Feasibility of A Novel On-Site Detection Method for Aflatoxin in Maize Flour from Markets and Selected Households in Kampala, Uganda
Abstract
:1. Introduction
2. Results
2.1. Validation of the Immunosensor
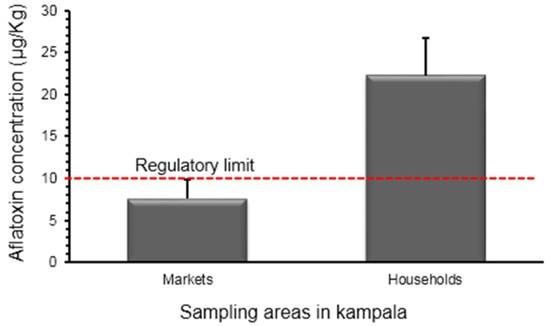
2.2. On-Site Detection
2.2.1. Market Samples
2.2.2. Households
2.2.3. Comparison between Aflatoxin Contamination of Households and Market Samples
2.3. On-Site Detection
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Validation of the Novel Immunosensor
4.3. On-Site Detection
4.4. Laboratory Control
4.4.1. HPLC
4.4.2. ELISA
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ranum, P.; Peña-Rosas, J.P.; Garcia-Casal, M.N. Global maize production, utilization, and consumption. Ann. N. Y. Acad. Sci. 2014, 1312, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macauley, H.; Ramadjita, T. Cereal crops: Rice, maize, millet, sorghum, wheat. Feed. Afr. 2015, 1–36. [Google Scholar]
- Atukwase, A.; Kaaya, A.N.; Muyanja, C. Factors associated with fumonisin contamination of maize in Uganda. J. Sci. Food Agrci. 2009, 89, 2393–2398. [Google Scholar] [CrossRef]
- De Groote, H.; Kimenju, S.C. Consumer preferences for maize products in urban Kenya. Food Nutr. Bull. 2012, 33, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Wacoo, A.P.; Wendiro, D.; Vuzi, P.C.; Hawumba, J.F. Methods for detection of aflatoxins in agricultural food crops. J. Appl. Chem. 2014, 2014, 1–15. [Google Scholar] [CrossRef]
- Battilani, P.; Toscano, P.; Van der Fels-Klerx, H.; Moretti, A.; Leggieri, M.C.; Brera, C.; Rortais, A.; Goumperis, T.; Robinson, T. Aflatoxin B1 contamination in maize in europe increases due to climate change. Sci. Rep. 2016, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hell, K.; Cardwell, K.; Setamou, M.; Poehling, H.-M. The influence of storage practices on aflatoxin contamination in maize in four agroecological zones of Benin, West Africa. J. Stored Prod. Res. 2000, 36, 365–382. [Google Scholar] [CrossRef]
- Shephard, G.S. Risk assessment of aflatoxins in food in Africa. Food Addit. Contam. 2008, 25, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, F. Global burden of aflatoxin-induced hepatocellular carcinoma: A risk assessment. Environ. Health Persp. 2010, 118, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Cardwell, K.; Hounsa, A.; Egal, S.; Turner, P.; Hall, A.; Wild, C. Dietary aflatoxin exposure and impaired growth in young children from Benin and Togo: Cross sectional study. BMJ 2002, 325, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Stoloff, L.; Van Egmond, H.; Park, D. Rationales for the establishment of limits and regulations for mycotoxins. Food Addit. Contam. 1991, 8, 213–221. [Google Scholar] [CrossRef] [PubMed]
- European Communities. Setting maximum levels for certain contaminants in foodstuffs. Commission Regulation (EC) NO. 1881/2006 of 19 December 2006. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Bandyopadhyay, R.; Ortega-Beltran, A.; Akande, A.; Mutegi, C.; Atehnkeng, J.; Kaptoge, L.; Senghor, A.; Adhikari, B.; Cotty, P. Biological control of aflatoxins in Africa: Current status and potential challenges in the face of climate change. World Mycotoxin J. 2016, 9, 771–789. [Google Scholar] [CrossRef]
- STANDARD, E.A. Milled Maize (Corn) Products—Specification; East Africa Community: Arusha, Tanzania, 2011; pp. 1–11. [Google Scholar]
- Kaaya, A.N.; Kyamuhangire, W. The effect of storage time and agroecological zone on mould incidence and aflatoxin contamination of maize from traders in Uganda. Int. J. Food Microbiol. 2006, 110, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Osuret, J.; Musinguzi, G.; Mukama, T.; Halage, A.A.; Natigo, A.K.; Ssempebwa, J.C.; Wang, J.-S. Aflatoxin contamination of selected staple foods sold for human consumption in Kampala markets, Uganda. J. Biol. Sci. 2016, 16, 1–5. [Google Scholar] [CrossRef]
- Moon, J.; Byun, J.; Kim, H.; Lim, E.-K.; Jeong, J.; Jung, J.; Kang, T. On-site detection of aflatoxin B1 in grains by a palm-sized surface plasmon resonance sensor. Sensors 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wacoo, P.A.; Ocheng, M.; Wendiro, D.; Vuzi, P.C.; Hawumba, F.J. Development and characterization of an electroless plated silver/cysteine sensor platform for the electrochemical determination of aflatoxin B1. J. Sens. 2016, 2015, 1–8. [Google Scholar] [CrossRef]
- Lewis, L.; Onsongo, M.; Njapau, H.; Schurz-Rogers, H.; Luber, G.; Kieszak, S.; Nyamongo, J.; Backer, L.; Dahiye, A.M.; Misore, A. Aflatoxin contamination of commercial maize products during an outbreak of acute aflatoxicosis in eastern and central Kenya. Environ. Health Persp. 2015, 113, 1763–1767. [Google Scholar]
- Hutchins, J.E.; Lee, Y.J.; Tyczkowska, K.; Hagler, W.M. Evaluation of silica cartridge purification and hemiacetal formation for liquid chromatographic determination of aflatoxins in corn. Arch. Environ. Contam. Toxicol. 1989, 18, 319–326. [Google Scholar] [CrossRef]
- Herzallah, S.M. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009, 114, 1141–1146. [Google Scholar] [CrossRef]
- Huybrechts, B. Evaluation of Immunoassay Kits for Aflatoxin Determination in Corn & Rice. CODA-CERVA Veterinary and Agrochemical Research Centre: NRL, Belgium. Available online: http://www. favvafsca. fgov.be/laboratories/approvedlaboratories/generalinformation/_documents/2012–05–04_Ev-aluation_immunoassay_kits_aflatoxin. pdf (accessed on 4 May 2012).
- Pei, S.C.; Zhang, Y.Y.; Eremin, S.A.; Lee, W.J. Detection of aflatoxin M1 in milk products from China by ELISA using monoclonal antibodies. Food Control 2009, 20, 1080–1085. [Google Scholar]
- Trombete, F.M.; Santos, T.B.; Direito, G.M.; Fraga, M.E.; Saldanha, T. In-house validation of a method for determining aflatoxins B1, B2, G1 and G2 in wheat and wheat by-products. Pesq. Agropecu. Trop. 2014, 44, 255–262. [Google Scholar] [CrossRef]
- Findlay, J.W.; Smith, W.C.; Lee, J.W.; Nordblom, G.D.; Das, I.; DeSilva, B.S.; Khan, M.N.; Bowsher, R.R. Validation of immunoassays for the bioanalysis: A pharmaceutical industry perspective. J. Pharm. Biomed. Anal. 2000, 21, 1249–1273. [Google Scholar] [CrossRef]
- Kos, J.J.; Hajnal, E.J.; Jajić, I.; Krstović, S.; Mastilović, J.; Šarić, B.; Jovanov, P. Comparison of ELISA, HPLC-FLD and HPLC-MS/MS methods for determination of aflatoxin M1 in natural contaminated milk samples. Acta Chim. Slov. 2016, 63, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Owino, J.H.; Ignaszak, A.; Al-Ahmed, A.; Baker, P.G.; Alemu, H.; Ngila, J.C.; Iwuoha, E.I. Modelling of the impedimetric responses of an aflatoxin B1 immunosensor prepared on an electrosynthetic polyaniline platform. Anal. Bioanal. Chem. 2007, 388, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Perrone, G.; Haidukowski, M.; Stea, G.; Epifani, F.; Bandyopadhyay, R.; Leslie, J.F.; Logrieco, A. Population structure and aflatoxin production by Aspergillus flavus from maize in Nigeria and Ghana. Food Microbiol. 2014, 41, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Probst, C.; Bandyopadhyay, R.; Cotty, P. Diversity of aflatoxin-producing fungi and their impact on food safety in sub-saharan Africa. Int. J. Food Microbiol. 2014, 174, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Magzoub, A. Compendium of rural women’s technologies and innovations. In Portable Electrochemical Aflatoxin Testing Kit, Uganda; IFAD, Ed.; IFAD: Rome, Italy, 2016; p. 8. [Google Scholar]
- IFAD. Technologies for Value Addition. In Portable Electrochemical Aflatoxin Testing Kit, Uganda; IFAD: Rome, Italy, 2015; p. 12. [Google Scholar]
- Shabani, I.; Kimanya, M.E.; Gichuhi, P.N.; Bonsi, C.; Bovell-Benjamin, A.C. Maize storage and consumption practices of farmers in handeni district, tanzania: Corollaries for mycotoxin contamination. Open J. Prev. Med. 2015, 5, 330–339. [Google Scholar] [CrossRef]
- Siwela, A.H.; Siwela, M.; Matindi, G.; Dube, S.; Nziramasanga, N. Decontamination of aflatoxin-contaminated maize by dehulling. J. Sci. Food Agrci. 2005, 85, 2535–2538. [Google Scholar] [CrossRef]
- Mienda, B.S. Preliminary report of dehulling effect on the occurrence and distribution of Aspergillus flavus in maize grains stored in Mubi market. Adv. Appl. Sci. Res. 2011, 2, 612–616. [Google Scholar]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Kilonzo, R.M.; Imungi, J.K.; Muiru, W.M.; Lamuka, P.O.; Njage, P.M.K. Household dietary exposure to aflatoxins from maize and maize products in Kenya. Food Addit. Contam. 2014, 31, 2055–2062. [Google Scholar] [CrossRef] [PubMed]
- Tittlemier, S.; Varga, E.; Scott, P.; Krska, R. Sampling of cereals and cereal-based foods for the determination of ochratoxin A: An overview. Food Addit. Contam. 2011, 28, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Guideline, I.H.T. In Validation of analytical procedures: Text and methodology Q2 (R1). In Proceedings of the International Conference on Harmonization, Geneva, Switzerland, November 2005; pp. 11–12. [Google Scholar]
- Sapsford, K.E.; Taitt, C.R.; Fertig, S.; Moore, M.H.; Lassman, M.E.; Maragos, C.M.; Shriver-Lake, L.C. Indirect competitive immunoassay for detection of aflatoxin B1 in corn and nut products using the array biosensor. Biosens. Bioelectron. 2006, 21, 2298–2305. [Google Scholar] [CrossRef] [PubMed]
- Muscarella, M.; Iammarino, M.; Nardiello, D.; Lo Magro, S.; Palermo, C.; Centonze, D.; Palermo, D. Validation of a confirmatory analytical method for the determination of aflatoxins B1, B2, G1 and G2 in foods and feed materials by HPLC with on-line photochemical derivatization and fluorescence detection. Food AddiT. Contam. 2009, 26, 1402–1410. [Google Scholar] [CrossRef]
- Raugel, P.-J. R-biopharm. In Rapid Food Analysis and Hygiene Monitoring; Springer: Berlin, Germany, 1999; pp. 500–554. [Google Scholar]






| Parameters | Values |
|---|---|
| Limit of Detection (LOD) (µg/kg) | 0.7 |
| Linear range (µg/kg) | 0.7 ± 0.1 to 11 ± 0.3 |
| Precision (CV) (%) | 0.3 (intra-day) |
| Accuracy | 1.5 (inter-day) |
| aflatoxin B1 standard(µg/kg) | 2 |
| recovery (%) | 99.0 ± 1.5 |
| aflatoxin B1 standard(µg/kg) | 5 |
| recovery (%) | 88.2 ± 0.8 |
| aflatoxin B1 standard(µg/kg) | 10 |
| recovery (%) | 70.5 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paul Wacoo, A.; Wendiro, D.; Nanyonga, S.; Hawumba, J.F.; Sybesma, W.; Kort, R. Feasibility of A Novel On-Site Detection Method for Aflatoxin in Maize Flour from Markets and Selected Households in Kampala, Uganda. Toxins 2018, 10, 327. https://doi.org/10.3390/toxins10080327
Paul Wacoo A, Wendiro D, Nanyonga S, Hawumba JF, Sybesma W, Kort R. Feasibility of A Novel On-Site Detection Method for Aflatoxin in Maize Flour from Markets and Selected Households in Kampala, Uganda. Toxins. 2018; 10(8):327. https://doi.org/10.3390/toxins10080327
Chicago/Turabian StylePaul Wacoo, Alex, Deborah Wendiro, Sarah Nanyonga, Joseph F. Hawumba, Wilbert Sybesma, and Remco Kort. 2018. "Feasibility of A Novel On-Site Detection Method for Aflatoxin in Maize Flour from Markets and Selected Households in Kampala, Uganda" Toxins 10, no. 8: 327. https://doi.org/10.3390/toxins10080327
APA StylePaul Wacoo, A., Wendiro, D., Nanyonga, S., Hawumba, J. F., Sybesma, W., & Kort, R. (2018). Feasibility of A Novel On-Site Detection Method for Aflatoxin in Maize Flour from Markets and Selected Households in Kampala, Uganda. Toxins, 10(8), 327. https://doi.org/10.3390/toxins10080327