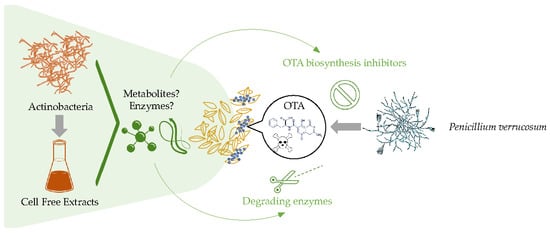
Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules
Abstract
:1. Introduction
2. Results
2.1. Antagonistic Evaluation of Actinobacteria and Their Cell Free Extracts (CFE)
2.2. Mycotoxin Degradation Assay
2.3. Global Analysis of Strains: Heatmap Analysis and Pearson Correlation Index
- -
- P. verrucosum growth in dual culture or with CFE added to the medium (Category 1);
- -
- OTA specific production during P. verrucosum growth confronted with bacteria, or with CFE added to the medium (Category 2); and
- -
- OTA degradation by actinobacteria in solid and liquid medium (Category 3).
2.4. Search for Degradation by-Products by HPLC
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Actinobacteria Strains
5.2. Actinobacteria Spore Numeration by Flux Cytometer
5.3. Pathogen Strains
5.4. Antagonistic Evaluation
5.4.1. Actinobacteria Cells
5.4.2. Cell Free Extracts
5.4.3. Pathogen Surface Growth Measurement and OTA Specific Production Calculation
5.5. Mycotoxin Degradation Assay
5.5.1. Screening of OTA Degradation by Actinobacteria and Their CFEs
5.5.2. Degradation Assays for the Search of Degradation by-Products
5.6. Mycotoxin and Degradation By-Products Analysis
5.6.1. Sample Extraction
5.6.2. OTA Quantification Using Fluorescence HPLC
5.6.3. Search for Degradation By-Products by HPLC-MS/MS
5.7. Data Analysis and Data Visualization
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eskola, M.; Kos, G.; Elliott, C.T.; Hajšlová, J.; Mayar, S.; Krska, R. Worldwide contamination of food-crops with mycotoxins: Validity of the widely cited ‘FAO estimate’ of 25. Crit. Rev. Food Sci. Nutr. 2019, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Groopman, J.D.; Pestka, J.J. Public Health Impacts of Foodborne Mycotoxins. Annu. Rev. Food Sci. Technol. 2014, 5, 351–372. [Google Scholar] [CrossRef] [Green Version]
- Raters, M.; Matissek, R. Thermal stability of aflatoxin B1 and ochratoxin A. Mycotoxin Res. 2008, 24, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Duarte, S.C.; Pena, A.; Lino, C.M. A review on ochratoxin A occurrence and effects of processing of cereal and cereal derived food products. Food Microbiol. 2010, 27, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D. Fungi and mycotoxins in grain: Implications for stored product research. J. Stored Prod. Res. 1995, 31, 1–16. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; Freire, F.; da, C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control. 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Van Egmond, H.P.; Schothorst, R.C.; Jonker, M.A. Regulations relating to mycotoxins in food: Perspectives in a global and European context. Anal. Bioanal. Chem. 2007, 389, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Buzby, J.C. International Trade and Food Safety: Economic Theory and Case Studies. Unites States 609 Department of Agriculture. 2003. Report Number 828. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.201.8435&rep=rep1&type=pdf (accessed on 4 May 2020).
- Grenier, B.; Loureiro-Bracarense, A.-P.; Leslie, J.F.; Oswald, I.P. Physical and Chemical Methods for Mycotoxin Decontamination in Maize. Mycotoxin Reduct. Grain Chains 2014, 116–129. [Google Scholar] [CrossRef]
- Amézqueta, S.; González-Peñas, E.; Murillo-Arbizu, M.; López de Cerain, A. Ochratoxin A decontamination: A review. Food Control. 2009, 20, 326–333. [Google Scholar] [CrossRef]
- Karlovsky, P.; Suman, M.; Berthiller, F.; De Meester, J.; Eisenbrand, G.; Perrin, I.; Oswald, I.P.; Speijers, G.; Chiodini, A.; Recker, T.; et al. Impact of food processing and detoxification treatments on mycotoxin contamination. Mycotoxin Res. 2016, 32, 179–205. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Lescure, G.; Agoulon, A.; Svinareff, P.; Orange, N.; Feuilloley, M. Application of the pulsed light technology to mycotoxin degradation and inactivation. J. Appl. Toxicol. 2013, 33, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Paterson, R.R.M.; Venâncio, A. Biodegradation of Ochratoxin A for Food and Feed Decontamination. Toxins (Basel) 2010, 2, 1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Li, C.; Zhang, B.; Zhou, Z.; Shen, Y.; Liao, X.; Yang, J.; Wang, Y.; Li, X.; Li, Y.; et al. Advances in Biodetoxification of Ochratoxin A-A Review of the Past Five Decades. Front. Microbiol. 2018, 9, 1386. [Google Scholar] [CrossRef]
- El Khoury, R.; Mathieu, F.; Atoui, A.; Kawtharani, H.; El Khoury, A.; Afif, C.; Maroun, R.G.; El Khoury, A. Ability of soil isolated actinobacterial strains to prevent, bind and biodegrade ochratoxin A. Toxins (Basel) 2017, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Liang, Z.; Li, J.; Hao, J.; Xu, Y.; Huang, K.; Tian, J.; He, X.; Xu, W. Ochratoxin A biocontrol and biodegradation by bacillus subtilis CW 14. J. Sci. Food Agric. 2014, 94, 1879–1885. [Google Scholar] [CrossRef]
- Péteri, Z.; Téren, J.; Vágvölgyi, C.; Varga, J. Ochratoxin degradation and adsorption caused by astaxanthin-producing yeasts. Food Microbiol. 2007, 24, 205–210. [Google Scholar] [CrossRef]
- Shetty, P.H.; Jespersen, L. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 2006, 17, 48–55. [Google Scholar] [CrossRef]
- Shapira, R. Control of mycotoxins in storage and techniques for their decontamination. Mycotoxins Food 2004, 190–226. [Google Scholar] [CrossRef]
- Vanhoutte, I.; Audenaert, K.; De Gelder, L. Biodegradation of mycotoxins: Tales from known and unexplored worlds. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Jacquard, N.C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.A.; Strub, C.; Fontana, A.; Schorr-Galindo, S. Crop molds and mycotoxins: Alternative management using biocontrol. Biol. Control 2017, 104, 10–27. [Google Scholar] [CrossRef]
- Rodriguez, H.; Reveron, I.; Doria, F.; Constantini, A.; De Las Rivas, B.; Muñoz, R.; Garcia-Moruno, E. Degradation of ochratoxin a by brevibacterium species. J. Agric. Food Chem. 2011, 59, 10755–10760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cserháti, M.; Kriszt, B.; Krifaton, C.; Szoboszlay, S.; Háhn, J.; Tóth, S.; Nagy, I.; Kukolya, J. Mycotoxin-degradation profile of Rhodococcus strains. Int. J. Food Microbiol. 2013, 166, 176–185. [Google Scholar] [CrossRef]
- Karlovsky, P. Biological Detoxification of Fungal Toxins and its Use in Plant Breeding, Feed and Food Production. Nat Toxins. 1999, 7, 1–23. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Cheng, Z.; Zhou, Z.; Ma, L. High-performance liquid chromatography-tandem mass spectrometry method for simultaneous detection of ochratoxin A and relative metabolites in Aspergillus species and dried vine fruits. Food Addit. Contam.—Part A Chem. Anal. Control. Expo. Risk Assess. 2016, 33, 1355–1366. [Google Scholar] [CrossRef]
- Zhu, Y.; Hassan, Y.I.; Lepp, D.; Shao, S.; Zhou, T. Strategies and Methodologies for Developing Microbial Detoxification Systems to Mitigate Mycotoxins. Toxins (Basel) 2017, 9, 130. [Google Scholar] [CrossRef] [Green Version]
- Patharajan, S.; Reddy, K.R.N.; Karthikeyan, V.; Spadaro, D.; Lore, A.; Gullino, M.L.; Garibaldi, A. Potential of yeast antagonists on invitro biodegradation of ochratoxin A. Food Control 2011, 22, 290–296. [Google Scholar] [CrossRef]
- Bazin, I.; Faucet-Marquis, V.; Monje, M.C.; El Khoury, M.; Marty, J.L.; Pfohl-Leszkowicz, A. Impact of pH on the stability and the cross-reactivity of ochratoxin A and citrinin. Toxins (Basel) 2013, 5, 2324–2340. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Wu, F.; Liu, F.; Wang, Q.; Zhang, X.; Nimal, J.; Zhao, Y.; Xing, F.; Yin, W.-B.; et al. A consensus ochratoxin A biosynthetic pathway: Insights from the genome sequence of Aspergillus ochraceus and a comparative genomic analysis. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mycotoxins and Other Fungal/Bacterial Metabolites from Several Food Matrices by LC/MS/MS (TN-1119) | Phenomenex UHPLC, HPLC, SPE and GC. Available online: https://www.phenomenex.com/ViewDocument/?id=mycotoxins+and+other+fungal_bacterial+metabolites+from+several+food+matrices+(tn-1119) (accessed on 1 April 2020).
- Ostry, V.; Malir, F.; Ruprich, J. Producers and important dietary sources of ochratoxin A and citrinin. Toxins (Basel) 2013, 5, 1574–1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Nimal Selvaraj, J.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A producing fungi, biosynthetic pathway and regulatory mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoury, R.; Choque, E.; El Khoury, A.; Snini, S.P.; Cairns, R.; Andriantsiferana, C.; Mathieu, F. OTA Prevention and Detoxification by Actinobacterial Strains and Activated Carbon Fibers: Preliminary Results. Toxins (Basel) 2018, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoll, D.A.; Link, S.; Kulling, S.; Geisen, R.; Schmidt-Heydt, M. Comparative proteome analysis of Penicillium verrucosum grown under light of short wavelength shows an induction of stress-related proteins associated with modified mycotoxin biosynthesis. Int. J. Food Microbiol. 2014, 175, 20–29. [Google Scholar] [CrossRef]
- Schmidt-Heydt, M.; Stoll, D.; Schütz, P.; Geisen, R. Oxidative stress induces the biosynthesis of citrinin by Penicillium verrucosum at the expense of ochratoxin. Int. J. Food Microbiol. 2015, 192, 1–6. [Google Scholar] [CrossRef]
- Kotzybik, K.; Gräf, V.; Kugler, L.; Stoll, D.A.; Greiner, R.; Geisen, R.; Schmidth-Heydt, M. Influence of different nanomaterials on growth and mycotoxin production of Penicillium verrucosum. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [Green Version]
- Stoll, D.; Schmidt-Heydt, M.; Geisen, R. Differences in the regulation of ochratoxin a by the HOG pathway in Penicillium and Aspergillus in response to high osmolar environments. Toxins (Basel) 2013, 5, 1282–1298. [Google Scholar] [CrossRef] [Green Version]
- Venkatesh, N.; Keller, N.P. Mycotoxins in Conversation With Bacteria and Fungi. Front. Microbiol. 2019, 10, 403. [Google Scholar] [CrossRef]
- Ferrigo, D.; Mondin, M.; Scopel, C.; Dal Maso, E.; Stefenatti, M.; Raiola, A.; Causin, R. Effects of a prothioconazole- and tebuconazole-based fungicide on Aspergillus flavus development under laboratory and field conditions. Eur. J. Plant Pathol. 2019, 155, 151–161. [Google Scholar] [CrossRef]
- Cubaiu, L.; Abbas, H.; Dobson, A.; Budroni, M.; Migheli, Q. A Saccharomyces cerevisiae Wine Strain Inhibits Growth and Decreases Ochratoxin A Biosynthesis by Aspergillus carbonarius and Aspergillus ochraceus. Toxins (Basel) 2012, 4, 1468–1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshinari, T.; Noda, Y.; Yoda, K.; Sezaki, H.; Nagasawa, H.; Sakuda, S. Inhibitory activity of blasticidin A, a strong aflatoxin production inhibitor, on protein synthesis of yeast: Selective inhibition of aflatoxin production by protein synthesis inhibitors. J. Antibiot. (Tokyo) 2010, 63, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Li, Y.; Banakar, S.P.; Liu, L.; Shao, C.; Li, Z.; Wang, C. New Metabolites From the Co-culture of Marine-Derived Actinomycete Streptomyces rochei MB037 and Fungus Rhinocladiella similis 35. Front. Microbiol. 2019, 10, 915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martínez-Buitrago, P.A.; Ramos, F.A.; Castellanos, L. Binary co-culture selection from marine-derived microorganisms for differential production of specialized metabolites. Quim. Nova 2019, 42, 713–719. [Google Scholar] [CrossRef]
- Wakefield, J.; Hassan, H.M.; Jaspars, M.; Ebel, R.; Rateb, M.E. Dual Induction of New Microbial Secondary Metabolites by Fungal Bacterial Co-cultivation. Front. Microbiol. 2017, 8, 1284. [Google Scholar] [CrossRef] [Green Version]
- Marmann, A.; Aly, A.H.; Lin, W.; Wang, B.; Proksch, P. Co-cultivation—A powerful emerging tool for enhancing the chemical diversity of microorganisms. Marine Drugs 2014, 12, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Varga, J.; Rigó, K.; Téren, J. Degradation of ochratoxin A by Aspergillus species. Int. J. Food Microbiol. 2000, 59, 1–7. [Google Scholar] [CrossRef]
- Gama, A.R.; Brito-Cunha, C.C.Q.; Campos, I.T.N.; de Souza, G.R.L.; Carneiro, L.C.; Bataus, L.A.M. Streptomyces thermocerradoensis I3 secretes a novel bifunctional xylanase/endoglucanase under solid-state fermentation. Biotechnol. Prog. 2019, 36, e2934. [Google Scholar] [CrossRef]
- Zhang, H.H.; Wang, Y.; Zhao, C.; Wang, J.; Zhang, X.L. Biodegradation of ochratoxin A by Alcaligenes faecalis isolated from soil. J. Appl. Microbiol. 2017, 123, 661–668. [Google Scholar] [CrossRef]
- Nazari, B.; Saito, A.; Kobayashi, M.; Miyashita, K.; Wang, Y.; Fujii, T. High expression levels of chitinase genes in Streptomyces coelicolor A3(2) grown in soil. FEMS Microbiol. Ecol. 2011, 77, 623–635. [Google Scholar] [CrossRef]
- Bejaoui, H.; Mathieu, F.; Taillandier, P.; Lebrihi, A. Biodegradation of ochratoxin A by Aspergillus section Nigri species isolated from French grapes: A potential means of ochratoxin A decontamination in grape juices and musts. FEMS Microbiol. Lett. 2006, 255, 203–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautbergue, T.; Puel, O.; Tadrist, S.; Meneghetti, L.; Péan, M.; Delaforge, M.; Debrauwer, L.; Oswald, I.P.; Jamin, E.L. Evidencing 98 secondary metabolites of Penicillium verrucosum using substrate isotopic labeling and high-resolution mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1071, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, J.; Zhang, H.; Li, C.; Zhang, X. Ochratoxin A is degraded by Yarrowia lipolytica and generates non-toxic degradation products. World Mycotoxin, J. 2016, 9, 269–278. [Google Scholar] [CrossRef]
- Yang, Z.; Xue, K.S.; Sun, X.; Tang, L.; Wang, J.-S. Multi-Toxic Endpoints of the Foodborne Mycotoxins in Nematode Caenorhabditis elegans. Toxins (Basel) 2015, 7, 5224–5235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, M.; Gonzalez, N.; Mintz, K.; Jaja-Chimedza, A.; Lawrence De Jesus, C.; Lydon, C.; Welch, A.Z.; Berry, J.P. Teratogenicity of Ochratoxin A and the Degradation Product, Ochratoxin α, in the Zebrafish (Danio rerio) Embryo Model of Vertebrate Development. Toxins (Basel) 2016, 8, 40. [Google Scholar] [CrossRef] [Green Version]
- Harris, J.P.; Mantle, P.G. Biosynthesis of ochratoxins by Aspergillus ochraceus. Phytochemistry 2001, 58, 709–716. [Google Scholar] [CrossRef]







| Cluster | Specific features | Subcluster | Specific Features | |
|---|---|---|---|---|
| I | Limited effect on P. verrucosum growth (dual culture and CFEs) | A | - | Highest increase in OTA specific production (dual culture) Two strains degrading OTA in liquid medium (IX56, IX42) |
| B | 1 | No or moderate increase in OTA specific production | ||
| 2 | Only cluster with strains able to highly degrade OTA on solid medium Strong degradation capacities in liquid medium | |||
| 3 | High inhibition of OTA specific production (dual culture and CFEs) | |||
| II | Strong effect on P. verrucosum growth (dual culture) High degradation of OTA in liquid medium | A | 1 | Highest inhibition of OTA specific production (dual culture) |
| 2 | Moderate inhibition of OTA specific production (dual culture) | |||
| B | 1 | Strong increase in OTA specific production | ||
| 2 | Moderate increase of OTA specific production (dual culture and CFEs) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos-Avelar, I.; Colas de la Noue, A.; Durand, N.; Fay, B.; Martinez, V.; Fontana, A.; Strub, C.; Schorr-Galindo, S. Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules. Toxins 2020, 12, 296. https://doi.org/10.3390/toxins12050296
Campos-Avelar I, Colas de la Noue A, Durand N, Fay B, Martinez V, Fontana A, Strub C, Schorr-Galindo S. Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules. Toxins. 2020; 12(5):296. https://doi.org/10.3390/toxins12050296
Chicago/Turabian StyleCampos-Avelar, Ixchel, Alexandre Colas de la Noue, Noel Durand, Blandine Fay, Véronique Martinez, Angélique Fontana, Caroline Strub, and Sabine Schorr-Galindo. 2020. "Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules" Toxins 12, no. 5: 296. https://doi.org/10.3390/toxins12050296
APA StyleCampos-Avelar, I., Colas de la Noue, A., Durand, N., Fay, B., Martinez, V., Fontana, A., Strub, C., & Schorr-Galindo, S. (2020). Minimizing Ochratoxin A Contamination through the Use of Actinobacteria and Their Active Molecules. Toxins, 12(5), 296. https://doi.org/10.3390/toxins12050296