Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains
Abstract
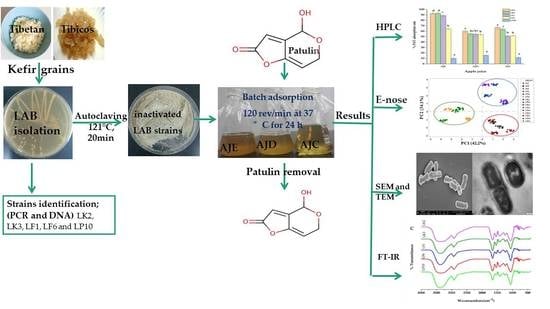
:1. Introduction
2. Results
2.1. Strains Identification
2.2. HPLC Analysis
2.3. The Effect of the Adsorption Process on °Brix and pH
2.4. Juice Aroma Analysis
2.5. Morphological Analysis of Bacterial Cells by SEM and TEM
2.6. FTIR Analysis
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Materials
5.2. Kefir Grains
5.3. Isolation and Enumeration of LABs from Grains
5.4. Identification of Bacteria Strains
5.5. Juice Preparation
5.6. Heat-Inactivated Bacterial Cell Preparation
5.7. Patulin Binding Assay
5.8. Patulin Releasing Assay
5.9. Extraction and Cleanup
5.10. HPLC Analysis
5.11. Characterization of Bacterial Cell Wall
5.12. Juice Quality Specification
5.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ioi, J.D.; Zhou, T.; Tsao, R.; Marcone, M.F. Mitigation of Patulin in fresh and processed foods and beverages. Toxins 2017, 9, 157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef] [Green Version]
- Shephard, G.S.; Leggott, N.L. Chromatographic determination of the mycotoxin patulin in fruit and fruit juices. J. Chromatogr. A 2000, 882, 17–22. [Google Scholar] [CrossRef]
- Stott, W.T.; Bullerman, L.B. Patulin: A Mycotoxin of Potential Concern in Foods. J. Milk Food Technol. 1975, 38, 695–705. [Google Scholar] [CrossRef]
- Ciegler, A.; Detroy, R.W.; Lillehoj, E.B. Patulin, Penicillic Acid, and other Carcinogenic Lactones; Academic Press: New York, NY, USA, 1966; Volume 6, pp. 409–434. [Google Scholar]
- Siddiquee, S. Recent Advancements on the role of biologically active secondary metabolites from Aspergillus. In New and Future Developments in Microbial Biotechnology and Bioengineering: Penicillium System Properties and Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 69–94. [Google Scholar]
- Ülger, T.G.; Uçar, A.; Çakıroğlu, F.P.; Yilmaz, S. Genotoxic effects of mycotoxins. Toxicon 2020, 185, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Puel, O.; Galtier, P.; Oswald, I.P. Biosynthesis and toxicological effects of patulin. Toxins 2010, 2, 613–631. [Google Scholar] [CrossRef] [Green Version]
- Saleh, I.; Goktepe, I. The characteristics, occurrence, and toxicological effects of patulin. Food Chem. Toxicol. 2019, 129, 301–311. [Google Scholar] [CrossRef]
- Guerra-Moreno, A.; Hanna, J. Induction of proteotoxic stress by the mycotoxin patulin. Toxicol. Lett. 2017, 276, 85–91. [Google Scholar] [CrossRef]
- Lee, H.S.; Daniels, B.H.; Salas, E.; Bollen, A.W.; Debnath, J.; Margeta, M. Clinical utility of LC3 and p62 immunohistochemistry in diagnosis of drug-induced autophagic vacuolar myopathies: A case-control study. PLoS ONE 2012, 7, e36221. [Google Scholar] [CrossRef] [PubMed]
- Jha, S.N. Rapid Detection of Food Adulterants and Contaminants; Academic Press: London, UK, 2015. [Google Scholar]
- Yue, T.; Guo, C.; Yuan, Y.; Wang, Z.; Luo, Y.; Wang, L. Adsorptive removal of patulin from apple juice using ca-alginate-activated carbon beads. J. Food Sci. 2013, 78, T1629–T1635. [Google Scholar] [CrossRef]
- Zheng, X.; Wei, W.; Rao, S.; Gao, L.; Li, H.; Yang, Z. Degradation of patulin in fruit juice by a lactic acid bacteria strain Lactobacillus casei YZU01. Food Control 2020, 112, 107147. [Google Scholar] [CrossRef]
- Kozlovskii, A.G.; Zhelifonova, V.P.; Antipova, T.V. Fungi of the genus Penicillium as producers of physiologically active compounds (Review). Appl. Biochem. Microbiol. 2013, 49, 1–10. [Google Scholar] [CrossRef]
- Commission, C.A. Code of Practice for the Prevention and Reduction of Patulin Contamination in Apple Juice and Apple Juice Ingredients in other Beverages. Codex Aliment. 2003, 50, 1–8. [Google Scholar]
- Sant’Ana, A.S.; Simas, R.C.; Almeida, C.A.A.; Cabral, E.C.; Rauber, R.H.; Mallmann, C.A.; Eberlin, M.N.; Rosenthal, A.; Massaguer, P.R. Influence of package, type of apple juice and temperature on the production of patulin by Byssochlamys nivea and Byssochlamys fulva. Int. J. Food Microbiol. 2010, 142, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Moake, M.M.; Padilla-Zakour, O.I.; Worobo, R.W. Comprehensive review of patulin control methods in foods. Compr. Rev. Food Sci. Food Saf. 2005, 4, 8–21. [Google Scholar] [CrossRef]
- De Clercq, N.; Vlaemynck, G.; Van Pamel, E.; Colman, D.; Heyndrickx, M.; Van Hove, F.; De Meulenaer, B.; Devlieghere, F.; Van Coillie, E. Patulin production by Penicillium expansum isolates from apples during different steps of long-term storage. World Mycotoxin J. 2016, 9, 379–388. [Google Scholar] [CrossRef]
- Chen, H.; Cheng, Z.; Wisniewski, M.; Liu, Y.; Liu, J. Ecofriendly hot water treatment reduces postharvest decay and elicits defense response in kiwifruit. Environ. Sci. Pollut. Res. 2015, 22, 15037–15045. [Google Scholar] [CrossRef]
- Tannous, J.; Atoui, A.; El Khoury, A.; Francis, Z.; Oswald, I.P.; Puel, O.; Lteif, R. A study on the physicochemical parameters for Penicillium expansum growth and patulin production: Effect of temperature, pH, and water activity. Food Sci. Nutr. 2016, 4, 611–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Egmond, H.P. Aflatoxin M1: Occurrence, toxicity, regulation. In Mycotoxins in Dairy Products; Elsevier: London, UK, 1989; pp. 11–55. [Google Scholar]
- Yuan, Y.; Zhuang, H.; Zhang, T.; Liu, J. Patulin content in apple products marketed in Northeast China. Food Control 2010, 21, 1488–1491. [Google Scholar] [CrossRef]
- Sajid, M.; Mehmood, S.; Yuan, Y.; Yue, T. Mycotoxin patulin in food matrices: Occurrence and its biological degradation strategies. Drug Metab. Rev. 2019, 51, 105–120. [Google Scholar]
- Brackett, R.E.; Marth, E.H. Ascorbic Acid and Ascorbate Cause Disappearance of Patulin from Buffer Solutions and Apple Juice. J. Food Prot. 1979, 42, 864–866. [Google Scholar] [CrossRef]
- Dong, Q.; Manns, D.C.; Feng, G.; Yue, T.; Churey, J.J.; Worobo, R.W. Reduction of patulin in apple cider by UV radiation. J. Food Prot. 2010, 73, 69–74. [Google Scholar] [CrossRef]
- Koutchma, T. Ultraviolet Light for Destruction of Mycotoxins and Chemicals. In Reference Module in Food Science; Elsevier: London, UK, 2016. [Google Scholar]
- Diao, E.; Hou, H.; Hu, W.; Dong, H.; Li, X. Removing and detoxifying methods of patulin: A review. Trends Food Sci. Technol. 2018, 81, 139–145. [Google Scholar] [CrossRef]
- Frémy, J.M.; Castegnaro, M.J.J.; Gleizes, E.; De Meo, M.; Laget, M. Procedures for destruction of patulin in laboratory wastes. Food Addit. Contam. 1995, 12, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wei, W.; Zhou, W.; Li, H.; Rao, S.; Gao, L.; Yang, Z. Prevention and detoxification of patulin in apple and its products: A review. Food Res. Int. 2021, 140, 110034. [Google Scholar] [CrossRef]
- Yazici, S.; Velioglu, Y.S. Effect of thiamine hydrochloride, pyridoxine hydrochloride and calcium-d-pantothenate on the patulin content of apple juice concentrate. Nahr. Food 2002, 46, 256–257. [Google Scholar] [CrossRef]
- Taheur, F.B.; Kouidhi, B.; Al Qurashi, Y.M.A.; Salah-Abbès, J.B.; Chaieb, K. Review: Biotechnology of mycotoxins detoxification using microorganisms and enzymes. Toxicon 2019, 160, 12–22. [Google Scholar] [CrossRef]
- Tabatabaie, F.; Mortazavi, S.A.; Tabatabaee, F.; Ebadi, A.G. Reduction of patulin in apple juice after treatment with SO2 and heat. Indian J. Sci. Technol. 2010, 3, 596–597. [Google Scholar] [CrossRef]
- Zhang, H.; Dong, M.; Yang, Q.; Apaliya, M.T.; Li, J.; Zhang, X. Biodegradation of zearalenone by Saccharomyces cerevisiae: Possible involvement of ZEN responsive proteins of the yeast. J. Proteomics 2016, 143, 416–423. [Google Scholar] [CrossRef]
- Sajid, M.; Mehmood, S.; Niu, C.; Yuan, Y.; Yue, T. Effective adsorption of patulin from apple juice by using non-cytotoxic heat-inactivated cells and spores of Alicyclobacillus strains. Toxins 2018, 10, 344. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yuan, Y.; Yue, T.; Hatab, S.; Wang, Z. Binding mechanism of patulin to heat-treated yeast cell. Lett. Appl. Microbiol. 2012, 55, 453–459. [Google Scholar] [CrossRef]
- Hatab, S.; Yue, T.; Mohamad, O. Removal of patulin from apple juice using inactivated lactic acid bacteria. J. Appl. Microbiol. 2012, 112, 892–899. [Google Scholar] [CrossRef]
- Hatab, S.; Yue, T.; Mohamad, O. Reduction of Patulin in Aqueous Solution by Lactic Acid Bacteria. J. Food Sci. 2012, 77, M238–M241. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Li, C.; Liu, L.; Tan, Y.; Meng, Y. The ability of Lactobacillus rhamnosus to bind patulin and its application in apple juice. Acta Aliment. 2020, 49, 93–102. [Google Scholar] [CrossRef]
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S.; Attar, H. Patulin removal from synbiotic apple juice using Lactobacillus plantarum ATCC 8014. J. Appl. Microbiol. 2019, 126, 1149–1160. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, X.; Hatab, S.; Wang, Z.; Wang, Y.; Luo, Y.; Yue, T. Patulin reduction in apple juice by inactivated Alicyclobacillus spp. Lett. Appl. Microbiol. 2014, 59, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Gu, F.; He, J.; Xiao, J.; Chen, Q.; Ruan, H.; He, G. Metagenome analysis of bacterial diversity in Tibetan kefir grains. Eur. Food Res. Technol. 2013, 236, 549–556. [Google Scholar] [CrossRef]
- Taheur, F.B.; Fedhila, K.; Chaieb, K.; Kouidhi, B.; Bakhrouf, A.; Abrunhosa, L. Adsorption of aflatoxin B1, zearalenone and ochratoxin A by microorganisms isolated from Kefir grains. Int. J. Food Microbiol. 2017, 251, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Garofalo, C.; Osimani, A.; Milanović, V.; Aquilanti, L.; De Filippis, F.; Stellato, G.; Di Mauro, S.; Turchetti, B.; Buzzini, P.; Ercolini, D.; et al. Bacteria and yeast microbiota in milk kefir grains from different Italian regions. Food Microbiol. 2015, 49, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Inês, A.; Rodrigues, A.I.; Guimarães, A.; Pereira, V.L.; Parpot, P.; Mendes-Faia, A.; Venâncio, A. Biodegradation of ochratoxin A by Pediococcus parvulus isolated from Douro wines. Int. J. Food Microbiol. 2014, 188, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Elsanhoty, R.M.; Ramadan, M.F.; El-Gohery, S.S.; Abol-Ela, M.F.; Azeke, M.A. Ability of selected microorganisms for removing aflatoxins invitro and fate of aflatoxins in contaminated wheat during baladi bread baking. Food Control 2013, 33, 287–292. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, J.; Jia, Y.; Pan, Y.; Wang, Y. Lactobacillus kefiranofaciens, the sole dominant and stable bacterial species, exhibits distinct morphotypes upon colonization in Tibetan kefir grains. Heliyon 2018, 4, e00649. [Google Scholar] [CrossRef]
- Korsak, N.; Taminiau, B.; Leclercq, M.; Nezer, C.; Crevecoeur, S.; Ferauche, C.; Detry, E.; Delcenserie, V.; Daube, G. Short communication: Evaluation of the microbiota of kefir samples using metagenetic analysis targeting the 16S and 26S ribosomal DNA fragments. J. Dairy Sci. 2015, 98, 3684–3689. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Wang, X.; Sun, G.; Qin, B.; Xiao, J.; Yan, S.; Pan, Y.; Wang, Y. Fine structure of Tibetan kefir grains and their yeast distribution, diversity, and shift. PLoS ONE 2014, 9, 0101387. [Google Scholar]
- Angelescu, I.R.; Zamfir, M.; Stancu, M.M.; Grosu-Tudor, S.S. Identification and probiotic properties of lactobacilli isolated from two different fermented beverages. Ann. Microbiol. 2019, 69, 1557–1565. [Google Scholar]
- Alexandraki, V.; Kazou, M.; Angelopoulou, A.; Arena, M.P.; Capozzi, V.; Russo, P.; Fiocco, D.; Spano, G.; Papadimitriou, K.; Tsakalidou, E. The Microbiota of Non-cow Milk and Products. In Non-Bovine Milk and Milk Products; Academic Press: London, UK, 2016; pp. 117–159. [Google Scholar]
- Guo, C.; Guo, M.; Zhang, S.; Qin, D.; Yang, Y.; Li, M. Assessment of patulin adsorption efficacy from aqueous solution by water-insoluble corn flour. J. Food Saf. 2018, 38, e12397. [Google Scholar]
- Leggott, N.L.; Vismer, H.F.; Sydenham, E.W.; Shephard, G.S.; Rheeder, J.P.; Marasas, W.F.O. Occurrence of patulin in the commercial processing of apple juice. S. Afr. J. Sci. 2000, 96, 241–243. [Google Scholar]
- Wang, L.; Yue, T.; Yuan, Y.; Wang, Z.; Ye, M.; Cai, R. A new insight into the adsorption mechanism of patulin by the heat-inactive lactic acid bacteria cells. Food Control 2015, 50, 104–110. [Google Scholar]
- Wang, L.; Wang, Z.; Yuan, Y.; Cai, R.; Niu, C.; Yue, T. Identification of key factors involved in the biosorption of patulin by inactivated lactic acid bacteria (LAB) cells. PLoS ONE 2015, 10, e0143431. [Google Scholar]
- Haddi, Z.; Alami, H.; El Bari, N.; Tounsi, M.; Barhoumi, H.; Maaref, A.; Jaffrezic-Renault, N.; Bouchikhi, B. Electronic nose and tongue combination for improved classification of Moroccan virgin olive oil profiles. Food Res. Int. 2013, 54, 1488–1498. [Google Scholar] [CrossRef]
- Aith Barbará, J.; Primieri Nicolli, K.; Souza-Silva, É.A.; Camarão Telles Biasoto, A.; Welke, J.E.; Alcaraz Zini, C. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef]
- Averbeck, M.; Schieberle, P.H. Characterisation of the key aroma compounds in a freshly reconstituted orange juice from concentrate. Eur. Food Res. Technol. 2009, 229, 611–622. [Google Scholar] [CrossRef]
- Biasoto, A.C.T.; de Sampaio, K.L.; Marques, E.J.N.; da Silva, M.A.A.P. Dynamics of the loss and emergence of volatile compounds during the concentration of cashew apple juice (Anacardium occidentale L.) and the impact on juice sensory quality. Food Res. Int. 2015, 69, 224–234. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Yue, T.; Hatab, S.; Yuan, Y. Ability of inactivated yeast powder to adsorb patulin from apple juice. J. Food Prot. 2012, 75, 585–590. [Google Scholar] [CrossRef]
- Topcu, A.; Bulat, T.; Wishah, R.; Boyacı, I.H. Detoxification of aflatoxin B1 and patulin by Enterococcus faecium strains. Int. J. Food Microbiol. 2010, 139, 202–205. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, A.; Garcia, H.S.; Steele, J.L. Screening of Lactobacillus casei strains for their ability to bind aflatoxin B1. Food Chem. Toxicol. 2009, 47, 1064–1068. [Google Scholar] [CrossRef]
- Pierides, M.; El-Nezami, H.; Peltonen, K.; Salminen, S.; Ahokas, J. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Prot. 2000, 63, 645–650. [Google Scholar] [CrossRef]
- Wei, C.; Yu, L.; Qiao, N.; Wang, S.; Tian, F.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. The characteristics of patulin detoxification by Lactobacillus plantarum 13M5. Food Chem. Toxicol. 2020, 146, 111787. [Google Scholar] [CrossRef]
- Fuchs, S.; Sontag, G.; Stidl, R.; Ehrlich, V.; Kundi, M.; Knasmüller, S. Detoxification of patulin and ochratoxin A, two abundant mycotoxins, by lactic acid bacteria. Food Chem. Toxicol. 2008, 46, 1398–1407. [Google Scholar] [CrossRef]
- Zanirati, D.F.; Abatemarco, M., Jr.; de Cicco Sandes, S.H.; Nicoli, J.R.; Nunes, Á.C.; Neumann, E. Selection of lactic acid bacteria from Brazilian kefir grains for potential use as starter or probiotic cultures. Anaerobe 2015, 32, 70–76. [Google Scholar] [CrossRef]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germanà, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, H.; Li, X.; Zhang, Q. Determination of trace patulin in apple-based food matrices. Food Chem. 2017, 233, 290–301. [Google Scholar] [CrossRef]
- Liu, M.; Wang, J.; Wang, X.; Zhu, W.; Yao, X.; Su, L.; Sun, J.; Yue, T.; Wang, J. Highly efficient and cost-effective removal of patulin from apple juice by surface engineering of diatomite with sulfur-functionalized graphene oxide. Food Chem. 2019, 300, 125111. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Qi, Y.; Zhang, J.; Liu, M.; Wei, X.; Fan, M. Effect of reduced glutathione on the quality characteristics of apple wine during alcoholic fermentation. Food Chem. 2019, 300, 125130. [Google Scholar] [CrossRef]







| Strains | Bacteria Height (nm) | Bacteria Diameter (nm) | Bacteria Thickness (nm) |
|---|---|---|---|
| LK2 | 1935.45 ± 19.36 bc | 885.96 ± 16.73 c | 45.39 ± 4.76 a |
| LK3 | 2381.66 ± 106.4 d | 831.33 ± 94.36 bc | 47.65 ± 6.18 a |
| LF1 | 2125.54 ± 320.92 cd | 701.49 ± 34.13 a | 49.45 ± 7.35 a |
| LF6 | 1814.23 ± 2.97 ab | 754.31 ± 35.37 ab | 44.02 ± 1.39 a |
| LP10 | 1552.78 ± 138 a | 717.44 ± 36.87 a | 42.73 ± 1.6 a |
| Functional Groups | Wavenumber (cm−1) | ||||
|---|---|---|---|---|---|
| LK2 | LK3 | LF1 | LF6 | LP10 | |
| O-H/N-H stretching | (3325.29567) 3377.374 | (3369.65866) 3387.0181 | (3363.8721) 3371.5875 | (3367.7298) 3371.5875 | (3371.5874) 3383.1604 |
| C-H stretch | (2964.60525) * | (2960.74759) * | (2964.60525) * | (2960.74759) * | (*) * |
| C-H stretch | (2929.88638) 2935.6729 | (2929.88638) 2937.6017 | (2929.88638) 2929.8864 | (2926.02873) 2935.6729 | (2926.02873) 2931.8152 |
| C=O amide 1 | (1658.7902) 1653.00371 | (1656.8614) 1653.0037 | (1658.7902) 1654.9325 | (1651.07488) 1656.8614 | (1645.2884) 1653.0037 |
| N-H amide 2 | (1537.27416) 1539.203 | (1537.27416) 1537.2742 | (1531.4877) 1539.203 | (1539.20298) 1537.2742 | (1535.34533) 1541.1318 |
| N-O asymmetric stretch | (*) * | (*) 1448.5482 | (*) * | (*) * | (*) * |
| O-H deformation | (1456.26348) 1411.9005 | (1450.477) 1408.0428 | (1452.4058) 1409.9717 | (1454.33465) 1409.9717 | (1454.33465) 1406.114 |
| C-C stretch | (1382.96809) * | (1394.54105) * | (1394.54105) * | (1398.3987) * | (1396.46988) * |
| C-N amide 3 | (1238.30616) 1240.235 | (1236.37733) 1242.1638 | (1234.44851) 1236.3773 | (1236.3773) 1236.3773 | (1238.3061) 1234.44 |
| C-O polysaccharides | (1064.71184) 1062.783 | (1064.71184) 1062.783 | (1060.85419) 1062.783 | (1062.78301) 1060.8542 | (1060.85419) 1058.9254 |
| O-H bend | (*) 923.9076 | (*) 920.0499 | (*) 923.9076 | (*) 921.9787 | (*) 918.1211 |
| O-H bend | (*) 869.9004 | (*) 875.6869 | (*) 871.8293 | (*) 864.114 | (*) 862.18513 |
| C-Cl stretch | (*) 815.8933 | (*) 819.751 | (*) 819.751 | (*) 819.751 | (*) 821.6798 |
| C-Cl stretch | (*) 773.4591 | (*) 775.388 | (*) 775.388 | (*) 779.2456 | (*) 773.459144 |
| C-Br Alkyl Halide | (*) 704.0214 | (*) 707.8791 | (*) 709.8079 | (*) 704.0214 | (*) 704.021415 |
| C-Br Alkyl Halide | (*) 630.726 | (*) 623.0107 | (*) 630.726 | (*) 630.726 | (*) 628.7972 |
| C-Br Alkyl Halide | (592.149519) * | (570.932435) * | (599.864822) * | (582.50539) * | (580.576564) * |
| C-Br Alkyl Halide | (*) 516.9253 | (*) * | (*) * | (*) 516.9253 | (*) 516.9253 |
| C-I Alkyl Halide | (*) 451.3452 | (*) 455.2029 | (*) 455.2029 | (*) 449.4164 | (*) 455.2029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahati, P.; Zeng, X.; Uzizerimana, F.; Tsoggerel, A.; Awais, M.; Qi, G.; Cai, R.; Yue, T.; Yuan, Y. Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains. Toxins 2021, 13, 434. https://doi.org/10.3390/toxins13070434
Bahati P, Zeng X, Uzizerimana F, Tsoggerel A, Awais M, Qi G, Cai R, Yue T, Yuan Y. Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains. Toxins. 2021; 13(7):434. https://doi.org/10.3390/toxins13070434
Chicago/Turabian StyleBahati, Pascaline, Xuejun Zeng, Ferdinand Uzizerimana, Ariunsaikhan Tsoggerel, Muhammad Awais, Guo Qi, Rui Cai, Tianli Yue, and Yahong Yuan. 2021. "Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains" Toxins 13, no. 7: 434. https://doi.org/10.3390/toxins13070434
APA StyleBahati, P., Zeng, X., Uzizerimana, F., Tsoggerel, A., Awais, M., Qi, G., Cai, R., Yue, T., & Yuan, Y. (2021). Adsorption Mechanism of Patulin from Apple Juice by Inactivated Lactic Acid Bacteria Isolated from Kefir Grains. Toxins, 13(7), 434. https://doi.org/10.3390/toxins13070434