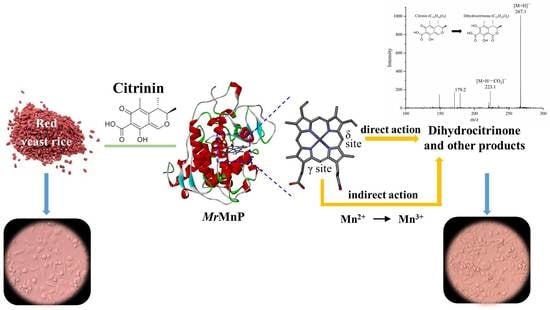
Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of the Buffer Used on CIT Degradation by MrMnP
2.2. Effect of Mn2+ on CIT Degradation by MrMnP
2.3. CIT Was Detoxified after MrMnP Treatment
2.4. Structural Analysis of the Degradation Products
2.5. Removal of CIT in Red Yeast Rice
3. Conclusions
4. Materials and Methods
4.1. Strains and Plasmids
4.2. Expression and Purification of MrMnP
4.3. Degradation of CIT by MrMnP
4.4. Effect of the Buffer Systems on Degradation of CIT by MrMnP
4.5. Effect of Mn2+ on Degradation of CIT
4.6. Toxicity Assay
4.7. HPLC and LC-MS/MS Analyses
4.8. Determining CIT Degradation by MrMnP in Red Yeast Rice
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CIT | Citrinin |
| MnP | Manganese peroxidases |
| ABTS | 2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
| MTT | 3-(4-dimethylthiazolyl-2-yl)-2-diphenyltetrazolium |
| H2O2 | Hydrogen peroxide |
| MES | 2-Morpholinoethanesulphonic acid |
| HEPES | 2-[4-(2-Hydroxyethyl)-1-piperazinyl] ethanesulfonic acid |
References
- He, Y.; Cox, R.J. The molecular steps of citrinin biosynthesis in fungi. Chem. Sci. 2016, 7, 2119–2127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, Y.; Iguchi, H.; Kamisuki, S.; Sugawara, F.; Furuichi, T.; Shinoda, Y. Low doses of the mycotoxin citrinin protect cortical neurons against glutamate-induced excitotoxicity. J. Toxicol. Sci. 2016, 41, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Corte, B.L. Underexplored opportunities for natural products in drug discovery. J. Med. Chem. 2016, 59, 9295–9304. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.H.; Li, X.H.; Xu, Y.; Xu, Y.; Pan, Z.N.; Sun, S.C. Citrinin exposure disrupts organelle distribution and functions in mouse oocytes. Environ. Res. 2020, 185, 109476. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.W.G.; Islam, M.T.; Ali, E.S.; Uddin, S.J.; Santos, J.V.O.; de Alencar, M.; Junior, A.L.G.; Paz, M.; de Brito, M.; JMC, E.S.; et al. A comprehensive review on biological properties of citrinin. Food Chem. Toxicol. 2017, 110, 130–141. [Google Scholar] [CrossRef]
- European Commission. Commission Regulation (EU) 2019/1901 of 7 November 2019 amending Regulation (EC) No 1881/2006 as regards maximum levels of citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Off. J. Eur. Union 2019, 62, 2–4. [Google Scholar]
- European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union 2006, 364, 5–24. [Google Scholar]
- Silva, L.J.G.; Pereira, A.; Pena, A.; Lino, C.M. Citrinin in foods and supplements: A review of occurrence and analytical methodologies. Foods 2020, 10, 14. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; di Mavungu, J.D.; Huybrechts, B.; Tangni, E.K.; Devreese, M.; Croubels, S.; De Saeger, S. Development and validation of an LC-MS/MS method for the simultaneous determination of citrinin and ochratoxin a in a variety of feed and foodstuffs. J. Chromatogr. A 2018, 1580, 100–109. [Google Scholar] [CrossRef]
- Kononenko, G.P.; Burkin, A.A. A survey on the occurrence of citrinin in feeds and their ingredients in Russia. Mycotoxin Res. 2008, 24, 3–6. [Google Scholar] [CrossRef]
- Meerpoel, C.; Vidal, A.; Tangni, E.K.; Huybrechts, B.; Couck, L.; De Rycke, R.; De Bels, L.; De Saeger, S.; Van den Broeck, W.; Devreese, M.; et al. A study of carry-over and histopathological effects after chronic dietary intake of citrinin in pigs, broiler chickens and laying hens. Toxins 2020, 12, 719. [Google Scholar] [CrossRef]
- BenáTrivedi, A. Formation of a new toxic compound, citrinin H1, from citrinin on mild heating in water. J. Chem. Soc. Perkin Trans. 1 1993, 18, 2167–2171. [Google Scholar]
- Magro, M.; Moritz, D.E.; Bonaiuto, E.; Baratella, D.; Terzo, M.; Jakubec, P.; Malina, O.; Cepe, K.; Aragao, G.M.F.; Zboril, R.; et al. Citrinin mycotoxin recognition and removal by naked magnetic nanoparticles. Food Chem. 2016, 203, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.L.; Chen, W.P.; Wang, J.J.; Pan, T.M. A simple and rapid approach for removing citrinin while retaining monacolin K in red mold rice. J. Agric. Food Chem. 2007, 55, 11101–11108. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, L.; Wang, Y.; Gao, H.; Li, X.; Huang, X.; Huang, T. Effects of rutin and its derivatives on citrinin production by Monascus aurantiacus Li AS3.4384 in liquid fermentation using different types of media. Food Chem. 2019, 284, 205–212. [Google Scholar] [CrossRef]
- Wang, K.; Lin, Z.; Zhang, H.; Zhang, X.; Zheng, X.; Zhao, L.; Yang, Q.; Ahima, J.; Boateng, N.A.S. Investigating proteome and transcriptome response of Cryptococcus podzolicus Y3 to citrinin and the mechanisms involved in its degradation. Food Chem. 2019, 283, 345–352. [Google Scholar] [CrossRef]
- Kanpiengjai, A.; Mahawan, R.; Lumyong, S.; Khanongnuch, C. A soil bacterium Rhizobium borbori and its potential for citrinin-degrading application. Ann. Microbiol. 2015, 66, 807–816. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Sheu, S.-C.; Mau, J.-L.; Hsieh, P.-C. Isolation and characterization of a strain of Klebsiella pneumoniae with citrinin-degrading activity. World J. Microbiol. Biotechnol. 2010, 27, 487–493. [Google Scholar] [CrossRef]
- Roy, B.P.; Paice, M.G.; Archibald, F.S.; Misra, S.K.; Misiak, L.E. Creation of metal-complexing agents, reduction of manganese dioxide, and promotion of manganese peroxidase-mediated Mn(III) production by cellobiose:quinone oxidoreductase from Trametes versicolor. J. Biol. Chem. 1994, 269, 19745–19750. [Google Scholar] [CrossRef]
- Wang, X.; Qin, X.; Hao, Z.; Luo, H.; Yao, B.; Su, X. Degradation of four major mycotoxins by eight manganese peroxidases in presence of a dicarboxylic acid. Toxins 2019, 11, 566. [Google Scholar] [CrossRef] [Green Version]
- Bronikowski, A.; Koschorreck, K.; Urlacher, V.B. Redesign of a New Manganese Peroxidase Highly Expressed in Pichia pastoris towards a Lignin-Degrading Versatile Peroxidase. Chembiochem 2018, 19, 2481–2489. [Google Scholar] [CrossRef]
- Wang, S.; Wang, X.; Penttinen, L.; Luo, H.; Zhang, Y.; Liu, B.; Yao, B.; Hakulinen, N.; Zhang, W.; Su, X. Patulin detoxification by recombinant manganese peroxidase from Moniliophthora roreri expressed by Pichia pastoris. Toxins 2022, 14, 440. [Google Scholar] [CrossRef]
- Gumiero, A.; Murphy, E.J.; Metcalfe, C.L.; Moody, P.C.; Raven, E.L. An analysis of substrate binding interactions in the heme peroxidase enzymes: A structural perspective. Arch. Biochem. Biophys. 2010, 500, 13–20. [Google Scholar] [CrossRef]
- Smith, M.C.; Gheux, A.; Coton, M.; Madec, S.; Hymery, N.; Coton, E. In vitro co-culture models to evaluate acute cytotoxicity of individual and combined mycotoxin exposures on Caco-2, THP-1 and HepaRG human cell lines. Chem. Biol. Interact. 2018, 281, 51–59. [Google Scholar] [CrossRef]
- Romero, A.; Ares, I.; Ramos, E.; Castellano, V.; Martinez, M.; Martinez-Larranaga, M.R.; Anadon, A.; Martinez, M.A. Mycotoxins modify the barrier function of Caco-2 cells through differential gene expression of specific claudin isoforms: Protective effect of illite mineral clay. Toxicology 2016, 353–354, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.Y.; Turner, P.C.; El-Nezami, H. Individual and combined cytotoxic effects of Fusarium toxins (deoxynivalenol, nivalenol, zearalenone and fumonisins B1) on swine jejunal epithelial cells. Food Chem. Toxicol. 2013, 57, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Malysheva, S.V.; Di Mavungu, J.D.; Boonen, J.; De Spiegeleer, B.; Goryacheva, I.Y.; Vanhaecke, L.; De Saeger, S. Improved positive electrospray ionization of patulin by adduct formation: Usefulness in liquid chromatography–tandem mass spectrometry multi-mycotoxin analysis. J. Chromatogr. A 2012, 1270, 334–339. [Google Scholar] [CrossRef] [Green Version]
- Gerding, J.; Cramer, B.; Humpf, H.U. Determination of mycotoxin exposure in Germany using an LC-MS/MS multibiomarker approach. Mol. Nutr. Food Res. 2014, 58, 2358–2368. [Google Scholar] [CrossRef] [PubMed]
- Patterson, M.F.; Damoglou, A.P. Conversion of the mycotoxin citrinin into dihydrocitrinone and ochratoxin A by Penicillium viridicatum. Appl. Microbiol. Biotechnol. 1987, 26, 574–578. [Google Scholar] [CrossRef]
- Foellmann, W.; Behm, C.; Degen, G. Metabolic conversion of the mycotoxin citrinin to dihydrocitrinone: Impact on toxicity. In Proceedings of the Naunyn-Schmiedebergs Archives of Pharmacology, Halle/Saale, Germany, 5–7 March 2013; p. S22. [Google Scholar]
- Follmann, W.; Behm, C.; Degen, G.H. Toxicity of the mycotoxin citrinin and its metabolite dihydrocitrinone and of mixtures of citrinin and ochratoxin A in vitro. Arch. Toxicol. 2014, 88, 1097–1107. [Google Scholar] [CrossRef]
- Bergmann, D.; Hubner, F.; Wibbeling, B.; Daniliuc, C.; Cramer, B.; Humpf, H.U. Large-scale total synthesis of (13)C3-labeled citrinin and its metabolite dihydrocitrinone. Mycotoxin Res. 2018, 34, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Kapich, A.N.; Korneichik, T.V.; Hatakka, A.; Hammel, K.E. Oxidizability of unsaturated fatty acids and of a non-phenolic lignin structure in the manganese peroxidase-dependent lipid peroxidation system. Enzyme Microb. Technol. 2010, 46, 136–140. [Google Scholar] [CrossRef]
- Kong, W.; Chen, H.; Lyu, S.; Ma, F.; Yu, H.; Zhang, X. Characterization of a novel manganese peroxidase from white-rot fungus Echinodontium taxodii 2538, and its use for the degradation of lignin-related compounds. Process Biochem. 2016, 51, 1776–1783. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Su, X. Linking enzymatic oxidative degradation of lignin to organics detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crestini, C.; D’Annibale, A.; Sermanni, G.G.; Saladino, R. The reactivity of phenolic and non-phenolic residual kraft lignin model compounds with Mn (II)-peroxidase from Lentinula edodes. Bioorg. Med. Chem. 2000, 8, 433–438. [Google Scholar] [CrossRef]
- Stojanović, M.; Lopičić, Z.; Milojković, J.; Lačnjevac, Č.; Mihajlović, M.; Petrović, M.; Kostić, A. Biomass waste material as potential adsorbent for sequestering pollutants. Zaštita Mater. 2012, 53, 231–237. [Google Scholar]
- Twaruzek, M.; Altyn, I.; Kosicki, R. Dietary supplements based on red yeast rice-A source of citrinin? Toxins 2021, 13, 497. [Google Scholar] [CrossRef]
- Qin, X.; Sun, X.; Huang, H.; Bai, Y.; Wang, Y.; Luo, H.; Yao, B.; Zhang, X.; Su, X. Oxidation of a non-phenolic lignin model compound by two Irpex lacteus manganese peroxidases: Evidence for implication of carboxylate and radicals. Biotechnol. Biofuels 2017, 10, 103. [Google Scholar] [CrossRef] [Green Version]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival: Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Wang, X.; Huang, H.; Tu, T.; Luo, H.; Zhang, Y.; Liu, B.; Yao, B.; Zhang, W.; Su, X. Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri. Toxins 2022, 14, 801. https://doi.org/10.3390/toxins14110801
Wang S, Wang X, Huang H, Tu T, Luo H, Zhang Y, Liu B, Yao B, Zhang W, Su X. Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri. Toxins. 2022; 14(11):801. https://doi.org/10.3390/toxins14110801
Chicago/Turabian StyleWang, Shuai, Xiaolu Wang, Huoqing Huang, Tao Tu, Huiying Luo, Yuhong Zhang, Bo Liu, Bin Yao, Wei Zhang, and Xiaoyun Su. 2022. "Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri" Toxins 14, no. 11: 801. https://doi.org/10.3390/toxins14110801
APA StyleWang, S., Wang, X., Huang, H., Tu, T., Luo, H., Zhang, Y., Liu, B., Yao, B., Zhang, W., & Su, X. (2022). Detoxification of the Mycotoxin Citrinin by a Manganese Peroxidase from Moniliophthora roreri. Toxins, 14(11), 801. https://doi.org/10.3390/toxins14110801