvonen, R. Rapid removal of lead and cadmium from water by specific lactic acid bacteria. Int. J. Food Microbiol. 2007, 114, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Kullisaar, T.; Songisepp, E.; Mikelsaar, M.; Zilmer, K.; Vihalemm, T.; Zilmer, M. Antioxidative probiotic fermented goats’ milk decreases oxidative stress-mediated atherogenicity in human subjects. Brit. J. Nutr. 2003, 90, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Mohtadi-Nia, J.; Homayouni-Rad, A.; Niafar, M.; Asghari-Jafarabadi, M.; Mofid, V. Probiotic yogurt improves antioxidant status in type 2 diabetic patients. Nutrition 2012, 28, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Monachese, M.; Burton, J.P.; Reid, G. Bioremediation and tolerance of humans to heavy metals through microbial processes: A potential role for probiotics? Appl. Environ. Microbiol. 2012, 78, 6397–6404. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against acute cadmium toxicity in mice. Appl. Environ. Microbiol. 2013, 79, 1508–1515. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, G.; Zhao, J.; Liu, X.; Narbad, A.; Chen, Y.Q.; Zhang, H.; Tian, F.; Chen, W. Protective effects of Lactobacillus plantarum CCFM8610 against chronic cadmium toxicity in mice: Intestinal sequestration is not the only route of protection. Appl. Environ. Microbiol. 2014, 80, 4063–4071. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Zhai, Q.; Zhao, J.; Liu, X.; Wang, G.; Zhang, H.; Zhang, H.; Chen, W. Lactobacillus plantarum CCFM8661 alleviates lead toxicity in mice. Biol. Trace Elem. Res. 2012, 150, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Jama, A.M.; Mitić-Ćulafić, D.; Kolarević, S.; Đurašević, S.F.; Knežević-Vukčević, J. Protective effect of probiotic bacteria against cadmium-induced genotoxicity in rat hepatocytes in vivo and in vitro. Arch. Biol. Sci. 2012, 64, 1197–1206. [Google Scholar] [CrossRef]
- Bisanz, J.E.; Enos, M.K.; Mwanga, J.R.; Changalucha, J.; Burton, J.P.; Gloor, G.B.; Reid, G. Randomized open-label pilot study of the influence of probiotics and the gut microbiome on toxic metal levels in Tanzanian pregnant women and school children. mBio 2014, 5, e01580-14. [Google Scholar] [CrossRef] [PubMed]
- Upasani, C.; Balaraman, R. Effect of vitamin E, vitamin C and spirulina on the levels of membrane bound enzymes and lipids in some organs of rats exposed to lead. Indian J. Pharmacol. 2001, 33, 185–191. [Google Scholar]
- Amin, A.; Hamza, A.A.; Daoud, S.; Hamza, W. Spirulina protects against cadmium-induced hepatotoxicity in rats. Am. J. Pharmacol. Toxicol. 2006, 1, 21–25. [Google Scholar] [CrossRef]
- Shim, J.-Y.; Om, A.-S. Chlorella vulgaris has preventive effect on cadmium induced liver damage in rats. Mol. Cell. Toxicol. 2008, 4, 138–143. [Google Scholar]
- Shim, J.-Y.; Shin, H.-S.; Han, J.-G.; Park, H.-S.; Lim, B.-L.; Chung, K.-W.; Om, A.-S. Protective effects of Chlorella vulgaris on liver toxicity in cadmium-administered rats. J. Med. Food 2008, 11, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Castro, N.; Escalona-Cardoso, G.; Hernández-Navarro, D.; Pérez-Pastén, R.; Chamorro-Cevallos, G. Spirulina (Arthrospira) protects against cadmium-induced teratogenic damage in mice. J. Med. Food 2011, 14, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Argüelles-Velázquez, N.; Alvarez-González, I.; Madrigal-Bujaidar, E.; Chamorro-Cevallos, G. Amelioration of cadmium-produced teratogenicity and genotoxicity in mice given Arthrospira maxima (Spirulina) treatment. Evid.-Based. Complement. Altern. 2013. [Google Scholar] [CrossRef]
- Yun, H.; Kim, I.; Kwon, S.; Kang, J.; Om, A. Protective effects of Chlorella Vulgaris against Lead-induced oxidative stress in rat brains. J. Health Sci. 2011, 57, 245–254. [Google Scholar] [CrossRef]
- Goldhaber, S.B. Trace element risk assessment: Essentiality vs. toxicity. Regul. Toxicol. Pharmacol. 2003, 38, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Greene, H.L.; Hambidge, K.; Schanler, R.; Tsang, R.C. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: Report of the subcommittee on pediatric parenteral nutrient requirements from the committee on clinical practice issues of the American society for clinical nutrition. Am. J. Clin. Nutr. 1988, 48, 1324–1342. [Google Scholar] [PubMed]
- Galati, G.; O’Brien, P.J. Potential toxicity of flavonoids and other dietary phenolics: Significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Q.; Narbad, A.; Chen, W. Dietary Strategies for the Treatment of Cadmium and Lead Toxicity. Nutrients 2015, 7, 552-571. https://doi.org/10.3390/nu7010552
Zhai Q, Narbad A, Chen W. Dietary Strategies for the Treatment of Cadmium and Lead Toxicity. Nutrients. 2015; 7(1):552-571. https://doi.org/10.3390/nu7010552
Chicago/Turabian StyleZhai, Qixiao, Arjan Narbad, and Wei Chen. 2015. "Dietary Strategies for the Treatment of Cadmium and Lead Toxicity" Nutrients 7, no. 1: 552-571. https://doi.org/10.3390/nu7010552
APA StyleZhai, Q., Narbad, A., & Chen, W. (2015). Dietary Strategies for the Treatment of Cadmium and Lead Toxicity. Nutrients, 7(1), 552-571. https://doi.org/10.3390/nu7010552