Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Plasma Measurements
2.3. RPAT SVF Cell and Adipocyte Isolation
2.4. Adipocyte Size Analysis
2.5. SVF Cell Composition Analysis by Flow Cytometry
2.6. RPAT SVF Cell Culture and Proliferation
2.7. Cell Differentiation Assay
2.8. Determinations
2.8.1. RNA Isolation and Real-Time Quantitative PCR
2.8.2. Immunofluorescence Assay
2.8.3. Leptin Measurement
2.8.4. Cellular Lipid Content
2.8.5. Cell Differentiation and Maturation
2.9. Statistical Analysis
3. Results
3.1. The MSG Rat Phenotype
3.2. Proliferation Capacity of SVF Cells from MSG Rats at 30 Days of Age
3.3. Enhanced Terminal Differentiation of SVF Cells from MSG Rats at 30 Days of Age
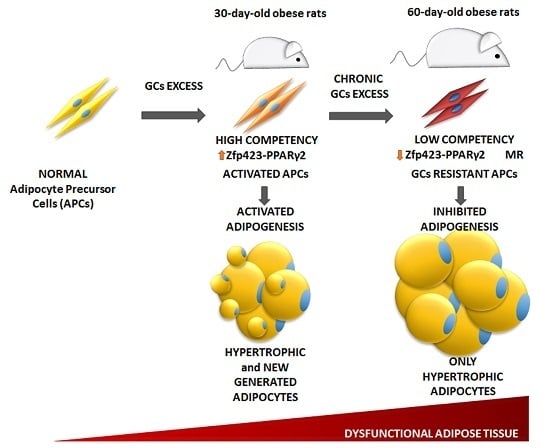
3.4. Age-Dependent Effect of GCs on APCs’ Competency and Adipogenic Potential
3.5. High Peripheral GC Levels Do Not Modify APC Number over Development
3.6. Relationship between APCs’ Competency and Adipogenic Capacity in MSG Rats
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Peckett, A.J.; Wright, D.C.; Riddell, M.C. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism 2011, 60, 1500–1510. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Fried, S.K. Integration of hormonal and nutrient signals that regulate leptin synthesis and secretion. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1230–E1238. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Pramyothin, P.; Karastergiou, K.; Fried, S.K. Deconstructing the roles of glucocorticoids in adipose tissue biology and the development of central obesity. Biochim. Biophys. Acta 2014, 1842, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Rebuffé-Scrive, M.; Krotkiewski, M.; Elfverson, J.; Björntorp, P. Muscle and adipose tissue morphology and metabolism in Cushing’s syndrome. J. Clin. Endocrinol. Metab. 1988, 67, 1122–1128. [Google Scholar] [CrossRef] [PubMed]
- Mayo-Smith, W.; Hayes, C.W.; Biller, B.M.; Klibanski, A.; Rosenthal, H.; Rosenthal, D.I. Body fat distribution measured with CT: Correlations in healthy subjects, patients with anorexia nervosa, and patients with Cushing syndrome. Radiology 1989, 170, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Chanson, P.; Salenave, S. Metabolic syndrome in Cushing’s syndrome. Neuroendocrinology 2010, 92 (Suppl. 1), 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Grant, L.D.; Bissette, G.; Ervin, G.N.; Harrell, L.E.; Prange, A.J. Growth, endocrinological and behavioral deficits after monosodium l-glutamate in the neonatal rat: Possible involvement of arcuate dopamine neuron damage. Psychoneuroendocrinology 1977, 2, 179–196. [Google Scholar] [CrossRef]
- Perelló, M.; Gaillard, R.C.; Chisari, A.; Spinedi, E. Adrenal enucleation in MSG-damaged hyperleptinemic male rats transiently restores adrenal sensitivity to leptin. Neuroendocrinology 2003, 78, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Moreno, G.; Perelló, M.; Camihort, G.; Luna, G.; Console, G.; Gaillard, R.C.; Spinedi, E. Impact of transient correction of increased adrenocortical activity in hypothalamo-damaged, hyperadipose female rats. Int. J. Obes. 2006, 30, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Joe, A.W.B.; Yi, L.; Even, Y.; Vogl, A.W.; Rossi, F.M.V. Depot-specific differences in adipogenic progenitor abundance and proliferative response to high-fat diet. Stem Cells 2009, 27, 2563–2570. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Grayburn, P.; Chen, S.; Ravazzola, M.; Orci, L.; Unger, R.H. Adipogenic capacity and the susceptibility to type 2 diabetes and metabolic syndrome. Proc. Natl. Acad. Sci. USA 2008, 105, 6139–6144. [Google Scholar] [CrossRef] [PubMed]
- Franck, N.; Stenkula, K.G.; Ost, A.; Lindström, T.; Strålfors, P.; Nystrom, F.H. Insulin-induced GLUT4 translocation to the plasma membrane is blunted in large compared with small primary fat cells isolated from the same individual. Diabetologia 2007, 50, 1716–1722. [Google Scholar] [CrossRef] [PubMed]
- Skurk, T.; Alberti-Huber, C.; Herder, C.; Hauner, H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Wåhlen, K.; Sjölin, E.; Löfgren, P. Role of fat cell size for plasma leptin in a large population based sample. Exp. Clin. Endocrinol. Diabetes 2011, 119, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Cristancho, A.G.; Lazar, M.A. Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 2011, 12, 722–734. [Google Scholar] [CrossRef] [PubMed]
- Sengenès, C.; Lolmède, K.; Zakaroff-Girard, A.; Busse, R.; Bouloumié, A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J. Cell. Physiol. 2005, 205, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Arany, Z.; Seale, P.; Mepani, R.J.; Ye, L.; Conroe, H.M.; Roby, Y.A.; Kulaga, H.; Reed, R.R.; Spiegelman, B.M. Transcriptional control of preadipocyte determination by Zfp423. Nature 2010, 464, 619–623. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef] [PubMed]
- Smas, C.M.; Chen, L.; Zhao, L.; Latasa, M.J.; Sul, H.S. Transcriptional repression of pref-1 by glucocorticoids promotes 3T3-L1 adipocyte differentiation. J. Biol. Chem. 1999, 274, 12632–12641. [Google Scholar] [CrossRef] [PubMed]
- Mulholland, D.J.; Dedhar, S.; Coetzee, G.A.; Nelson, C.C. Interaction of nuclear receptors with the Wnt/beta-catenin/Tcf signaling axis: Wnt you like to know? Endocr. Rev. 2005, 26, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Li, H.; Yang, J.; Qi, X.; Zu, X.; Yang, J.; Zhong, J.; Cao, R.; Liu, J.; Wen, G. Wnt/β-catenin signaling pathway and lipolysis enzymes participate in methylprednisolone induced fat differential distribution between subcutaneous and visceral adipose tissue. Steroids 2014, 84, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. TEM 2009, 20, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hudak, C.; Sul, H.S. Role of preadipocyte factor 1 in adipocyte differentiation. Clin. Lipidol. 2010, 5, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Pantoja, C.; Huff, J.T.; Yamamoto, K.R. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell 2008, 19, 4032–4041. [Google Scholar] [CrossRef] [PubMed]
- Caprio, M.; Fève, B.; Claës, A.; Viengchareun, S.; Lombès, M.; Zennaro, M.C. Pivotal role of the mineralocorticoid receptor in corticosteroid-induced adipogenesis. FASEB J. 2007, 21, 2185–2194. [Google Scholar] [CrossRef] [PubMed]
- Hoppmann, J.; Perwitz, N.; Meier, B.; Fasshauer, M.; Hadaschik, D.; Lehnert, H.; Klein, J. The balance between gluco- and mineralo-corticoid action critically determines inflammatory adipocyte responses. J. Endocrinol. 2010, 204, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Fried, S.K. The glucocorticoid receptor, not the mineralocorticoid receptor, plays the dominant role in adipogenesis and adipokine production in human adipocytes. Int. J. Obes. 2014, 38, 1228–1233. [Google Scholar] [CrossRef] [PubMed]
- Zubiría, M.G.; Vidal-Bravo, J.; Spinedi, E.; Giovambattista, A. Relationship between impaired adipogenesis of retroperitoneal adipose tissue and hypertrophic obesity: Role of endogenous glucocorticoid excess. J. Cell. Mol. Med. 2014, 18, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
- Giovambattista, A.; Gaillard, R.C.; Spinedi, E. Ghrelin gene-related peptides modulate rat white adiposity. Vitam. Horm. 2008, 77, 171–205. [Google Scholar] [PubMed]
- Tchoukalova, Y.D.; Harteneck, D.A.; Karwoski, R.A.; Tarara, J.; Jensen, M.D. A quick, reliable, and automated method for fat cell sizing. J. Lipid Res. 2003, 44, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Sengenès, C.; Decaunes, P.; Zakaroff-Girard, A.; Bourlier, V.; Lafontan, M.; Galitzky, J.; Bouloumié, A. Evidence of in situ proliferation of adult adipose tissue-derived progenitor cells: Influence of fat mass microenvironment and growth. J. Clin. Endocrinol. Metab. 2008, 93, 4098–4106. [Google Scholar] [CrossRef] [PubMed]
- Giovambattista, A.; Piermaría, J.; Suescun, M.O.; Calandra, R.S.; Gaillard, R.C.; Spinedi, E. Direct effect of ghrelin on leptin production by cultured rat white adipocytes. Obesity (Silver Spring) 2006, 14, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Dodson, M.V.; Jiang, Z. Cellular and molecular comparison of redifferentiation of intramuscular- and visceral-adipocyte derived progeny cells. Int. J. Biol. Sci. 2010, 6, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Grégoire, F.; Todoroff, G.; Hauser, N.; Remacle, C. The stroma-vascular fraction of rat inguinal and epididymal adipose tissue and the adipoconversion of fat cell precursors in primary culture. Biol. Cell 1990, 69, 215–222. [Google Scholar] [CrossRef]
- Wang, Y.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Hu, F.B. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am. J. Clin. Nutr. 2005, 81, 555–563. [Google Scholar] [PubMed]
- Tankó, L.B.; Bagger, Y.Z.; Alexandersen, P.; Larsen, P.J.; Christiansen, C. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation 2003, 107, 1626–1631. [Google Scholar] [CrossRef] [PubMed]
- Macotela, Y.; Emanuelli, B.; Mori, M.A.; Gesta, S.; Schulz, T.J.; Tseng, Y.H.; Kahn, C.R. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes 2012, 61, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Schmid, P.; Pfeiffer, E.F. Glucocorticoids and insulin promote the differentiation of human adipocyte precursor cells into fat cells. J. Clin. Endocrinol. Metab. 1987, 64, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Gong, D.W.; Burkey, B.F.; Fried, S.K. Pathways regulated by glucocorticoids in omental and subcutaneous human adipose tissues: A microarray study. Am. J. Physiol. Endocrinol. Metab. 2011, 300, E571–E580. [Google Scholar] [CrossRef] [PubMed]
- Geer, E.B.; Shen, W.; Gallagher, D.; Punyanitya, M.; Looker, H.C.; Post, K.D.; Freda, P.U. MRI assessment of lean and adipose tissue distribution in female patients with Cushing’s disease. Clin. Endocrinol. (Oxf.) 2010, 73, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Rockall, A.G.; Sohaib, S.A.; Evans, D.; Kaltsas, G.; Isidori, A.M.; Monson, J.P.; Besser, G.M.; Grossman, A.B.; Reznek, R.H. Computed tomography assessment of fat distribution in male and female patients with Cushing’s syndrome. Eur. J. Endocrinol. 2003, 149, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Perelló, M.; Moreno, G.; Gaillard, R.C.; Spinedi, E. Glucocorticoid-dependency of increased adiposity in a model of hypothalamic obesity. Neuro Endocrinol. Lett. 2004, 25, 119–126. [Google Scholar] [PubMed]
- Miranville, A.; Heeschen, C.; Sengenès, C.; Curat, C.A.; Busse, R.; Bouloumié, A. Improvement of postnatal neovascularization by human adipose tissue-derived stem cells. Circulation 2004, 110, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Rodeheffer, M.S.; Birsoy, K.; Friedman, J.M. Identification of white adipocyte progenitor cells in vivo. Cell 2008, 135, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Maumus, M.; Peyrafitte, J.; D’Angelo, R.; Fournier-Wirth, C.; Bouloumié, A.; Casteilla, L.; Sengenès, C.; Bourin, P. Native human adipose stromal cells: Localization, morphology and phenotype. Int. J. Obes. 2011, 35, 1141–1153. [Google Scholar] [CrossRef] [PubMed]
- Tchoukalova, Y.; Koutsari, C.; Jensen, M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia 2007, 50, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Elgazar-Carmon, V.; Rudich, A.; Hadad, N.; Levy, R. Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 2008, 49, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, S.; Oh, D.Y.; Bandyopadhyay, G.; Li, D.; Xu, J.; McNelis, J.; Lu, M.; Li, P.; Yan, Q.; Zhu, Y.; et al. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 2012, 18, 1407–1412. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Manabe, I.; Nagasaki, M.; Eto, K.; Yamashita, H.; Ohsugi, M.; Otsu, M.; Hara, K.; Ueki, K.; Sugiura, S.; et al. CD8+ effector T cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat. Med. 2009, 15, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. (Lausanne) 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P.S. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Lacasa, D.; Taleb, S.; Keophiphath, M.; Miranville, A.; Clement, K. Macrophage-secreted factors impair human adipogenesis: Involvement of proinflammatory state in preadipocytes. Endocrinology 2007, 148, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Kumar, P.A.; Fan, Y.; Sperling, M.A.; Menon, R.K. A novel effect of growth hormone on macrophage modulates macrophage-dependent adipocyte differentiation. Endocrinology 2010, 151, 2189–2199. [Google Scholar] [CrossRef] [PubMed]
- Barahona, M.J.; Sucunza, N.; Resmini, E.; Fernández-Real, J.M.; Ricart, W.; Moreno-Navarrete, J.M.; Puig, T.; Farrerons, J.; Webb, S.M. Persistent body fat mass and inflammatory marker increases after long-term cure of Cushing’s syndrome. J. Clin. Endocrinol. Metab. 2009, 94, 3365–3371. [Google Scholar] [CrossRef] [PubMed]
- Setola, E.; Losa, M.; Lanzi, R.; Lucotti, P.; Monti, L.D.; Castrignanò, T.; Galluccio, E.; Giovanelli, M.; Piatti, P. Increased insulin-stimulated endothelin-1 release is a distinct vascular phenotype distinguishing Cushing’s disease from metabolic syndrome. Clin. Endocrinol. 2007, 66, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Rondinone, C.M.; Rodbard, D.; Baker, M.E. Aldosterone stimulated differentiation of mouse 3T3-L1 cells into adipocytes. Endocrinology 1993, 132, 2421–2426. [Google Scholar] [PubMed]








| Primers (5′-3′) | GBAN | bp | |
|---|---|---|---|
| β-actin (ACTβ) | se, AGCCATGTACGTAGCCATCC | NM_031144 | 115 |
| as, ACCCTCATAGATGGGCACAG | |||
| Adiponectin (Adipoq) | se, AATCCTGCCCAGTCATGAAG | NM_144744 | 159 |
| as, TCTCCAGGAGTGCCATCTCT | |||
| CCAAT/enhancer binding protein alpha (C/EBPα) | se, CTGCGAGCACGAGACGTCTATAG | NM_012524 | 159 |
| as, TCCCGGGTAGTCAAAGTCACC | |||
| Glucocorticoid Receptor (GR) | se, TGCCCAGCATGCCGCTATCG | NW_047512 | 170 |
| as, GGGGTGAGCTGTGGTAATGCTGC | |||
| Mineralocorticoid Receptor (MR) | se, TCGCTCCGACCAAGGAGCCA | NM_013131 | 193 |
| as, TTCGCTGCCAGGCGGTTGAG | |||
| Leptin (Ob) | se, GAGACCTCCTCCATCTGCTG | NM_013076 | 192 |
| as, CTCAGCATTCAGGGCTAAGG | |||
| Peroxisome proliferator-activated receptor gamma 2 (PPAR-γ2) | se, AGGGGCCTGGACCTCTGCTG | NW_047696 | 185 |
| as, TCCGAAGTTGGTGGGCCAGA | |||
| Preadipocyte Factor 1 (Pref-1) | se, TGCTCCTGCTGGCTTTCGGC | NM_053744 | 113 |
| as, CCAGCCAGGCTCACACCTGC | |||
| Wingless-type MMTV integration site family member 10b (Wnt-10b) | se, AGGGGCTGCACATCGCCGTTC | NW_047784 | 175 |
| as, ACTGCGTGCATGACACCAGCAG | |||
| Zinc finger protein 423 (Zfp423) | se, CCGCGATCGGTGAAAGTTG | NM_053583.2 | 121 |
| as, CACGGCTGGATTTCCGATCA |
| CTR 30-Day-Old | MSG 30-Day-Old | CTR 60-Day-Old | MSG 60-Day-Old | |
|---|---|---|---|---|
| Body Weight (BW, g) | 78.84 ± 1.78 | 71.23 ± 1.56 * | 312.34 ± 9.32 a,b,c | 242.50 ± 5.47 *,a,b,c |
| Insulin (INS, ng/mL) | 0.25 ± 0.02 | 0.31 ± 0.04 | 2.01 ± 0.05 a | 2.42 ± 0.35 a |
| Glucose (Glu, g/L) | 1.26 ± 0.03 | 1.27 ± 0.03 | 1.17 ± 0.03 | 1.14 ± 0.06 |
| Triglyceride (TG, g/L) | 0.82 ± 0.06 | 1.19 ± 0.11 | 1.16 ± 0.14 | 1.24 ± 0.22 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zubiría, M.G.; Alzamendi, A.; Moreno, G.; Portales, A.; Castrogiovanni, D.; Spinedi, E.; Giovambattista, A. Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model. Nutrients 2016, 8, 410. https://doi.org/10.3390/nu8070410
Zubiría MG, Alzamendi A, Moreno G, Portales A, Castrogiovanni D, Spinedi E, Giovambattista A. Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model. Nutrients. 2016; 8(7):410. https://doi.org/10.3390/nu8070410
Chicago/Turabian StyleZubiría, María Guillermina, Ana Alzamendi, Griselda Moreno, Andrea Portales, Daniel Castrogiovanni, Eduardo Spinedi, and Andrés Giovambattista. 2016. "Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model" Nutrients 8, no. 7: 410. https://doi.org/10.3390/nu8070410
APA StyleZubiría, M. G., Alzamendi, A., Moreno, G., Portales, A., Castrogiovanni, D., Spinedi, E., & Giovambattista, A. (2016). Relationship between the Balance of Hypertrophic/Hyperplastic Adipose Tissue Expansion and the Metabolic Profile in a High Glucocorticoids Model. Nutrients, 8(7), 410. https://doi.org/10.3390/nu8070410