Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip
Abstract
:1. Introduction
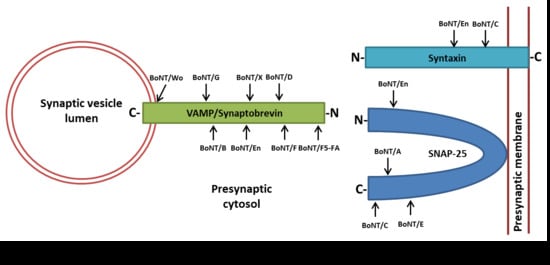
2. BoNTs’ Molecular Architecture and Mechanism of Nerve Endings Intoxication
3. Botulinum Neurotoxins Variability and Classification
4. BoNT/H (Also Known as BoNT/FA or BoNT/HA): A Complicated Story
5. Identification and Characterization of BoNT/X
6. Discovery and Biological Characterization of the First Non-Clostridial Botulinum-Like Toxin
7. The First Complete Bont Locus in a Non-Clostridial Organism
8. Engineering BoNTs: Potential for Novel Therapeutic Applications
9. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Pirazzini, M.; Rossetto, O.; Eleopra, R.; Montecucco, C. Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol. Rev. 2017, 69, 200–235. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Pirazzini, M.; Montecucco, C. Botulinum neurotoxins: Genetic, structural and mechanistic insights. Nat. Rev. Microbiol. 2014, 12, 535–549. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A.; Montecucco, C. Botulism. Handb. Clin. Neurol. 2008, 91, 333–368. [Google Scholar] [PubMed]
- Sutton, R.B.; Fasshauer, D.; Jahn, R.; Brunger, A.T. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 a resolution. Nature 1998, 395, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C.; Rizo, J. Synaptic vesicle exocytosis. Cold Spring Harb. Perspect. Biol. 2011, 3. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Matteoli, M.; Montecucco, C. Neurotoxins affecting neuroexocytosis. Physiol. Rev. 2000, 80, 717–766. [Google Scholar] [CrossRef] [PubMed]
- Hallett, M.; Albanese, A.; Dressler, D.; Segal, K.R.; Simpson, D.M.; Truong, D.; Jankovic, J. Evidence-based review and assessment of botulinum neurotoxin for the treatment of movement disorders. Toxicon 2013, 67, 94–114. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Dressler, D.; Hallett, M.; Jankovic, J.; Schiavo, G.; Segal, K.R.; Truong, D. Evidence-based review and assessment of botulinum neurotoxin for the treatment of secretory disorders. Toxicon 2013, 67, 141–152. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Botulinum toxin: State of the art. Mov. Disord. 2017, 32, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Seveso, M.; Caccin, P.; Schiavo, G.; Montecucco, C. Tetanus and botulinum neurotoxins: Turning bad guys into good by research. Toxicon 2001, 39, 27–41. [Google Scholar] [CrossRef]
- Caleo, M.; Restani, L. Exploiting botulinum neurotoxins for the study of brain physiology and pathology. Toxins 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Rothman, J.E. The principle of membrane fusion in the cell (nobel lecture). Angew. Chem. Int. Ed. Engl. 2014, 53, 12676–12694. [Google Scholar] [CrossRef] [PubMed]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. JAMA 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Hill, K.K.; Raphael, B.H. Historical and current perspectives on clostridium botulinum diversity. Res. Microbiol. 2015, 166, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Peck, M.W.; Smith, T.J.; Anniballi, F.; Austin, J.W.; Bano, L.; Bradshaw, M.; Cuervo, P.; Cheng, L.W.; Derman, Y.; Dorner, B.G.; et al. Historical perspectives and guidelines for botulinum neurotoxin subtype nomenclature. Toxins 2017, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Rasotto, M.B. On botulinum neurotoxin variability. MBio 2015, 6, e02131-14. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Doxey, A.C. Genomic insights into the evolution and ecology of botulinum neurotoxins. Pathog. Dis. 2018. [Google Scholar] [CrossRef] [PubMed]
- Doxey, A.C.; Mansfield, M.J.; Montecucco, C. Discovery of novel bacterial toxins by genomics and computational biology. Toxicon 2018, 147, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lacy, D.B.; Tepp, W.; Cohen, A.C.; DasGupta, B.R.; Stevens, R.C. Crystal structure of botulinum neurotoxin type a and implications for toxicity. Nat. Struct. Biol. 1998, 5, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.; Eswaramoorthy, S. Structural analysis of the catalytic and binding sites of clostridium botulinum neurotoxin b. Nat. Struct. Biol. 2000, 7, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A. Two feet on the membrane: Uptake of clostridial neurotoxins. Curr. Top. Microbiol. Immunol. 2016, 406, 1–37. [Google Scholar]
- Binz, T.; Rummel, A. Cell entry strategy of clostridial neurotoxins. J. Neurochem. 2009, 109, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Muraro, L.; Tosatto, S.; Motterlini, L.; Rossetto, O.; Montecucco, C. The n-terminal half of the receptor domain of botulinum neurotoxin a binds to microdomains of the plasma membrane. Biochem. Biophys. Res. Commun. 2009, 380, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Varnum, S.M. The receptor binding domain of botulinum neurotoxin serotype c binds phosphoinositides. Biochimie 2012, 94, 920–923. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Zhang, S.; Mahrhold, S.; Lam, K.; Stern, D.; Bagramyan, K.; Perry, K.; Kalkum, M.; Rummel, A.; Dong, M.; et al. N-linked glycosylation of SV2 is required for binding and uptake of botulinum neurotoxin A. Nat. Struct. Mol. Biol. 2016, 23, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C.; Zanotti, G. Botulinum neurotoxin a1 likes it double sweet. Nat. Struct. Mol. Biol. 2016, 23, 619. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Montal, M. Molecular dissection of botulinum neurotoxin reveals interdomain chaperone function. Toxicon 2013, 75, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Montal, M. Botulinum neurotoxin: A marvel of protein design. Annu. Rev. Biochem. 2010, 79, 591–617. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Leka, O.; Zanetti, G.; Rossetto, O.; Montecucco, C. On the translocation of botulinum and tetanus neurotoxins across the membrane of acidic intracellular compartments. Biochim. Biophys. Acta 2016, 1858, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Pantano, S.; Montecucco, C. The blockade of the neurotransmitter release apparatus by botulinum neurotoxins. Cell. Mol. Life Sci. 2013, 71, 793–811. [Google Scholar] [CrossRef] [PubMed]
- Binz, T. Clostridial neurotoxin light chains: Devices for SNARE cleavage mediated blockade of neurotransmission. Curr. Top. Microbiol. Immunol. 2013, 364, 139–157. [Google Scholar] [PubMed]
- Schiavo, G.; Rossetto, O.; Santucci, A.; DasGupta, B.R.; Montecucco, C. Botulinum neurotoxins are zinc proteins. J. Biol. Chem. 1992, 267, 23479–23483. [Google Scholar] [PubMed]
- Simpson, L. The life history of a botulinum toxin molecule. Toxicon 2013, 68, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O. Challenges in searching for therapeutics against botulinum neurotoxins. Exp. Opin. Drug Discov. 2017, 12, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Rossetto, O.; Montecucco, C. Hsp90 and thioredoxin-thioredoxin reductase enable the catalytic activity of clostridial neurotoxins inside nerve terminals. Toxicon 2017, 147, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Azarnia Tehran, D.; Zanetti, G.; Lista, F.; Binz, T.; Shone, C.C.; Rossetto, O.; Montecucco, C. The thioredoxin reductase—Thioredoxin redox system cleaves the interchain disulphide bond of botulinum neurotoxins on the cytosolic surface of synaptic vesicles. Toxicon 2015, 107, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, C. How do tetanus and botulinum toxins bind to neuronal membranes? Trends Biochem. Sci. 1986, 11, 314–317. [Google Scholar] [CrossRef]
- Benoit, R.M.; Frey, D.; Hilbert, M.; Kevenaar, J.T.; Wieser, M.M.; Stirnimann, C.U.; McMillan, D.; Ceska, T.; Lebon, F.; Jaussi, R.; et al. Structural basis for recognition of synaptic vesicle protein 2c by botulinum neurotoxin a. Nature 2014, 505, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, R.A.; Benoit, R.M. Botulinum neurotoxins: New questions arising from structural biology. Trends Biochem. Sci. 2014, 39, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 is the protein receptor for botulinum neurotoxin a. Science 2006, 312, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Janz, R.; Chapman, E.R. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin e into neurons. Mol. Biol. Cell 2008, 19, 5226–5237. [Google Scholar] [CrossRef] [PubMed]
- Nishiki, T.; Kamata, Y.; Nemoto, Y.; Omori, A.; Ito, T.; Takahashi, M.; Kozaki, S. Identification of protein receptor for clostridium botulinum type b neurotoxin in rat brain synaptosomes. J. Biol. Chem. 1994, 269, 10498–10503. [Google Scholar] [PubMed]
- Peng, L.; Berntsson, R.P.; Tepp, W.H.; Pitkin, R.M.; Johnson, E.A.; Stenmark, P.; Dong, M. Botulinum neurotoxin d-c uses synaptotagmin i and ii as receptors, and human synaptotagmin ii is not an effective receptor for type b, d-c and g toxins. J. Cell Sci. 2012, 125, 3233–3242. [Google Scholar] [CrossRef] [PubMed]
- Rummel, A.; Karnath, T.; Henke, T.; Bigalke, H.; Binz, T. Synaptotagmins i and ii act as nerve cell receptors for botulinum neurotoxin g. J. Biol. Chem. 2004, 279, 30865–30870. [Google Scholar] [CrossRef] [PubMed]
- Mahrhold, S.; Bergstrom, T.; Stern, D.; Dorner, B.G.; Astot, C.; Rummel, A. Only the complex n559-glycan in the synaptic vesicle glycoprotein 2c mediates high affinity binding to botulinum neurotoxin serotype a1. Biochem. J. 2016, 473, 2645–2654. [Google Scholar] [CrossRef] [PubMed]
- Pucic, M.; Pinto, S.; Novokmet, M.; Knezevic, A.; Gornik, O.; Polasek, O.; Vlahovicek, K.; Wang, W.; Rudd, P.M.; Wright, A.F.; et al. Common aberrations from the normal human plasma n-glycan profile. Glycobiology 2010, 20, 970–975. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, A.; Polasek, O.; Gornik, O.; Rudan, I.; Campbell, H.; Hayward, C.; Wright, A.; Kolcic, I.; O’Donoghue, N.; Bones, J.; et al. Variability, heritability and environmental determinants of human plasma n-glycome. J. Proteom. Res. 2009, 8, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Lauc, G.; Pezer, M.; Rudan, I.; Campbell, H. Mechanisms of disease: The human n-glycome. Biochim. Biophys. Acta 2015, 1860, 1574–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Berntsson, R.P.A.; Tepp, W.H.; Tao, L.; Johnson, E.A.; Stenmark, P.; Dong, M. Structural basis for the unique ganglioside and cell membrane recognition mechanism of botulinum neurotoxin dc. Nat. Commun. 2017, 8, 1637. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kohda, T.; Umeda, K.; Yamamoto, H.; Mukamoto, M.; Kozaki, S. Characterization of the d/c mosaic neurotoxin produced by clostridium botulinum associated with bovine botulism in Japan. Vet. Microbiol. 2010, 140, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Kroken, A.R.; Karalewitz, A.P.; Fu, Z.; Kim, J.J.; Barbieri, J.T. Novel ganglioside-mediated entry of botulinum neurotoxin serotype d into neurons. J. Biol. Chem. 2011, 286, 26828–26837. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Tepp, W.H.; Johnson, E.A.; Dong, M. Botulinum neurotoxin d uses synaptic vesicle protein SV2 and gangliosides as receptors. PLoS Pathog. 2011, 7, e1002008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strotmeier, J.; Lee, K.; Volker, A.K.; Mahrhold, S.; Zong, Y.; Zeiser, J.; Zhou, J.; Pich, A.; Bigalke, H.; Binz, T.; et al. Botulinum neurotoxin serotype d attacks neurons via two carbohydrate-binding sites in a ganglioside-dependent manner. Biochem. J. 2010, 431, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, K.; Kohda, T.; Mukamoto, M.; Takeuchi, K.; Ihara, H.; Saito, M.; Kozaki, S. Binding of clostridium botulinum type c and d neurotoxins to ganglioside and phospholipid. Novel insights into the receptor for clostridial neurotoxins. J. Biol. Chem. 2005, 280, 35164–35171. [Google Scholar] [CrossRef] [PubMed]
- Kroken, A.R.; Karalewitz, A.P.; Fu, Z.; Baldwin, M.R.; Kim, J.J.; Barbieri, J.T. Unique ganglioside binding by botulinum neurotoxins c and d-sa. FEBS J. 2011, 278, 4486–4496. [Google Scholar] [CrossRef] [PubMed]
- Karalewitz, A.P.; Kroken, A.R.; Fu, Z.; Baldwin, M.R.; Kim, J.J.; Barbieri, J.T. Identification of a unique ganglioside binding loop within botulinum neurotoxins c and d-sa. Biochemistry 2010, 49, 8117–8126. [Google Scholar] [CrossRef] [PubMed]
- Colasante, C.; Rossetto, O.; Morbiato, L.; Pirazzini, M.; Molgo, J.; Montecucco, C. Botulinum neurotoxin type a is internalized and translocated from small synaptic vesicles at the neuromuscular junction. Mol. Neurobiol. 2013, 48, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.B.; Papadopulos, A.; Martin, S.; Matthews, D.R.; Morgan, G.P.; Nguyen, T.H.; Wang, T.; Nair, D.; Choquet, D.; Meunier, F.A. Botulinum neurotoxin type-a enters a non-recycling pool of synaptic vesicles. Sci. Rep. 2016, 6, 19654. [Google Scholar] [CrossRef] [PubMed]
- Harper, C.B.; Martin, S.; Nguyen, T.H.; Daniels, S.J.; Lavidis, N.A.; Popoff, M.R.; Hadzic, G.; Mariana, A.; Chau, N.; McCluskey, A.; et al. Dynamin inhibition blocks botulinum neurotoxin type a endocytosis in neurons and delays botulism. J. Biol. Chem. 2011, 286, 35966–35976. [Google Scholar] [CrossRef] [PubMed]
- Koriazova, L.K.; Montal, M. Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat. Struct. Biol. 2003, 10, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Rossetto, O.; Bolognese, P.; Shone, C.C.; Montecucco, C. Double anchorage to the membrane and intact inter-chain disulfide bond are required for the low ph induced entry of tetanus and botulinum neurotoxins into neurons. Cell. Microbiol. 2011, 13, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Suresh, S.; Liu, H.; Tepp, W.H.; Johnson, E.A.; Edwardson, J.M.; Chapman, E.R. Receptor binding enables botulinum neurotoxin b to sense low ph for translocation channel assembly. Cell Host Microbe 2011, 10, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Montal, M. Crucial role of the disulfide bridge between botulinum neurotoxin light and heavy chains in protease translocation across membranes. J. Biol. Chem. 2007, 282, 29604–29611. [Google Scholar] [CrossRef] [PubMed]
- Azarnia Tehran, D.; Pirazzini, M.; Leka, O.; Mattarei, A.; Lista, F.; Binz, T.; Rossetto, O.; Montecucco, C. Hsp90 is involved in the entry of clostridial neurotoxins into the cytosol of nerve terminals. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, G.; Azarnia Tehran, D.; Pirazzini, M.; Binz, T.; Shone, C.C.; Fillo, S.; Lista, F.; Rossetto, O.; Montecucco, C. Inhibition of botulinum neurotoxins interchain disulfide bond reduction prevents the peripheral neuroparalysis of botulism. Biochem. Pharmacol. 2015, 98, 522–530. [Google Scholar]
- Eleopra, R.; Montecucco, C.; Devigili, G.; Lettieri, C.; Rinaldo, S.; Verriello, L.; Pirazzini, M.; Caccin, P.; Rossetto, O. Botulinum neurotoxin serotype d is poorly effective in humans: An in vivo electrophysiological study. Clin. Neurophysiol. 2013, 124, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Van Ermengem, E. Ueber einen neuen anaëroben bacillus und seine beziehungen zum botulismus. Med. Microbiol. Immunol. 1897, 26, 1–56. [Google Scholar] [CrossRef]
- Erbguth, F.J. Historical notes on botulism, clostridium botulinum, botulinum toxin, and the idea of the therapeutic use of the toxin. Mov. Disord. 2004, 19, S2–S6. [Google Scholar] [CrossRef] [PubMed]
- Moriishi, K.; Koura, M.; Fujii, N.; Fujinaga, Y.; Inoue, K.; Syuto, B.; Oguma, K. Molecular cloning of the gene encoding the mosaic neurotoxin, composed of parts of botulinum neurotoxin types c1 and d, and pcr detection of this gene from clostridium botulinum type c organisms. Appl. Environ. Microbiol. 1996, 62, 662–667. [Google Scholar] [PubMed]
- Moriishi, K.; Koura, M.; Abe, N.; Fujii, N.; Fujinaga, Y.; Inoue, K.; Ogumad, K. Mosaic structures of neurotoxins produced from clostridium botulinum types c and d organisms. Biochim. Biophys. Acta 1996, 1307, 123–126. [Google Scholar] [CrossRef]
- Hedeland, M.; Moura, H.; Båverud, V.; Woolfitt, A.R.; Bondesson, U.; Barr, J.R. Confirmation of botulism in birds and cattle by the mouse bioassay and endopep-ms. J. Med. Microbiol. 2011, 60, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Kohda, T.; Seto, Y.; Mukamoto, M.; Kozaki, S. Improved detection methods by genetic and immunological techniques for botulinum c/d and d/c mosaic neurotoxins. Vet. Microbiol. 2013, 162, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Tsukamoto, K.; Kohda, T.; Matsui, M.; Mukamoto, M.; Kozaki, S. Characterization of the neurotoxin produced by isolates associated with avian botulism. Avian Dis. 2005, 49, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Woudstra, C.; Le Maréchal, C.; Souillard, R.; Bayon-Auboyer, M.-H.; Mermoud, I.; Desoutter, D.; Fach, P. New insights into the genetic diversity of clostridium botulinum group iii through extensive genome exploration. Front. Microbiol. 2016, 7, 757. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.H.; Sahl, J.W.; Smith, T.J.; Xie, G.; Foley, B.T.; Smith, L.A.; Fernandez, R.A.; Lindstrom, M.; Korkeala, H.; Keim, P.; et al. Comparative genomic analyses reveal broad diversity in botulinum-toxin-producing clostridia. BMC Genom. 2016, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.K.; Smith, T.J. Genetic diversity within clostridium botulinum serotypes, botulinum neurotoxin gene clusters and toxin subtypes. Curr. Top. Microbiol. Immunol. 2013, 364, 1–20. [Google Scholar] [PubMed]
- Hill, K.K.; Smith, T.J.; Helma, C.H.; Ticknor, L.O.; Foley, B.T.; Svensson, R.T.; Brown, J.L.; Johnson, E.A.; Smith, L.A.; Okinaka, R.T.; et al. Genetic diversity among botulinum neurotoxin-producing clostridial strains. J. Bacteriol. 2007, 189, 818–832. [Google Scholar] [CrossRef] [PubMed]
- Giordani, F.; Fillo, S.; Anselmo, A.; Palozzi, A.M.; Fortunato, A.; Gentile, B.; Azarnia Tehran, D.; Ciammaruconi, A.; Spagnolo, F.; Pittiglio, V.; et al. Genomic characterization of italian clostridium botulinum group i strains. Infect. Genet. Evol. 2015, 36, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Fillo, S.; Giordani, F.; Anniballi, F.; Gorge, O.; Ramisse, V.; Vergnaud, G.; Riehm, J.M.; Scholz, H.C.; Splettstoesser, W.D.; Kieboom, J.; et al. Clostridium botulinum group i strain genotyping by 15-locus multilocus variable-number tandem-repeat analysis. J. Clin. Microbiol. 2011, 49, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- Whitemarsh, R.C.; Tepp, W.H.; Johnson, E.A.; Pellett, S. Persistence of botulinum neurotoxin a subtypes 1-5 in primary rat spinal cord cells. PLoS ONE 2014, 9, e90252. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Krilich, J.; Pellett, S.; Baudys, J.; Tepp, W.H.; Barr, J.R.; Johnson, E.A.; Kalb, S.R. Comparison of the catalytic properties of the botulinum neurotoxin subtypes a1 and a5. Biochim. Biophys. Acta 2013, 1834, 2722–2728. [Google Scholar] [CrossRef] [PubMed]
- Whitemarsh, R.C.; Tepp, W.H.; Bradshaw, M.; Lin, G.; Pier, C.L.; Scherf, J.M.; Johnson, E.A.; Pellett, S. Characterization of botulinum neurotoxin a subtypes 1 through 5 by investigation of activities in mice, in neuronal cell cultures, and in vitro. Infect. Immun. 2013, 81, 3894–3902. [Google Scholar] [CrossRef] [PubMed]
- Pier, C.L.; Chen, C.; Tepp, W.H.; Lin, G.; Janda, K.D.; Barbieri, J.T.; Pellett, S.; Johnson, E.A. Botulinum neurotoxin subtype a2 enters neuronal cells faster than subtype a1. FEBS Lett. 2011, 585, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Tepp, W.H.; Whitemarsh, R.C.; Bradshaw, M.; Johnson, E.A. In vivo onset and duration of action varies for botulinum neurotoxin a subtypes 1-5. Toxicon 2015, 107, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Torii, Y.; Goto, Y.; Nakahira, S.; Kozaki, S.; Kaji, R.; Ginnaga, A. Comparison of systemic toxicity between botulinum toxin subtypes a1 and a2 in mice and rats. Basic Clin. Pharmacol. Toxicol. 2015, 116, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Kull, S.; Schulz, K.M.; Weisemann, J.; Kirchner, S.; Schreiber, T.; Bollenbach, A.; Dabrowski, P.W.; Nitsche, A.; Kalb, S.R.; Dorner, M.B.; et al. Isolation and functional characterization of the novel clostridium botulinum neurotoxin a8 subtype. PLoS ONE 2015, 10, e0116381. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Baudys, J.; Webb, R.P.; Wright, P.; Smith, T.J.; Smith, L.A.; Fernández, R.; Raphael, B.H.; Maslanka, S.E.; Pirkle, J.L.; et al. Discovery of a novel enzymatic cleavage site for botulinum neurotoxin f5. FEBS Lett. 2012, 586, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, S.; Kamata, Y.; Nishiki, T.; Kakinuma, H.; Maruyama, H.; Takahashi, H.; Karasawa, T.; Yamakawa, K.; Nakamura, S. Characterization of clostridium botulinum type b neurotoxin associated with infant botulism in japan. Infect. Immun. 1998, 66, 4811–4816. [Google Scholar] [PubMed]
- Bentivoglio, A.R.; Del Grande, A.; Petracca, M.; Ialongo, T.; Ricciardi, L. Clinical differences between botulinum neurotoxin type a and b. Toxicon 2015, 107, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.P.; Melicoff, E.; Padgett, D.; Liu, Y.; Teich, A.F.; Dickey, B.F.; Lin, W.; Adachi, R.; Sudhof, T.C. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J. Neurosci. 2006, 26, 13493–13504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Jahn, R.; Dahlstrom, A. Synaptotagmin i is present mainly in autonomic and sensory neurons of the rat peripheral nervous system. Neuroscience 1994, 63, 837–850. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Z.; Dong, M.; Sun, S.; Chapman, E.R.; Jackson, M.B. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry 2011, 50, 2711–2713. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, G.; Sikorra, S.; Rummel, A.; Krez, N.; Duregotti, E.; Negro, S.; Henke, T.; Rossetto, O.; Binz, T.; Pirazzini, M. Botulinum neurotoxin c mutants reveal different effects of syntaxin or snap-25 proteolysis on neuromuscular transmission. PLoS Pathog. 2017, 13, e1006567. [Google Scholar] [CrossRef] [PubMed]
- Dover, N.; Barash, J.R.; Hill, K.K.; Xie, G.; Arnon, S.S. Molecular characterization of a novel botulinum neurotoxin type h gene. J. Infect. Dis. 2014, 209, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Barash, J.R.; Arnon, S.S. A novel strain of clostridium botulinum that produces type b and type h botulinum toxins. J. Infect. Dis. 2013, 209, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.A. Validity of botulinum neurotoxin serotype h. J. Infect. Dis. 2014, 210, 992–993. [Google Scholar] [CrossRef] [PubMed]
- Maslanka, S.E.; Lúquez, C.; Dykes, J.K.; Tepp, W.H.; Pier, C.L.; Pellett, S.; Raphael, B.H.; Kalb, S.R.; Barr, J.R.; Rao, A.; et al. A novel botulinum neurotoxin, previously reported as serotype h, has a hybrid-like structure with regions of similarity to the structures of serotypes a and f and is neutralized with serotype a antitoxin. J. Infect. Dis. 2016, 213, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Kalb, S.R.; Baudys, J.; Raphael, B.H.; Dykes, J.K.; Luquez, C.; Maslanka, S.E.; Barr, J.R. Functional characterization of botulinum neurotoxin serotype h as a hybrid of known serotypes f and a (bont f/a). Anal. Chem. 2015, 87, 3911–3917. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Lam, K.; Perry, K.; Weisemann, J.; Rummel, A.; Jin, R. Crystal structure of the receptor-binding domain of botulinum neurotoxin type ha, also known as type fa or h. Toxins 2017, 9, 93. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Barash, J.R.; Lou, J.; Conrad, F.; Marks, J.D.; Arnon, S.S. Immunological characterization and neutralizing ability of monoclonal antibodies directed against botulinum neurotoxin type h. J. Infect. Dis. 2016, 213, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Tepp, W.H.; Bradshaw, M.; Kalb, S.R.; Dykes, J.K.; Lin, G.; Nawrocki, E.M.; Pier, C.L.; Barr, J.R.; Maslanka, S.E.; et al. Purification and characterization of botulinum neurotoxin fa from a genetically modified clostridium botulinum strain. mSphere 2016, 1, e00100-15. [Google Scholar] [CrossRef] [PubMed]
- Pellett, S.; Tepp, W.H.; Lin, G.; Johnson, E.A. Substrate cleavage and duration of action of botulinum neurotoxin type fa (“h, ha”). Toxicon 2018, 147, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Kakinuma, H.; Maruyama, H.; Takahashi, H.; Yamakawa, K.; Nakamura, S. The first case of type b infant botulism in japan. Pediatr. Int. 1996, 38, 541–543. [Google Scholar] [CrossRef]
- Umeda, K.; Seto, Y.; Kohda, T.; Mukamoto, M.; Kozaki, S. Stability of toxigenicity in proteolytic clostridium botulinum type b upon serial passage. Microbiol. Immunol. 2012, 56, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Masuyer, G.; Zhang, J.; Shen, Y.; Lundin, D.; Henriksson, L.; Miyashita, S.-I.; Martínez-Carranza, M.; Dong, M.; Stenmark, P. Identification and characterization of a novel botulinum neurotoxin. Nat. Commun. 2017, 8, 14130. [Google Scholar] [CrossRef] [PubMed]
- Pellizzari, R.; Rossetto, O.; Lozzi, L.; Giovedi, S.; Johnson, E.; Shone, C.C.; Montecucco, C. Structural determinants of the specificity for synaptic vesicle-associated membrane protein/synaptobrevin of tetanus and botulinum type b and g neurotoxins. J. Biol. Chem. 1996, 271, 20353–20358. [Google Scholar] [CrossRef] [PubMed]
- Rossetto, O.; Schiavo, G.; Montecucco, C.; Poulain, B.; Deloye, F.; Lozzi, L.; Shone, C.C. SNARE motif and neurotoxins. Nature 1994, 372, 415–416. [Google Scholar] [CrossRef] [PubMed]
- Breidenbach, M.A.; Brunger, A.T. Substrate recognition strategy for botulinum neurotoxin serotype a. Nature 2004, 432, 925–929. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Schmidt, J.J.; Stafford, R.G.; Swaminathan, S. Mode of VAMP substrate recognition and inhibition of Clostridium botulinum neurotoxin f. Nat. Struct. Mol. Biol. 2009, 16, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Zhang, S.; Barkho, S.; Shen, Y.; Henriksson, L.; Košenina, S.; Dong, M.; Stenmark, P. Structural characterisation of the catalytic domain of botulinum neurotoxin x—High activity and unique substrate specificity. Sci. Rep. 2018, 8, 4518. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Adams, J.B.; Doxey, A.C. Botulinum neurotoxin homologs in non-clostridium species. FEBS Lett. 2015, 589, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Tohno, M.; Kitahara, M.; Irisawa, T.; Masuda, T.; Uegaki, R.; Ohkuma, M.; Tajima, K. Lactobacillus silagei sp. nov., isolated from orchardgrass silage. Int. J. Syst. Evol. Microbiol. 2013, 63, 4613–4618. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Caccin, P.; Johnson, E.A.; Montecucco, C.; Rossetto, O. Site-directed mutagenesis identifies active-site residues of the light chain of botulinum neurotoxin type a. Biochem. Biophys. Res. Commun. 2001, 288, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Zornetta, I.; Azarnia Tehran, D.; Arrigoni, G.; Anniballi, F.; Bano, L.; Leka, O.; Zanotti, G.; Binz, T.; Montecucco, C. The first non clostridial botulinum-like toxin cleaves VAMP within the juxtamembrane domain. Sci. Rep. 2016, 6, 30257. [Google Scholar] [CrossRef] [PubMed]
- Bowen, M.; Brunger, A.T. Conformation of the synaptobrevin transmembrane domain. Proc. Natl. Acad. Sci. USA 2006, 103, 8378. [Google Scholar] [CrossRef] [PubMed]
- Fang, Q.; Zhao, Y.; Lindau, M. Juxtamembrane tryptophans of synaptobrevin 2 control the process of membrane fusion. FEBS Lett. 2013, 587, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Brunt, J.; Carter, A.T.; Stringer, S.C.; Peck, M.W. Identification of a novel botulinum neurotoxin gene cluster in enterococcus. FEBS Lett. 2018, 592, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.H.D.; Smith, T.J.; Foley, B.T.; Hill, K.; Keim, P.; Sahl, J.W. Botulinum-neurotoxin-like sequences identified from an Enterococcus sp. Genome assembly. bioRxiv 2017. [Google Scholar] [CrossRef]
- Zhang, S.; Lebreton, F.; Mansfield, M.J.; Miyashita, S.I.; Zhang, J.; Schwartzman, J.A.; Tao, L.; Masuyer, G.; Martinez-Carranza, M.; Stenmark, P.; et al. Identification of a botulinum neurotoxin-like toxin in a commensal strain of enterococcus faecium. Cell Host Microbe 2018, 23, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.B.; Rosenbaum, A.; Collins, C.C. Pharmacologic weakening of extraocular muscles. Investig. Ophthalmol. 1973, 12, 924–927. [Google Scholar]
- Scott, A.B. Botulinum toxin injection into extraocular muscles as an alternative to strabismus surgery. Ophthalmology 1980, 87, 1044–1049. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Quatrale, R.; Rossetto, O.; Montecucco, C.; Dressler, D. Clinical use of non-a botulinum toxins: Botulinum toxin type c and botulinum toxin type f. Neurotox. Res. 2006, 9, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Tugnoli, V.; Quatrale, R.; Rossetto, O.; Montecucco, C. Different types of botulinum toxin in humans. Mov. Disord. 2004, 19, S53–S59. [Google Scholar] [CrossRef] [PubMed]
- Eleopra, R.; Tugnoli, V.; Rossetto, O.; De Grandis, D.; Montecucco, C. Different time courses of recovery after poisoning with botulinum neurotoxin serotypes a and e in humans. Neurosci. Lett. 1998, 256, 135–138. [Google Scholar] [CrossRef]
- Eleopra, R.; Tugnoli, V.; Rossetto, O.; Montecucco, C.; De Grandis, D. Botulinum neurotoxin serotype c: A novel effective botulinum toxin therapy in human. Neurosci. Lett. 1997, 224, 91–94. [Google Scholar] [CrossRef]
- Peng, L.; Adler, M.; Demogines, A.; Borrell, A.; Liu, H.; Tao, L.; Tepp, W.H.; Zhang, S.-C.; Johnson, E.A.; Sawyer, S.L.; et al. Widespread sequence variations in VAMP1 across vertebrates suggest a potential selective pressure from botulinum neurotoxins. PLoS Pathog. 2014, 10, e1004177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, H.; Ida, T.; Tsutsuki, H.; Mori, M.; Matsumoto, T.; Kohda, T.; Mukamoto, M.; Goshima, N.; Kozaki, S.; Ihara, H. Specificity of botulinum protease for human VAMP family proteins. Microbiol. Immunol. 2012, 56, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Coffield, J.A.; Bakry, N.; Zhang, R.D.; Carlson, J.; Gomella, L.G.; Simpson, L.L. In vitro characterization of botulinum toxin types a, c and d action on human tissues: Combined electrophysiologic, pharmacologic and molecular biologic approaches. J. Pharmacol. Exp. Ther. 1997, 280, 1489–1498. [Google Scholar] [PubMed]
- Pellett, S.; Tepp, W.H.; Scherf, J.M.; Pier, C.L.; Johnson, E.A. Activity of botulinum neurotoxin type d (strain 1873) in human neurons. Toxicon 2015, 101, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.; Williams, P.T.; Katos, A.M.; Krasna, M.; Burrows, W.; Hilmas, C.J. Chapter 30—Botulinum toxin. In Handbook of Toxicology of Chemical Warfare Agents; Gupta, R.C., Ed.; Academic Press: San Diego, CA, USA, 2009; pp. 407–432. [Google Scholar]
- Carle, S.; Pirazzini, M.; Rossetto, O.; Barth, H.; Montecucco, C. High conservation of tetanus and botulinum neurotoxins cleavage sites on human SNARE proteins suggests that these pathogens exerted little or no evolutionary pressure on humans. Toxins 2017, 9, 404. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Chaddock, J.A.; Foster, K.A.; Acharya, K.R. Engineered botulinum neurotoxins as new therapeutics. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 27–51. [Google Scholar] [CrossRef] [PubMed]
- Sikorra, S.; Litschko, C.; Muller, C.; Thiel, N.; Galli, T.; Eichner, T.; Binz, T. Identification and characterization of botulinum neurotoxin a substrate binding pockets and their re-engineering for human snap-23. J. Mol. Biol. 2016, 428, 372–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Barbieri, J.T. Engineering botulinum neurotoxin to extend therapeutic intervention. Proc. Natl. Acad. Sci. USA 2009, 106, 9180–9184. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Meng, J.; Lawrence, G.W.; Zurawski, T.H.; Sasse, A.; Bodeker, M.O.; Gilmore, M.A.; Fernández-Salas, E.; Francis, J.; Steward, L.E.; et al. Novel chimeras of botulinum neurotoxins a and e unveil contributions from the binding, translocation, and protease domains to their functional characteristics. J. Biol. Chem. 2008, 283, 16993–17002. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zurawski, T.H.; Bodeker, M.O.; Meng, J.; Boddul, S.; Aoki, K.R.; Dolly, J.O. Longer-acting and highly potent chimaeric inhibitors of excessive exocytosis created with domains from botulinum neurotoxin A and B. Biochem. J. 2012, 444, 59. [Google Scholar] [CrossRef] [PubMed]
- Kutschenko, A.; Reinert, M.-C.; Krez, N.; Liebetanz, D.; Rummel, A. Bont/ab hybrid maintains similar duration of paresis as bont/a wild-type in murine running wheel assay. NeuroToxicology 2017, 59, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pirazzini, M.; Henke, T.; Rossetto, O.; Mahrhold, S.; Krez, N.; Rummel, A.; Montecucco, C.; Binz, T. Neutralisation of specific surface carboxylates speeds up translocation of botulinum neurotoxin type b enzymatic domain. FEBS Lett. 2013, 587, 3831–3836. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Peng, L.; Berntsson, R.P.A.; Liu, S.M.; Park, S.; Yu, F.; Boone, C.; Palan, S.; Beard, M.; Chabrier, P.-E.; et al. Engineered botulinum neurotoxin b with improved efficacy for targeting human receptors. Nat. Commun. 2017, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Bade, S.; Rummel, A.; Reisinger, C.; Karnath, T.; Ahnert-Hilger, G.; Bigalke, H.; Binz, T. Botulinum neurotoxin type d enables cytosolic delivery of enzymatically active cargo proteins to neurones via unfolded translocation intermediates. J. Neurochem. 2004, 91, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Masuyer, G.; Davies, J.R.; Moore, K.; Chaddock, J.A.; Ravi Acharya, K. Structural analysis of clostridium botulinum neurotoxin type d as a platform for the development of targeted secretion inhibitors. Sci. Rep. 2015, 5, 13397. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Cintron, E.J.; Beske, P.H.; Tenezaca, L.; Tran, B.Q.; Oyler, J.M.; Glotfelty, E.J.; Angeles, C.A.; Syngkon, A.; Mukherjee, J.; Kalb, S.R.; et al. Engineering botulinum neurotoxin c1 as a molecular vehicle for intra-neuronal drug delivery. Sci. Rep. 2017, 7, 42923. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, V.B.; Ovsepian, S.V.; Raghunath, A.; Huo, Q.; Lawrence, G.W.; Smith, L.; Dolly, J.O. Innocuous full-length botulinum neurotoxin targets and promotes the expression of lentiviral vectors in central and autonomic neurons. Gene Ther. 2011, 18, 656. [Google Scholar] [CrossRef] [PubMed]
- Nugent, M.; Wang, J.; Lawrence, G.; Zurawski, T.; Geoghegan, J.A.; Dolly, J.O. Conjugate of an igg binding domain with botulinum neurotoxin a lacking the acceptor moiety targets its SNARE protease into trka-expressing cells when coupled to anti-trka igg or fc-βngf. Bioconjug. Chem. 2017, 28, 1684–1692. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Maywood, E.S.; Restani, L.; Caleo, M.; Pirazzini, M.; Rossetto, O.; Hastings, M.H.; Niranjan, D.; Schiavo, G.; Davletov, B. Re-assembled botulinum neurotoxin inhibits cns functions without systemic toxicity. Toxins 2011, 3, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Darios, F.; Niranjan, D.; Ferrari, E.; Zhang, F.; Soloviev, M.; Rummel, A.; Bigalke, H.; Suckling, J.; Ushkaryov, Y.; Naumenko, N.; et al. Snare tagging allows stepwise assembly of a multimodular medicinal toxin. Proc. Natl. Acad. Sci. USA 2010, 107, 18197–18201. [Google Scholar] [CrossRef] [PubMed]
- Wentz, T.G.; Muruvanda, T.; Lomonaco, S.; Thirunavukkarasu, N.; Hoffmann, M.; Allard, M.W.; Hodge, D.R.; Pillai, S.P.; Hammack, T.S.; Brown, E.W.; et al. Closed genome sequence of Chryseobacterium piperi strain CTM(T)/ATCC BAA-1782, a gram-negative bacterium with clostridial neurotoxin-like coding sequences. Genome Announc. 2017, 5, e01296-17. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, M.J.; Wentz, T.G.; Zhang, S.; Lee, E.J.; Dong, M.; Sharma, S.K.; Doxey, A.C. Newly identified relatives of botulinum neurotoxins shed light on their molecular evolution. bioRxiv 2017. [Google Scholar] [CrossRef]


| Serotype | Protein Receptor | Ganglioside Receptor | HC-C GBP (SXWY) | Metalloprotease Motif (HExxH) | Substrates | Cleavage Sites |
|---|---|---|---|---|---|---|
| BoNT/A | N-glycosylated SV2A-B-C | GT1b GD1a | SNWY | HELIH | SNAP-23 | T202–R203 |
| SNAP-25 | Q197–R198 | |||||
| BoNT/B | Synaptotamin-1/2 | GT1b GD1a | SKWY | HELIH | VAMP-1 | Q78–F79 |
| VAMP-2 | Q76–F77 | |||||
| VAMP-3 | Q63–F64 | |||||
| BoNT/C | none * | GT1b GD1b | (W)KNY | HELNH | SNAP-25 | R198–A199 |
| Syntaxin-1A,-2,-3 | K253–A254 | |||||
| Syntaxin-1B | K252–A253 | |||||
| BoNT/D | N-glycosylated SV2A-B-C | GT1b GD1b GD2 | (W)VNY | HELTH | VAMP-1 | K61–L62 |
| VAMP-2 | K59–L60 | |||||
| VAMP-3 | K46–L47 | |||||
| BoNT/DC | Synaptotamin-1/2 | Sialic acid residue | SNYIS | HELTH | VAMP-1 | K61–L62 |
| VAMP-2 | K59–L60 | |||||
| VAMP-3 | K46–L47 | |||||
| BoNT/E | N-glycosylated SV2A-B | GT1b GD1a | STWY | HELIH | SNAP-23 SNAP-25 | K185–I186 R180–I181 |
| BoNT/F | N-glycosylated SV2A-B-C | GT1b GD1a | SSWY | HELIH | VAMP-1 | Q60–K61 |
| VAMP-2 | Q58-K59 | |||||
| VAMP-3 | Q45–K46 | |||||
| BoNT/F5 | Unknown (similar to BoNT/F?) | Unknown (similar to BoNT/F?) | SSWY | HELIH | VAMP-1 | L56–E57 |
| VAMP-2 | L54–E55 | |||||
| VAMP-3 | L41–E42 | |||||
| BoNT/H (BoNT/HA, BoNT/FA) | N-glycosylated SV2C | Unknown (similar to BoNT/A?) | SNWY | HELIH | VAMP-1 | L56–E57 |
| VAMP-2 | L54–E55 | |||||
| VAMP-3 | L41–E42 | |||||
| BoNT/G1 | Synaptotamin-1/2 | GT1b GD1a | SQWY | HELIH | VAMP-1 | A83–A84 |
| VAMP-2 | A81–A82 | |||||
| VAMP-3 | A68–A69 | |||||
| BoNT/X | Unknown | Unknown | SAWY | HELVH | VAMP-1 | R68–A69 |
| VAMP-2 | R66–A67 | |||||
| VAMP-3 | R53–A54 | |||||
| VAMP-4 | K86–S87 | |||||
| VAMP-5 | R40–S41 | |||||
| Ykt6 | K173–S174 | |||||
| BoNT/Wo | Unknown | Unknown | Not present ** | HEMTH | VAMP-2 | W89–W90 |
| BoNT/En (eBoNT/J) | Unknown | Unknown | SAWY | HELCH | SNAP-25 | K69–D70 |
| VAMP-1 | A69–D70 | |||||
| VAMP-2 | A67–D68 | |||||
| VAMP-3 | A54–D55 | |||||
| Syntaxin-1B | K145–D146 | |||||
| Syntaxin-4 | K191–D192 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tehran, D.A.; Pirazzini, M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins 2018, 10, 190. https://doi.org/10.3390/toxins10050190
Tehran DA, Pirazzini M. Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins. 2018; 10(5):190. https://doi.org/10.3390/toxins10050190
Chicago/Turabian StyleTehran, Domenico Azarnia, and Marco Pirazzini. 2018. "Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip" Toxins 10, no. 5: 190. https://doi.org/10.3390/toxins10050190
APA StyleTehran, D. A., & Pirazzini, M. (2018). Novel Botulinum Neurotoxins: Exploring Underneath the Iceberg Tip. Toxins, 10(5), 190. https://doi.org/10.3390/toxins10050190