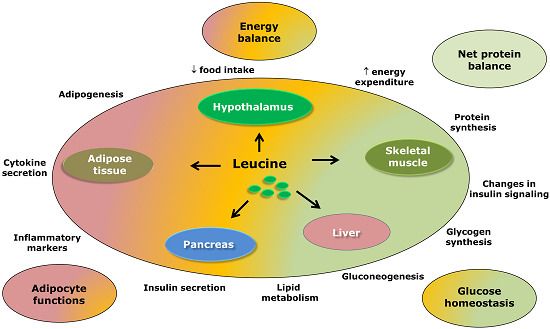
Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis
Abstract
:1. Introduction
2. Intracellular Mechanisms Activated by Leucine

3. Leucine-Responsive Tissues

4. Central Effects of Leucine

5. Does Leucine Regulate Food Intake?
| Reference | Route | Duration | Comments | Effects on Feeding |
|---|---|---|---|---|
| [40] | icv | Acute | - | Decreased |
| [17] | icv | Acute | - | Decreased |
| [43] | MBH | Acute/7 days | Food intake decreased in the first 2 days | Decreased |
| [44] | NTS | Acute | - | Decreased |
| [46] | icv | Acute | - | Decreased |
| [47] | icv | Acute | - | Decreased |
| Reference | Route | Duration | Comments | Effects on Feeding |
|---|---|---|---|---|
| [55] | Diet | 14 days | Normal and tumor-bearing pregnant rats | No changes |
| [56] | Diet | Acute | Overnight food-deprived adult and old rats | No changes |
| [57] | Diet | 20 days | Normal and tumor-bearing pregnant rats | No changes |
| [58] | Diet | 12 days | Young and tumor-bearing pregnant rats | No changes |
| [59] | Diet | 10 days | Adult and old rats | No changes |
| [60] | Diet | 14 days | Leucine increased nocturnal meal size | No changes |
| [61] | Diet | 9 weeks | Leucine + phenylalanine supplementation | No changes |
| [62] | Diet | 7 days | - | No changes |
| [17] | Diet | 3 weeks | Aversive behavior to leucine-rich diet in the 1°, but not in the 2° and 21° days. | Decreased |
| [63] | Diet | 12 weeks | Healthy elderly men. Energy intake and macronutrient composition were calculated from dietary intake records. | No changes |
| [64] | Diet | 8 weeks | Regular and high-fat diets | No changes |
| [65] | Diet | 21 days | Lactating rats | No changes |
| [66] | Diet | 5 weeks | - | No changes |
| [67] | Diet | 24 weeks | Elderly type 2 diabetic men; 3 days’ dietary intake records to evaluate energy and macronutrient intake. | No changes |
| [68] | Diet | 6 weeks | Previously obese rats | No changes |
| [21] | Diet | 6 weeks | Regular and high-fat diets | No changes |
| [50] | Diet | 7 days | HFD-fed mice; leucine produced similar effects as alanine supplementation. | Decreased |
| [51] | Diet | 20 weeks | Mice consuming an HFD | Decreased |
| [69] | Diet | 40 days | Old rats recovering from unilateral hind-limb casting | No changes |
| [70] | Diet | 9 months | Aging rats | No changes |
| [48] | Diet | 6 months | Increased food intake only in the first 2 weeks of supplementation | Increased/No changes |
| [71] | Diet | 8 weeks | Rats consuming an HFD | No changes |
| [46] | Diet | 4 days | Pronounced taste aversion | Decreased |
| [49] | Diet | 24 weeks | Leucine increased food intake only in some points along the experiment | Increased/No changes |
| [72] | Diet | 2 weeks | Nutritional recovery | No changes |
| [73] | Diet | 40 days | Adult rats recovering from unilateral hind-limb casting | No changes |
| [74] | Diet | 6 weeks | 30% calorie-restricted diet | No changes |
| [75] | Diet | 27 weeks | - | No changes |
| [47] | Diet | 12 days | - | No changes |
| [76] | Diet | 8 weeks | Non-obese, insulin-resistant rats | No changes |
| Reference | Route | Duration | Comments | Effects on Feeding |
|---|---|---|---|---|
| [27] | Water | 12 days | Leucine or norleucine supplementation | No changes |
| [54] | Water | 14 weeks | Increased in chow diet group. No change in HFD group. | Increased/No changes |
| [77] | Water | 14 weeks | Mice consuming an HFD | No changes |
| [52] | Water | 8 weeks | Food intake decreased in RCS10 mice, but no changes were observed in yellow agouti mice. | Decreased/No changes |
| [78] | Water | 8 weeks | Mice consuming an HFD | No changes |
| [79] | Water | 10 weeks | Offspring from HFD-fed mothers | No changes |
| [80] | Water | 8 weeks | Supplementation in normal and high-fat diets | No changes |
| [81] | Water | 17 weeks | Mice consuming normal and high-fat diets | No changes |
| [53] | Water | 9 weeks | Food intake decreased in males, but not females. No leucine effect in mice fed an HFD. | Decreased/No changes |
| [46] | Water | 18 days | - | No changes |
| [31] | Water | 6 weeks | Mice consuming normal and high-fat diets and ob/ob mice | No changes |
| [38] | Water | 6 weeks | Rats consuming normal and high-fat diets | No changes |
| [82] | Water | 21 weeks | Previously obese mice | No changes |
| [46] | Gavage | 3 days | - | No changes |
| [31] | Gavage | 2 days | - | No changes |
| [83] | Gavage | 10 days | Supplementation during skeletal muscle recovery | No changes |
| [46] | ip | 3 days | - | No changes |
| [46] | sc | 3 days | - | No changes |
6. The Effects of Leucine on Body Composition, Obesity, and Energy Expenditure
7. Regulation of Glucose Homeostasis by Leucine
8. Concluding Remarks

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garlick, P.J. The role of leucine in the regulation of protein metabolism. J. Nutr. 2005, 135, 1553S–1556S. [Google Scholar] [PubMed]
- Kimball, S.R.; Jefferson, L.S. Control of translation initiation through integration of signals generated by hormones, nutrients, and exercise. J. Biol. Chem. 2010, 285, 29027–29032. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.O.; Layman, D.K. Effects of leucine on in vitro protein synthesis and degradation in rat skeletal muscles. J. Nutr. 1984, 114, 1204–1212. [Google Scholar] [PubMed]
- Patti, M.E.; Brambilla, E.; Luzi, L.; Landaker, E.J.; Kahn, C.R. Bidirectional modulation of insulin action by amino acids. J. Clin. Investig. 1998, 101, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Kimball, S.R.; Vary, T.C.; Jefferson, L.S. Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eif4f formation. J. Nutr. 2000, 130, 139–145. [Google Scholar] [PubMed]
- Anthony, J.C.; Anthony, T.G.; Layman, D.K. Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J. Nutr. 1999, 129, 1102–1106. [Google Scholar] [PubMed]
- Kimball, S.R.; Shantz, L.M.; Horetsky, R.L.; Jefferson, L.S. Leucine regulates translation of specific mrnas in l6 myoblasts through mtor-mediated changes in availability of eif4e and phosphorylation of ribosomal protein s6. J. Biol. Chem. 1999, 274, 11647–11652. [Google Scholar] [CrossRef] [PubMed]
- Anthony, J.C.; Yoshizawa, F.; Anthony, T.G.; Vary, T.C.; Jefferson, L.S.; Kimball, S.R. Leucine stimulates translation initiation in skeletal muscle of postabsorptive rats via a rapamycin-sensitive pathway. J. Nutr. 2000, 130, 2413–2419. [Google Scholar] [PubMed]
- Xu, G.; Kwon, G.; Marshall, C.A.; Lin, T.A.; Lawrence, J.C., Jr.; McDaniel, M.L. Branched-chain amino acids are essential in the regulation of phas-i and p70 s6 kinase by pancreatic beta-cells. A possible role in protein translation and mitogenic signaling. J. Biol. Chem. 1998, 273, 28178–28184. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The ras-erk and pi3k-mtor pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Sancak, Y.; Peterson, T.R.; Shaul, Y.D.; Lindquist, R.A.; Thoreen, C.C.; Bar-Peled, L.; Sabatini, D.M. The rag gtpases bind raptor and mediate amino acid signaling to mtorc1. Science 2008, 320, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-trna synthetase is an intracellular leucine sensor for the mtorc1-signaling pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional transport of amino acids regulates mtor and autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, F.; Brule, S.; Hee Um, S.; Li, Y.; Masuda, K.; Roden, M.; Sun, X.J.; Krebs, M.; Polakiewicz, R.D.; Thomas, G.; et al. Identification of irs-1 ser-1101 as a target of s6k1 in nutrient- and obesity-induced insulin resistance. Proc. Natl. Acad. Sci. USA 2007, 104, 14056–14061. [Google Scholar] [CrossRef] [PubMed]
- Um, S.H.; Frigerio, F.; Watanabe, M.; Picard, F.; Joaquin, M.; Sticker, M.; Fumagalli, S.; Allegrini, P.R.; Kozma, S.C.; Auwerx, J.; et al. Absence of s6k1 protects against age- and diet-induced obesity while enhancing insulin sensitivity. Nature 2004, 431, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Ono, H.; Schwartz, G.J. Mediobasal hypothalamic p70 s6 kinase 1 modulates the control of energy homeostasis. Cell Metab. 2008, 8, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Ropelle, E.R.; Pauli, J.R.; Fernandes, M.F.; Rocco, S.A.; Marin, R.M.; Morari, J.; Souza, K.K.; Dias, M.M.; Gomes-Marcondes, M.C.; Gontijo, J.A.; et al. A central role for neuronal amp-activated protein kinase (ampk) and mammalian target of rapamycin (mtor) in high-protein diet-induced weight loss. Diabetes 2008, 57, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Bruckbauer, A.; Li, F.; Cao, Q.; Cui, X.; Wu, R.; Shi, H.; Zemel, M.B.; Xue, B. Leucine amplifies the effects of metformin on insulin sensitivity and glycemic control in diet-induced obese mice. Metabolism 2015, in press. [Google Scholar]
- Liang, C.; Curry, B.J.; Brown, P.L.; Zemel, M.B. Leucine modulates mitochondrial biogenesis and sirt1-ampk signaling in c2c12 myotubes. J. Nutr. Metab. 2014, 2014, 239750. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M.B. Synergistic effects of polyphenols and methylxanthines with leucine on ampk/sirtuin-mediated metabolism in muscle cells and adipocytes. PLoS ONE 2014, 9, e89166. [Google Scholar] [CrossRef] [PubMed]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. (Lond.) 2012, 9, 77. [Google Scholar] [CrossRef]
- Wilson, G.J.; Layman, D.K.; Moulton, C.J.; Norton, L.E.; Anthony, T.G.; Proud, C.G.; Rupassara, S.I.; Garlick, P.J. Leucine or carbohydrate supplementation reduces ampk and eef2 phosphorylation and extends postprandial muscle protein synthesis in rats. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1236–E1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F.; Huang, Z.; Li, H.; Yu, J.; Wang, C.; Chen, S.; Meng, Q.; Cheng, Y.; Gao, X.; Li, J.; et al. Leucine deprivation increases hepatic insulin sensitivity via gcn2/mtor/s6k1 and ampk pathways. Diabetes 2011, 60, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; McGrath, B.C.; Reinert, J.; Olsen, D.S.; Lei, L.; Gill, S.; Wek, S.A.; Vattem, K.M.; Wek, R.C.; Kimball, S.R.; et al. The gcn2 eif2alpha kinase is required for adaptation to amino acid deprivation in mice. Mol. Cell. Biol. 2002, 22, 6681–6688. [Google Scholar] [CrossRef] [PubMed]
- Gallinetti, J.; Harputlugil, E.; Mitchell, J.R. Amino acid sensing in dietary-restriction-mediated longevity: Roles of signal-transducing kinases gcn2 and tor. Biochem. J. 2013, 449, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maurin, A.C.; Benani, A.; Lorsignol, A.; Brenachot, X.; Parry, L.; Carraro, V.; Guissard, C.; Averous, J.; Jousse, C.; Bruhat, A.; et al. Hypothalamic eif2alpha signaling regulates food intake. Cell Rep. 2014, 6, 438–444. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Hutson, S.M.; Patson, B.J.; Vaval, A.; Vary, T.C. Tissue-specific effects of chronic dietary leucine and norleucine supplementation on protein synthesis in rats. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E824–E835. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Patson, B.J.; Anthony, J.; Vaval, A.; Jefferson, L.S.; Vary, T.C. Leucine is a direct-acting nutrient signal that regulates protein synthesis in adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E503–E513. [Google Scholar] [CrossRef] [PubMed]
- Anthony, T.G.; Anthony, J.C.; Yoshizawa, F.; Kimball, S.R.; Jefferson, L.S. Oral administration of leucine stimulates ribosomal protein mrna translation but not global rates of protein synthesis in the liver of rats. J. Nutr. 2001, 131, 1171–1176. [Google Scholar] [PubMed]
- Ijichi, C.; Matsumura, T.; Tsuji, T.; Eto, Y. Branched-chain amino acids promote albumin synthesis in rat primary hepatocytes through the mtor signal transduction system. Biochem. Biophs. Res. 2003, 303, 59–64. [Google Scholar] [CrossRef]
- Zampieri, T.T.; Pedroso, J.A.; Furigo, I.C.; Tirapegui, J.; Donato, J., Jr. Oral leucine supplementation is sensed by the brain but neither reduces food intake nor induces an anorectic pattern of gene expression in the hypothalamus. PLoS ONE 2013, 8, e84094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- She, P.; Reid, T.M.; Bronson, S.K.; Vary, T.C.; Hajnal, A.; Lynch, C.J.; Hutson, S.M. Disruption of bcatm in mice leads to increased energy expenditure associated with the activation of a futile protein turnover cycle. Cell Metab. 2007, 6, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Sweatt, A.J.; Wood, M.; Suryawan, A.; Wallin, R.; Willingham, M.C.; Hutson, S.M. Branched-chain amino acid catabolism: Unique segregation of pathway enzymes in organ systems and peripheral nerves. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E64–E76. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Espinosa, M.A.; Wallin, R.; Hutson, S.M.; Sweatt, A.J. Widespread neuronal expression of branched-chain aminotransferase in the cns: Implications for leucine/glutamate metabolism and for signaling by amino acids. J. Neurochem. 2007, 100, 1458–1468. [Google Scholar] [PubMed]
- Paxton, R.; Harris, R.A. Regulation of branched-chain alpha-ketoacid dehydrogenase kinase. Arch. Biochem. Biophys. 1984, 231, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.A.; Joshi, M.; Jeoung, N.H. Mechanisms responsible for regulation of branched-chain amino acid catabolism. Biochem. Biophys. Res. Commun. 2004, 313, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Imamura, W.; Yoshimura, R.; Takai, M.; Yamamura, J.; Kanamoto, R.; Kato, H. Adverse effects of excessive leucine intake depend on dietary protein intake: A transcriptomic analysis to identify useful biomarkers. J. Nutr. Sci. Vitaminol. 2013, 59, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, T.T.; Torres-Leal, F.L.; Campana, A.B.; Lima, F.B.; Donato, J., Jr. l-leucine supplementation worsens the adiposity of already obese rats by promoting a hypothalamic pattern of gene expression that favors fat accumulation. Nutrients 2014, 6, 1364–1373. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Meek, T.H.; Schwartz, M.W. Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 2014, 15, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Cota, D.; Proulx, K.; Smith, K.A.; Kozma, S.C.; Thomas, G.; Woods, S.C.; Seeley, R.J. Hypothalamic mtor signaling regulates food intake. Science 2006, 312, 927–930. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr.; Frazão, R.; Elias, C.F. The pi3k signaling pathway mediates the biological effects of leptin. Arq. Bras. Endocrinol. Metabol. 2010, 54, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Niswender, K.D.; Morton, G.J.; Stearns, W.H.; Rhodes, C.J.; Myers, M.G., Jr.; Schwartz, M.W. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 2001, 413, 794–795. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Jo, Y.H.; Li, X.; Schwartz, G.J. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J. Neurosci. 2009, 29, 8302–8311. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Schwartz, G.J. Brainstem nutrient sensing in the nucleus of the solitary tract inhibits feeding. Cell Metab. 2012, 16, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Karnani-Mahesh, M.; Apergis-Schoute, J.; Adamantidis, A.; Jensen, L.T.; de Lecea, L.; Fugger, L.; Burdakov, D. Activation of central orexin/hypocretin neurons by dietary amino acids. Neuron 2011, 72, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Goddeke, S.; Kruger, M.; Tups, A. Effect of central and peripheral leucine on energy metabolism in the djungarian hamster (phodopus sungorus). J. Comp. Physiol. B Biochem. Syst. Environ. Physiol. 2013, 183, 261–268. [Google Scholar] [CrossRef]
- Laeger, T.; Reed, S.D.; Henagan, T.M.; Fernandez, D.H.; Taghavi, M.; Addington, A.; Munzberg, H.; Martin, R.J.; Hutson, S.M.; Morrison, C.D. Leucine acts in the brain to suppress food intake but does not function as a physiological signal of low dietary protein. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R310–R320. [Google Scholar] [CrossRef] [PubMed]
- Zeanandin, G.; Balage, M.; Schneider, S.M.; Dupont, J.; Hebuterne, X.; Mothe-Satney, I.; Dardevet, D. Differential effect of long-term leucine supplementation on skeletal muscle and adipose tissue in old rats: An insulin signaling pathway approach. Age 2012, 34, 371–387. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Liu, R.; Ma, Y.; Guo, H.; Hao, L.; Yao, P.; Liu, L.; Sun, X.; He, K.; et al. Chronic leucine supplementation increases body weight and insulin sensitivity in rats on high-fat diet likely by promoting insulin signaling in insulin-target tissues. Mol. Nutr. Food Res. 2013, 57, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Dietary l: -leucine and l: -alanine supplementation have similar acute effects in the prevention of high-fat diet-induced obesity. Amino Acids 2012, 44, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Freudenberg, A.; Petzke, K.J.; Klaus, S. Comparison of high-protein diets and leucine supplementation in the prevention of metabolic syndrome and related disorders in mice. J. Nutr. Biochem. 2012, 23, 1524–1530. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Yu, Y.H.; Hou, J.; Zhang, Y. Chronic leucine supplementation improves glycemic control in etiologically distinct mouse models of obesity and diabetes mellitus. Nutr. Metab. (Lond.) 2010, 7, 57. [Google Scholar] [CrossRef]
- Drgonova, J.; Jacobsson, J.A.; Han, J.C.; Yanovski, J.A.; Fredriksson, R.; Marcus, C.; Schioth, H.B.; Uhl, G.R. Involvement of the neutral amino acid transporter slc6a15 and leucine in obesity-related phenotypes. PLoS ONE 2013, 8, e68245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, K.; LeBlanc, R.E.; Loh, D.; Schwartz, G.J.; Yu, Y.H. Increasing dietary leucine intake reduces diet-induced obesity and improves glucose and cholesterol metabolism in mice via multimechanisms. Diabetes 2007, 56, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Ventrucci, G.; Mello, M.A.; Gomes-Marcondes, M.C. Effect of a leucine-supplemented diet on body composition changes in pregnant rats bearing walker 256 tumor. Braz. J. Med. Biol. Res. 2001, 34, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, D.; Sornet, C.; Bayle, G.; Prugnaud, J.; Pouyet, C.; Grizard, J. Postprandial stimulation of muscle protein synthesis in old rats can be restored by a leucine-supplemented meal. J. Nutr. 2002, 132, 95–100. [Google Scholar] [PubMed]
- Ventrucci, G.; de Mello, M.A.; Gomes-Marcondes, M.C. Effects of leucine supplemented diet on intestinal absorption in tumor bearing pregnant rats. BMC Cancer 2002, 2, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes-Marcondes, M.C.; Ventrucci, G.; Toledo, M.T.; Cury, L.; Cooper, J.C. A leucine-supplemented diet improved protein content of skeletal muscle in young tumor-bearing rats. Braz. J. Med. Biol. Res. 2003, 36, 1589–1594. [Google Scholar] [CrossRef] [PubMed]
- Rieu, I.; Sornet, C.; Bayle, G.; Prugnaud, J.; Pouyet, C.; Balage, M.; Papet, I.; Grizard, J.; Dardevet, D. Leucine-supplemented meal feeding for ten days beneficially affects postprandial muscle protein synthesis in old rats. J. Nutr. 2003, 133, 1198–1205. [Google Scholar] [PubMed]
- Bassil, M.S.; Hwalla, N.; Obeid, O.A. Meal pattern of male rats maintained on histidine-, leucine-, or tyrosine-supplemented diet. Obesity (Silver Spring) 2007, 15, 616–623. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Pedrosa, R.G.; de Araujo, J.A., Jr.; Pires, I.S.; Tirapegui, J. Effects of leucine and phenylalanine supplementation during intermittent periods of food restriction and refeeding in adult rats. Life Sci. 2007, 81, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Zhong, B.; Sakai, S.; Saeki, T.; Kanamoto, R. Excess leucine intake induces serine dehydratase in rat liver. Biosc. Biotechnol. Biochem. 2007, 71, 2614–2617. [Google Scholar] [CrossRef]
- Verhoeven, S.; Vanschoonbeek, K.; Verdijk, L.B.; Koopman, R.; Wodzig, W.K.; Dendale, P.; van Loon, L.J. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men. Am. J. Clin. Nutr. 2009, 89, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Bong, H.Y.; Kim, J.Y.; Jeong, H.I.; Moon, M.S.; Kim, J.; Kwon, O. Effects of corn gluten hydrolyzates, branched chain amino acids, and leucine on body weight reduction in obese rats induced by a high fat diet. Nutr. Res. Pract. 2010, 4, 106–113. [Google Scholar] [CrossRef] [PubMed]
- López, N.; Sánchez, J.; Picó, C.; Palou, A.; Serra, F. Dietary l-leucine supplementation of lactating rats results in a tendency to increase lean/fat ratio associated to lower orexigenic neuropeptide expression in hypothalamus. Peptides 2010, 31, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Balage, M.; Dupont, J.; Mothe-Satney, I.; Tesseraud, S.; Mosoni, L.; Dardevet, D. Leucine supplementation in rats induced a delay in muscle ir/pi3k signaling pathway associated with overall impaired glucose tolerance. J. Nutr. Biochem. 2011, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; van Kranenburg, J.; Hartgens, F.; Wodzig, W.K.; Saris, W.H.; van Loon, L.J. Prolonged leucine supplementation does not augment muscle mass or affect glycemic control in elderly type 2 diabetic men. J. Nutr. 2011, 141, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Torres-Leal, F.L.; Fonseca-Alaniz, M.H.; Teodoro, G.F.; de Capitani, M.D.; Vianna, D.; Pantaleao, L.C.; Matos-Neto, E.M.; Rogero, M.M.; Donato, J., Jr.; Tirapegui, J. Leucine supplementation improves adiponectin and total cholesterol concentrations despite the lack of changes in adiposity or glucose homeostasis in rats previously exposed to a high-fat diet. Nutr. Metab. (Lond.) 2011, 8, 62. [Google Scholar] [CrossRef]
- Magne, H.; Savary-Auzeloux, I.; Migne, C.; Peyron, M.A.; Combaret, L.; Remond, D.; Dardevet, D. Contrarily to whey and high protein diets, dietary free leucine supplementation cannot reverse the lack of recovery of muscle mass after prolonged immobilization during ageing. J. Physiol. 2012, 590, 2035–2049. [Google Scholar] [CrossRef] [PubMed]
- Vianna, D.; Resende, G.F.; Torres-Leal, F.L.; Pantaleao, L.C.; Donato, J., Jr.; Tirapegui, J. Long-term leucine supplementation reduces fat mass gain without changing body protein status of aging rats. Nutrition 2012, 28, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Eller, L.K.; Saha, D.C.; Shearer, J.; Reimer, R.A. Dietary leucine improves whole-body insulin sensitivity independent of body fat in diet-induced obese sprague-dawley rats. J. Nutr. Biochem. 2013, 24, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.G.; Donato, J., Jr.; Pires, I.S.; Tirapegui, J. Leucine supplementation increases serum insulin-like growth factor 1 concentration and liver protein/rna ratio in rats after a period of nutritional recovery. Appl. Physiol. Nutr. Metab. 2013, 38, 694–697. [Google Scholar] [CrossRef] [PubMed]
- Savary-Auzeloux, I.; Magne, H.; Migne, C.; Oberli, M.; Breuille, D.; Faure, M.; Vidal, K.; Perrot, M.; Remond, D.; Combaret, L.; et al. A dietary supplementation with leucine and antioxidants is capable to accelerate muscle mass recovery after immobilization in adult rats. PLoS ONE 2013, 8, e81495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, J.A.; Nishimura, L.S.; de Matos-Neto, E.M.; Donato, J., Jr.; Tirapegui, J. Leucine improves protein nutritional status and regulates hepatic lipid metabolism in calorie-restricted rats. Cell Biochem. Funct. 2014, 32, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine supplementation differentially enhances pancreatic cancer growth in lean and overweight mice. Cancer Metab. 2014, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Li, W.; Xu, J.Y.; Han, S.; Qin, L.Q. Effects of whey protein and leucine supplementation on insulin resistance in non-obese insulin-resistant model rats. Nutrition 2014, 30, 1076–1080. [Google Scholar] [CrossRef] [PubMed]
- Nairizi, A.; She, P.; Vary, T.C.; Lynch, C.J. Leucine supplementation of drinking water does not alter susceptibility to diet-induced obesity in mice. J. Nutr. 2009, 139, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Emanuelli, B.; Bång, A.M.; Espinoza, D.O.; Boucher, J.; Beebe, K.; Gall, W.; Kahn, C.R. Dietary leucine-an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS ONE 2011, 6, e21187. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Simar, D.; Ting, J.H.; Erkelens, J.R.; Morris, M.J. Leucine improves glucose and lipid status in offspring from obese dams, dependent on diet type, but not caloric intake. J. Neuroendocrinol. 2012, 24, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xu, M.; Lee, J.; He, C.; Xie, Z. Leucine supplementation increases sirt1 expression and prevents mitochondrial dysfunction and metabolic disorders in high-fat diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1234–E1244. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Andre, C.; Elie, M.; Romero-Zerbo, S.Y.; Leste-Lasserre, T.; Belluomo, L.; Duchampt, A.; Clark, S.; Aubert, A.; et al. Leucine supplementation protects from insulin resistance by regulating adiposity levels. PLoS ONE 2013, 8, e74705. [Google Scholar] [CrossRef] [PubMed]
- Binder, E.; Bermudez-Silva, F.J.; Elie, M.; Leste-Lasserre, T.; Belluomo, I.; Clark, S.; Duchampt, A.; Mithieux, G.; Cota, D. Leucine supplementation modulates fuel substrates utilization and glucose metabolism in previously obese mice. Obesity (Silver Spring) 2014, 22, 713–720. [Google Scholar] [CrossRef]
- Pereira, M.G.; Baptista, I.L.; Carlassara, E.O.; Moriscot, A.S.; Aoki, M.S.; Miyabara, E.H. Leucine supplementation improves skeletal muscle regeneration after cryolesion in rats. PLoS ONE 2014, 9, e85283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murin, R.; Hamprecht, B. Metabolic and regulatory roles of leucine in neural cells. Neurochem. Res. 2008, 33, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sisk, C.L.; Nunez, A.A.; Thebert, M.M. Differential effects of electrolytic and chemical hypothalamic lesions on lh pulses in rats. Am. J. Physiol. 1988, 255, E583–E590. [Google Scholar] [PubMed]
- Pizzi, W.J.; Barnhart, J.E.; Fanslow, D.J. Monosodium glutamate admlinistration to the newborn reduces reproductive ability in female and male mice. Science 1977, 196, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K.; Walker, D.A. Potential importance of leucine in treatment of obesity and the metabolic syndrome. J. Nutr. 2006, 136, 319S–323S. [Google Scholar] [PubMed]
- Donato, J., Jr.; Pedrosa, R.G.; Cruzat, V.F.; Pires, I.S.; Tirapegui, J. Effects of leucine supplementation on the body composition and protein status of rats submitted to food restriction. Nutrition 2006, 22, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Fried, S.K.; Watford, M. Leucing weight with a futile cycle. Cell Metab. 2007, 6, 155–156. [Google Scholar] [CrossRef] [PubMed]
- Balage, M.; Dardevet, D. Long-term effects of leucine supplementation on body composition. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, P.; English, T.; Shi, J.; Smas, C.M.; Kandror, K.V. Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes 2010, 59, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Polak, P.; Cybulski, N.; Feige, J.N.; Auwerx, J.; Ruegg, M.A.; Hall, M.N. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008, 8, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, G.F.; Vianna, D.; Torres-Leal, F.L.; Pantaleao, L.C.; Matos-Neto, E.M.; Donato, J., Jr.; Tirapegui, J. Leucine is essential for attenuating fetal growth restriction caused by a protein-restricted diet in rats. J. Nutr. 2012, 142, 924–930. [Google Scholar] [CrossRef] [PubMed]
- El-Chaar, D.; Gagnon, A.; Sorisky, A. Inhibition of insulin signaling and adipogenesis by rapamycin: Effect on phosphorylation of p70 s6 kinase vs eif4e-bp1. Int. J. Obes. Relat. Metab. Disord. 2004, 28, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef] [PubMed]
- Newgard, C.B. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metab. 2012, 15, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Lynch, C.J.; Gern, B.; Lloyd, C.; Hutson, S.M.; Eicher, R.; Vary, T.C. Leucine in food mediates some of the postprandial rise in plasma leptin concentrations. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E621–E630. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, J.A.; Buonfiglio, D.C.; Cardinali, L.I.; Furigo, I.C.; Ramos-Lobo, A.M.; Tirapegui, J.; Elias, C.F.; Donato, J., Jr. Inactivation of socs3 in leptin receptor-expressing cells protects mice from diet-induced insulin resistance but does not prevent obesity. Mol. Metab. 2014, 3, 608–618. [Google Scholar] [CrossRef] [PubMed]
- She, P.; Van Horn, C.; Reid, T.; Hutson, S.M.; Cooney, R.N.; Lynch, C.J. Obesity-related elevations in plasma leucine are associated with alterations in enzymes involved in branched-chain amino acid metabolism. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1552–E1563. [Google Scholar] [CrossRef] [PubMed]
- Sans, M.D.; Tashiro, M.; Vogel, N.L.; Kimball, S.R.; D’Alecy, L.G.; Williams, J.A. Leucine activates pancreatic translational machinery in rats and mice through mtor independently of cck and insulin. J. Nutr. 2006, 136, 1792–1799. [Google Scholar] [PubMed]
- Filiputti, E.; Rafacho, A.; Araujo, E.P.; Silveira, L.R.; Trevisan, A.; Batista, T.M.; Curi, R.; Velloso, L.A.; Quesada, I.; Boschero, A.C.; et al. Augmentation of insulin secretion by leucine supplementation in malnourished rats: Possible involvement of the phosphatidylinositol 3-phosphate kinase/mammalian target protein of rapamycin pathway. Metabolism 2010, 59, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Van Loon, L.J.; Kruijshoop, M.; Verhagen, H.; Saris, W.H.; Wagenmakers, A.J. Ingestion of protein hydrolysate and amino acid-carbohydrate mixtures increases postexercise plasma insulin responses in men. J. Nutr. 2000, 130, 2508–2513. [Google Scholar] [PubMed]
- Van Loon, L.J.; Saris, W.H.; Verhagen, H.; Wagenmakers, A.J. Plasma insulin responses after ingestion of different amino acid or protein mixtures with carbohydrate. Am. J. Clin. Nutr. 2000, 72, 96–105. [Google Scholar] [PubMed]
- Rachdi, L.; Aiello, V.; Duvillie, B.; Scharfmann, R. l-leucine alters pancreatic beta-cell differentiation and function via the mtor signaling pathway. Diabetes 2012, 61, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, R.G.; Donato, J.; Pires, I.S.; Tirapegui, J. Leucine supplementation favors liver protein status but does not reduce body fat in rats during 1 week of food restriction. Appl. Physiol. Nutr. Metab. 2010, 35, 180–183. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, J.A., Jr.; Falavigna, G.; Rogero, M.M.; Pires, I.S.; Pedrosa, R.G.; Castro, I.A.; Donato, J., Jr.; Tirapegui, J. Effect of chronic supplementation with branched-chain amino acids on the performance and hepatic and muscle glycogen content in trained rats. Life Sci. 2006, 79, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Krebs, M.; Krssak, M.; Bernroider, E.; Anderwald, C.; Brehm, A.; Meyerspeer, M.; Nowotny, P.; Roth, E.; Waldhausl, W.; Roden, M. Mechanism of amino acid-induced skeletal muscle insulin resistance in humans. Diabetes 2002, 51, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Shah, O.J.; Wang, Z.; Hunter, T. Inappropriate activation of the tsc/rheb/mtor/s6k cassette induces irs1/2 depletion, insulin resistance, and cell survival deficiencies. Curr. Biol. 2004, 14, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Carvalheira, J.B.; Tambascia, R.C.; Bezerra, R.M.; Amaral, M.E.; Carneiro, E.M.; Folli, F.; Franchini, K.G.; Saad, M.J. Regulation of insulin signalling by hyperinsulinaemia: Role of irs-1/2 serine phosphorylation and the mtor/p70 s6k pathway. Diabetologia 2005, 48, 506–518. [Google Scholar] [CrossRef] [PubMed]
- Giraud, J.; Leshan, R.; Lee, Y.H.; White, M.F. Nutrient-dependent and insulin-stimulated phosphorylation of insulin receptor substrate-1 on serine 302 correlates with increased insulin signaling. J. Biol. Chem. 2004, 279, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Gao, Z.; Yin, J.; Quon, M.J.; Ye, J. S6k directly phosphorylates irs-1 on ser-270 to promote insulin resistance in response to tnf-(alpha) signaling through ikk2. J. Biol. Chem. 2008, 283, 35375–35382. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S.; Peraldi, P.; Budavari, A.; Ellis, R.; White, M.F.; Spiegelman, B.M. Irs-1-mediated inhibition of insulin receptor tyrosine kinase activity in tnf-alpha- and obesity-induced insulin resistance. Science 1996, 271, 665–668. [Google Scholar] [CrossRef] [PubMed]
- Hirosumi, J.; Tuncman, G.; Chang, L.; Gorgun, C.Z.; Uysal, K.T.; Maeda, K.; Karin, M.; Hotamisligil, G.S. A central role for jnk in obesity and insulin resistance. Nature 2002, 420, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, T.R.; Forny-Germano, L.; Sathler, L.B.; Brito-Moreira, J.; Houzel, J.C.; Decker, H.; Silverman, M.A.; Kazi, H.; Melo, H.M.; McClean, P.L.; et al. An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by alzheimer’s disease- associated abeta oligomers. J. Clin. Investig. 2012, 122, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Zemel, M.B.; Bruckbauer, A. Effects of a leucine and pyridoxine-containing nutraceutical on fat oxidation, and oxidative and inflammatory stress in overweight and obese subjects. Nutrients 2012, 4, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Donato, J., Jr. The central nervous system as a promising target to treat diabetes mellitus. Curr. Top. Med. Chem. 2012, 12, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T.; Collins, F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Vianna, C.R.; Donato, J., Jr.; Kim, M.H.; Chuang, J.C.; Lee, C.E.; Lauzon, D.A.; Lin, P.; Brule, L.J.; Scott, M.M.; et al. Direct leptin action on pomc neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012, 122, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Murphy, K.G.; Bloom, S.R. Gut hormones and the regulation of energy homeostasis. Nature 2006, 444, 854–859. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Reimer, R.A. Dairy protein and leucine alter glp-1 release and mrna of genes involved in intestinal lipid metabolism in vitro. Nutrition 2009, 25, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Stoffers, D.A.; Habener, J.F.; Bonner-Weir, S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes 1999, 48, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, D.A.; Kahn, S.E.; Leusner, C.R.; Ensinck, J.W. Glucagon-like peptide 1 enhances glucose tolerance both by stimulation of insulin release and by increasing insulin-independent glucose disposal. J. Clin. Investig. 1994, 93, 2263–2266. [Google Scholar] [CrossRef] [PubMed]
- Layman, D.K. The role of leucine in weight loss diets and glucose homeostasis. J. Nutr. 2003, 133, 261S–267S. [Google Scholar] [PubMed]
- Layman, D.K.; Boileau, R.A.; Erickson, D.J.; Painter, J.E.; Shiue, H.; Sather, C.; Christou, D.D. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J. Nutr. 2003, 133, 411–417. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedroso, J.A.B.; Zampieri, T.T.; Donato, J., Jr. Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients 2015, 7, 3914-3937. https://doi.org/10.3390/nu7053914
Pedroso JAB, Zampieri TT, Donato J Jr. Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients. 2015; 7(5):3914-3937. https://doi.org/10.3390/nu7053914
Chicago/Turabian StylePedroso, João A.B., Thais T. Zampieri, and Jose Donato, Jr. 2015. "Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis" Nutrients 7, no. 5: 3914-3937. https://doi.org/10.3390/nu7053914
APA StylePedroso, J. A. B., Zampieri, T. T., & Donato, J., Jr. (2015). Reviewing the Effects of l-Leucine Supplementation in the Regulation of Food Intake, Energy Balance, and Glucose Homeostasis. Nutrients, 7(5), 3914-3937. https://doi.org/10.3390/nu7053914