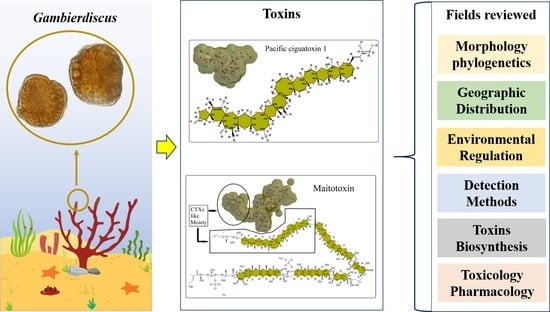
Gambierdiscus and Its Associated Toxins: A Minireview
Abstract
:1. Introduction
2. Taxonomy and Phylogenetics of Gambierdiscus
3. Geographic Distribution and Role of Environmental Factors
4. Gambierdiscus-Associated Toxins
5. Toxin Detection Methods
6. Toxin Biosynthesis
7. Toxicology and Pharmacology
8. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoppenrath, M.; Murray, S.A.; Chomérat, N.; Horiguchi, T. Marine Benthic Dinoflagellates—Unveiling Their Worldwide Biodiversity; Kleine Senckenberg-Reihe; Band 54; Schweizerbart: Stuttgart, Germany, 2014; ISBN 978-3-510-61402-8. [Google Scholar]
- Faust, M.A. Observation of sand-dwelling toxic dinoflagellates (Dinophyceae) from widely differing sites, including two new species. J. Phycol. 1995, 31, 996–1003. [Google Scholar] [CrossRef]
- Rains, L.K.; Parsons, M.L. Gambierdiscus species exhibit different epiphytic behaviors toward a variety of macroalgal hosts. Harmful Algae 2015, 49, 29–39. [Google Scholar] [CrossRef]
- Strachan, L.C.; Lewis, R.J.; Nicholson, G.M. Differential actions of pacific ciguatoxin-1 on sodium channel subtypes in mammalian sensory neurons. J. Pharmacol. Exp. Ther. 1999, 288, 379–388. [Google Scholar]
- Birinyi-Strachan, L.C.; Gunning, S.J.; Lewis, R.J.; Nicholson, G.M. Block of voltage-gated potassium channels by Pacific ciguatoxin-1 contributes to increased neuronal excitability in rat sensory neurons. Toxicol. Appl. Pharmacol. 2005, 204, 175–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munday, R.; Murray, S.; Rhodes, L.L.; Larsson, M.E.; Harwood, D.T. Ciguatoxins and Maitotoxins in Extracts of Sixteen Gambierdiscus Isolates and One Fukuyoa Isolate from the South Pacific and Their Toxicity to Mice by Intraperitoneal and Oral Administration. Mar. Drugs 2017, 15, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhodes, L.; Harwood, T.; Smith, K.F.; Argyle, P.A.; Munday, R. Production of ciguatoxin and maitotoxin by strains of Gambierdiscus australes, G. pacificus and G. polynesiensis (Dinophyceae) isolated from Rarotonga, Cook Islands. Harmful Algae 2014, 39, 185–190. [Google Scholar] [CrossRef]
- Leite, I.d.P.; Sdiri, K.; Taylor, A.; Viallon, J.; Gharbia, H.B.; Mafra Júnior, L.L.; Swarzenski, P.; Oberhaensli, F.; Darius, H.T.; Chinain, M.; et al. Experimental Evidence of Ciguatoxin Accumulation and Depuration in Carnivorous Lionfish. Toxins 2021, 13, 564. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.R.; Estevez, P.; Castro, D.; Solino, L.; Gouveia, N.; Santos, C.; Rodrigues, S.M.; Leao, J.M.; Gago-Martinez, A. New Insights into the Occurrence and Toxin Profile of Ciguatoxins in Selvagens Islands (Madeira, Portugal). Toxins 2018, 10, 524. [Google Scholar] [CrossRef] [Green Version]
- Pisapia, F.; Holland, W.C.; Hardison, D.R.; Litaker, R.W.; Fraga, S.; Nishimura, T.; Adachi, M.; Nguyen-Ngoc, L.; Séchet, V.; Amzil, Z.; et al. Toxicity screening of 13 Gambierdiscus strains using neuro-2a and erythrocyte lysis bioassays. Harmful Algae 2017, 63, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Chateau-Degat, M.-L.; Chinain, M.; Cerf, N.; Gingras, S.; Hubert, B.; Dewailly, É. Seawater temperature, Gambierdiscus spp. variability and incidence of ciguatera poisoning in French Polynesia. Harmful Algae 2005, 4, 1053–1062. [Google Scholar] [CrossRef]
- Lehane, L.; Lewis, R.J. Ciguatera: Recent advances but the risk remains. Int. J. Food Microbiol. 2000, 61, 91–125. [Google Scholar] [CrossRef]
- Soliño, L.; Costa, P.R. Global impact of ciguatoxins and ciguatera fish poisoning on fish, fisheries and consumers. Environ. Res. 2020, 182, 109111. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M.A.; Fernandez, M.; Backer, L.C.; Dickey, R.W.; Bernstein, J.; Schrank, K.; Kibler, S.; Stephan, W.; Gribble, M.O.; Bienfang, P.; et al. An Updated Review of Ciguatera Fish Poisoning: Clinical, Epidemiological, Environmental, and Public Health Management. Mar. Drugs 2017, 15, 72. [Google Scholar] [CrossRef] [PubMed]
- L’Herondelle, K.; Talagas, M.; Mignen, O.; Misery, L.; Le Garrec, R. Neurological Disturbances of Ciguatera Poisoning: Clinical Features and Pathophysiological Basis. Cells 2020, 9, 2291. [Google Scholar] [CrossRef]
- Tudó, À.; Gaiani, G.; Rey Varela, M.; Tsumuraya, T.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Diogène, J. Further Advance of Gambierdiscus species in the Canary Islands, with the First Report of Gambierdiscus belizeanus. Toxins 2020, 12, 692. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.-Z. Neurotoxins from Marine Dinoflagellates: A Brief Review. Mar. Drugs 2008, 6, 349–371. [Google Scholar] [CrossRef] [PubMed]
- Rossignoli, A.E.; Tudó, A.; Bravo, I.; Díaz, P.A.; Diogène, J.; Riobó, P. Toxicity Characterisation of Gambierdiscus species from the Canary Islands. Toxins 2020, 12, 134. [Google Scholar] [CrossRef] [Green Version]
- Lyu, Y.; Richlen, M.L.; Sehein, T.R.; Chinain, M.; Adachi, M.; Nishimura, T.; Xu, Y.; Parsons, M.L.; Smith, T.B.; Zheng, T.; et al. LSU rDNA based RFLP assays for the routine identification of Gambierdiscus species. Harmful Algae 2017, 66, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Larsson, M.E.; Laczka, O.F.; Harwood, D.T.; Lewis, R.J.; Himaya, S.W.A.; Murray, S.A.; Doblin, M.A. Toxicology of Gambierdiscus spp. (Dinophyceae) from Tropical and Temperate Australian Waters. Mar. Drugs 2018, 16, 7. [Google Scholar] [CrossRef] [Green Version]
- Neves, R.A.F.; Pardal, M.A.; Nascimento, S.M.; Silva, A.; Oliveira, P.J.; Rodrigues, E.T. High sensitivity of rat cardiomyoblast H9c2(2-1) cells to Gambierdiscus toxic compounds. Aquat. Toxicol. 2020, 223, 105475. [Google Scholar] [CrossRef]
- Roué, M.; Darius, H.T.; Viallon, J.; Ung, A.; Gatti, C.; Harwood, D.T.; Chinain, M. Application of solid phase adsorption toxin tracking (SPATT) devices for the field detection of Gambierdiscus toxins. Harmful Algae 2018, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Gingold, D.B.; Strickland, M.J.; Hess, J.J. Ciguatera Fish Poisoning and Climate Change: Analysis of National Poison Center Data in the United States, 2001–2011. Environ. Health Perspect. 2014, 122, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Soliño, L.; Costa, P.R. Differential toxin profiles of ciguatoxins in marine organisms: Chemistry, fate and global distribution. Toxicon 2018, 150, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Pasinszki, T.; Lako, J.; Dennis, T.E. Advances in Detecting Ciguatoxins in Fish. Toxins 2020, 12, 494. [Google Scholar] [CrossRef]
- Nakahara, H.; Sakami, T.; Chinain, M.; Ishida, Y. The role of macroalgae in epiphytism of the toxic dinoflagellate Gambierdiscus toxicus (Dinophyceae). Phycol. Res. 1996, 44, 113–117. [Google Scholar] [CrossRef]
- Adachi, R.; Fukuyo, Y. The Thecal Structure of a Marine Toxic Dinoflagellate Gambierdiscus toxicus gen. et sp. nov. Collected in a Ciguatera-endemic Area. Bull. Jpn. Soc. Sci. Fish 1979, 45, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Aligizaki, K.; Nikolaidis, G. Morphological identification of two tropical dinoflagellates of the genera Gambierdiscus and Sinophysis in the Mediterranean Sea. J. Biol. Res.-Thessalon. 2008, 9, 75–82. [Google Scholar]
- Nascimento, S.M.; Melo, G.; Salgueiro, F.; Diniz, B.d.S.; Fraga, S. Morphology of Gambierdiscus excentricus (Dinophyceae) with emphasis on sulcal plates. Phycologia 2015, 54, 628–639. [Google Scholar] [CrossRef]
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Chinain, M.; Holmes, M.J.; Holland, W.C.; Tester, P.A. Taxonomy of Gambierdiscus including four new species, Gambierdiscus caribaeus, Gambierdiscus carolinianus, Gambierdiscus carpenteri and Gambierdiscus ruetzleri (Gonyaulacales, Dinophyceae). Phycologia 2009, 48, 344–390. [Google Scholar] [CrossRef]
- Hoppenrath, M.; Kretzschmar, A.L.; Kaufmann, M.J.; Murray, S.A. Morphological and molecular phylogenetic identification and record verification of Gambierdiscus excentricus (Dinophyceae) from Madeira Island (NE Atlantic Ocean). Mar. Biodivers. Rec. 2019, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available online: https://www.algaebase.org (accessed on 18 May 2022).
- Jang, S.H.; Jeong, H.J.; Yoo, Y.D. Gambierdiscus jejuensis sp. nov., an epiphytic dinoflagellate from the waters of Jeju Island, Korea, effect of temperature on the growth, and its global distribution. Harmful Algae 2018, 80, 149–157. [Google Scholar] [CrossRef]
- Rhodes, L.; Smith, K.F.; Verma, A.; Curley, B.G.; Harwood, D.T.; Murray, S.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; et al. A new species of Gambierdiscus (Dinophyceae) from the south-west Pacific: Gambierdiscus honu sp. nov. Harmful Algae 2017, 65, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Fraga, S.; Rodríguez, F.; Caillaud, A.; Diogène, J.; Raho, N.; Zapata, M. Gambierdiscus excentricus sp. nov. (Dinophyceae), a benthic toxic dinoflagellate from the Canary Islands (NE Atlantic Ocean). Harmful Algae 2011, 11, 10–22. [Google Scholar] [CrossRef] [Green Version]
- Fraga, S.; Rodríguez, F. Genus Gambierdiscus in the Canary Islands (NE Atlantic Ocean) with Description of Gambierdiscus silvae sp. nov., a New Potentially Toxic Epiphytic Benthic Dinoflagellate. Protist 2014, 165, 839–853. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Rhodes, L.; Verma, A.; Curley, B.G.; Harwood, D.T.; Kohli, G.S.; Solomona, D.; Rongo, T.; Munday, R.; Murray, S.A. A new Gambierdiscus species (Dinophyceae) from Rarotonga, Cook Islands: Gambierdiscus cheloniae sp. nov. Harmful Algae 2016, 60, 45–56. [Google Scholar] [CrossRef]
- Fraga, S.; Rodríguez, F.; Riobó, P.; Bravo, I. Gambierdiscus balechii sp. nov (Dinophyceae), a new benthic toxic dinoflagellate from the Celebes Sea (SW Pacific Ocean). Harmful Algae 2016, 58, 93–105. [Google Scholar] [CrossRef]
- Kretzschmar, A.L.; Verma, A.; Harwood, T.; Hoppenrath, M.; Murray, S. Characterization of Gambierdiscus lapillus sp. nov. (Gonyaulacales, Dinophyceae): A new toxic dinoflagellate from the Great Barrier Reef (Australia). J. Phycol. 2017, 53, 283–297. [Google Scholar] [CrossRef]
- Chinain, M.; Germain, M.; Sako, Y.; Pauillac, S.; Legrand, A. Genetic diversity in French Polynesian strains of the ciguatera-causing dinoflagellate Gambierdiscus toxicus: RFLP and sequence analysis on the SSU and LSU rRNA genes. In Harmful Algae; United Nations Educational, Scientific, and Cultural Organization: Paris, France, 1998; pp. 287–290. [Google Scholar]
- Leung, P.T.Y.; Yan, M.; Lam, V.T.T.; Yiu, S.K.F.; Chen, C.-Y.; Murray, J.S.; Harwood, D.T.; Rhodes, L.L.; Lam, P.K.S.; Wai, T.-C. Phylogeny, morphology and toxicity of benthic dinoflagellates of the genus Fukuyoa (Goniodomataceae, Dinophyceae) from a subtropical reef ecosystem in the South China Sea. Harmful Algae 2018, 74, 78–97. [Google Scholar] [CrossRef]
- Subba Rao, D.V. (Ed.) Dinoflagellates: Classification, Evolution, Physiology and Ecological Significance; Marine and Freshwater Biology; Nova Science Publishers: New York, NY, USA, 2020; ISBN 978-1-5361-7888-3. [Google Scholar]
- Tester, P.; Wickliffe, L.; Jossart, J.; Rhodes, L.; Enevoldsen, H.; Adachi, M.; Nishimura, T.; Rodriguez, F.; Chinain, M.; Litaker, W. Global distribution of the genera Gambierdiscus and Fukuyoa. Harmful Algae 2018, 138. [Google Scholar] [CrossRef]
- Tudó, À.; Toldrà, A.; Rey, M.; Todolí, I.; Andree, K.B.; Fernández-Tejedor, M.; Campàs, M.; Sureda, F.X.; Diogène, J. Gambierdiscus and Fukuyoa as potential indicators of ciguatera risk in the Balearic Islands. Harmful Algae 2020, 99, 101913. [Google Scholar] [CrossRef]
- Gaiani, G.; Leonardo, S.; Tudó, À.; Toldrà, A.; Rey, M.; Andree, K.B.; Tsumuraya, T.; Hirama, M.; Diogène, J.; O’Sullivan, C.K.; et al. Rapid detection of ciguatoxins in Gambierdiscus and Fukuyoa with immunosensing tools. Ecotoxicol. Environ. Saf. 2020, 204, 111004. [Google Scholar] [CrossRef] [PubMed]
- Gómez, F.; Qiu, D.; Lopes, R.M.; Lin, S. Fukuyoa paulensis gen. et sp. nov., a New Genus for the Globular Species of the Dinoflagellate Gambierdiscus (Dinophyceae). PLoS ONE 2015, 10, e0119676. [Google Scholar] [CrossRef] [PubMed]
- Villareal, T.A.; Hanson, S.; Qualia, S.; Jester, E.L.E.; Granade, H.R.; Dickey, R.W. Petroleum production platforms as sites for the expansion of ciguatera in the northwestern Gulf of Mexico. Harmful Algae 2007, 6, 253–259. [Google Scholar] [CrossRef]
- Litaker, R.W.; Holland, W.C.; Hardison, D.R.; Pisapia, F.; Hess, P.; Kibler, S.R.; Tester, P.A. Ciguatoxicity of Gambierdiscus and Fukuyoa species from the Caribbean and Gulf of Mexico. PLoS ONE 2017, 12, e0185776. [Google Scholar] [CrossRef]
- Holland, W.C.; Litaker, R.W.; Tomas, C.R.; Kibler, S.R.; Place, A.R.; Davenport, E.D.; Tester, P.A. Differences in the toxicity of six Gambierdiscus (Dinophyceae) species measured using an in vitro human erythrocyte lysis assay. Toxicon 2013, 65, 15–33. [Google Scholar] [CrossRef]
- Chinain, M.; Faust, M.A.; Pauillac, S. Morphology and molecular analyses of three toxic species of Gambierdiscus (Dinophyceae): G. pacificus, sp. nov., G. australes, sp. nov., and G. polynesiensis, sp. nov. J. Phycol. 1999, 35, 1282–1296. [Google Scholar] [CrossRef]
- Gatti, C.M.I.; Lonati, D.; Darius, H.T.; Zancan, A.; Roué, M.; Schicchi, A.; Locatelli, C.A.; Chinain, M. Tectus niloticus (Tegulidae, Gastropod) as a Novel Vector of Ciguatera Poisoning: Clinical Characterization and Follow-Up of a Mass Poisoning Event in Nuku Hiva Island (French Polynesia). Toxins 2018, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Sato, S.; Tawong, W.; Sakanari, H.; Uehara, K.; Shah, M.M.R.; Suda, S.; Yasumoto, T.; Taira, Y.; Yamaguchi, H.; et al. Genetic Diversity and Distribution of the Ciguatera-Causing Dinoflagellate Gambierdiscus spp. (Dinophyceae) in Coastal Areas of Japan. PLoS ONE 2013, 8, e60882. [Google Scholar] [CrossRef] [Green Version]
- IOC-UNESCO. The Harmful Algal Event Database (HAEDAT). Available online: https://obis.org (accessed on 23 August 2021).
- VIshwas, C.; Achuthankutty, C.T. IndOBIS Catalogue of Life. Available online: http://www.indobis.org/ (accessed on 23 August 2021).
- Litaker, R.W.; Vandersea, M.W.; Faust, M.A.; Kibler, S.R.; Nau, A.W.; Holland, W.C.; Chinain, M.; Holmes, M.J.; Tester, P.A. Global distribution of ciguatera causing dinoflagellates in the genus Gambierdiscus. Toxicon 2010, 56, 711–730. [Google Scholar] [CrossRef]
- Xu, Y.; Richlen, M.L.; Morton, S.L.; Mak, Y.L.; Chan, L.L.; Tekiau, A.; Anderson, D.M. Distribution, abundance and diversity of Gambierdiscus spp. from a ciguatera-endemic area in Marakei, Republic of Kiribati. Harmful Algae 2014, 34, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Hallegraeff, G. Transport of toxic dinoflagellates via ships’ ballast water:bioeconomic risk assessment and efficacy of possible ballast water management strategies. Mar. Ecol. Prog. Ser. 1998, 168, 297–309. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, F.; Fraga, S.; Ramilo, I.; Rial, P.; Figueroa, R.I.; Riobó, P.; Bravo, I. Canary Islands (NE Atlantic) as a biodiversity “hotspot” of Gambierdiscus: Implications for future trends of ciguatera in the area. Harmful Algae 2017, 67, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Harris, A.J.; Blair, C.; He, X. RASP (Reconstruct Ancestral State in Phylogenies): A tool for historical biogeography. Mol. Phylogenet. Evol. 2015, 87, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Bomber, J.W.; Guillard, R.R.L.; Nelson, W.G. Rôles of temperature, salinity, and light in seasonality, growth, and toxicity of ciguatera-causing Gambierdiscus toxicus Adachi et Fukuyo (Dinophyceae). J. Exp. Mar. Biol. Ecol. 1988, 115, 53–65. [Google Scholar] [CrossRef]
- Morton, S.L.; Norris, D.R.; Bomber, J.W. Effect of temperature, salinity and light intensity on the growth and seasonality of toxic dinoflagellates associated with ciguatera. J. Exp. Mar. Biol. Ecol. 1992, 157, 79–90. [Google Scholar] [CrossRef]
- Kibler, S.R.; Litaker, R.W.; Holland, W.C.; Vandersea, M.W.; Tester, P.A. Growth of eight Gambierdiscus (Dinophyceae) species: Effects of temperature, salinity and irradiance. Harmful Algae 2012, 19, 1–14. [Google Scholar] [CrossRef]
- Parsons, M.L.; Aligizaki, K.; Bottein, M.-Y.D.; Fraga, S.; Morton, S.L.; Penna, A.; Rhodes, L. Gambierdiscus and Ostreopsis: Reassessment of the state of knowledge of their taxonomy, geography, ecophysiology, and toxicology. Harmful Algae 2012, 14, 107–129. [Google Scholar] [CrossRef]
- Yoshimatsu, T.; Yamaguchi, H.; Iwamoto, H.; Nishimura, T.; Adachi, M. Effects of temperature, salinity and their interaction on growth of Japanese Gambierdiscus spp. (Dinophyceae). Harmful Algae 2014, 35, 29–37. [Google Scholar] [CrossRef]
- Sparrow, L.; Momigliano, P.; Russ, G.R.; Heimann, K. Effects of temperature, salinity and composition of the dinoflagellate assemblage on the growth of Gambierdiscus carpenteri isolated from the Great Barrier Reef. Harmful Algae 2017, 65, 52–60. [Google Scholar] [CrossRef]
- Xu, Y.; Richlen, M.L.; Liefer, J.D.; Robertson, A.; Kulis, D.; Smith, T.B.; Parsons, M.L.; Anderson, D.M. Influence of Environmental Variables on Gambierdiscus spp. (Dinophyceae) Growth and Distribution. PLoS ONE 2016, 11, e0153197. [Google Scholar] [CrossRef]
- Tawong, W.; Yoshimatsu, T.; Yamaguchi, H.; Adachi, M. Temperature and salinity effects and toxicity of Gambierdiscus caribaeus (Dinophyceae) from Thailand. Phycologia 2016, 55, 274–278. [Google Scholar] [CrossRef]
- Kibler, S.R.; Tester, P.A.; Kunkel, K.E.; Moore, S.K.; Litaker, R.W. Effects of ocean warming on growth and distribution of dinoflagellates associated with ciguatera fish poisoning in the Caribbean. Ecol. Modell. 2015, 316, 194–210. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Sievert, S.M. Pan-genome analyses identify lineage- and niche-specific markers of evolution and adaptation in Epsilonproteobacteria. Front. Microbiol. 2014, 5, 110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezuidt, O.K.; Pierneef, R.; Gomri, A.M.; Adesioye, F.; Makhalanyane, T.P.; Kharroub, K.; Cowan, D.A. The Geobacillus Pan-Genome: Implications for the Evolution of the Genus. Front. Microbiol. 2016, 7, 723. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, T.; Wang, J.; Wang, P.; Qiu, Y.; Zhao, W.; Pang, S.; Li, X.; Wang, H.; Song, J.; et al. Pan-genome of Raphanus highlights genetic variation and introgression among domesticated, wild, and weedy radishes. Mol. Plant 2021, 14, 2032–2055. [Google Scholar] [CrossRef]
- Tester, P.A.; Litaker, R.W.; Berdalet, E. Climate change and harmful benthic microalgae. Harmful Algae 2020, 91, 101655. [Google Scholar] [CrossRef]
- Núñez-Vázquez, E.J.; Almazán-Becerril, A.; López-Cortés, D.J.; Heredia-Tapia, A.; Hernández-Sandoval, F.E.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Gárate-Lizárraga, I.; García-Mendoza, E.; Salinas-Zavala, C.A.; et al. Ciguatera in Mexico (1984–2013). Mar. Drugs 2018, 17, 13. [Google Scholar] [CrossRef] [Green Version]
- Boada, L.D.; Zumbado, M.; Luzardo, O.P.; Almeida-González, M.; Plakas, S.M.; Granade, H.R.; Abraham, A.; Jester, E.L.E.; Dickey, R.W. Ciguatera fish poisoning on the West Africa Coast: An emerging risk in the Canary Islands (Spain). Toxicon 2010, 56, 1516–1519. [Google Scholar] [CrossRef]
- Leynse, A.K.; Parsons, M.L.; Thomas, S.E. Differences in the photoacclimation and photoprotection exhibited by two species of the ciguatera causing dinoflagellate genus, Gambierdiscus. Harmful Algae 2017, 70, 90–97. [Google Scholar] [CrossRef]
- Loeffler, C.; Richlen, M.; Brandt, M.; Smith, T. Effects of grazing, nutrients, and depth on the ciguatera-causing dinoflagellate Gambierdiscus in the US Virgin Islands. Mar. Ecol. Prog. Ser. 2015, 531, 91–104. [Google Scholar] [CrossRef]
- Longo, S.; Sibat, M.; Darius, H.T.; Hess, P.; Chinain, M. Effects of pH and Nutrients (Nitrogen) on Growth and Toxin Profile of the Ciguatera-Causing Dinoflagellate Gambierdiscus polynesiensis (Dinophyceae). Toxins 2020, 12, 767. [Google Scholar] [CrossRef] [PubMed]
- Lewis, R.J.; Vernoux, J.-P.; Brereton, I.M. Structure of Caribbean Ciguatoxin Isolated from Caranx latus. J. Am. Chem. Soc. 1998, 120, 5914–5920. [Google Scholar] [CrossRef]
- Hamilton, B.; Hurbungs, M.; Vernoux, J.-P.; Jones, A.; Lewis, R.J. Isolation and characterisation of Indian Ocean ciguatoxin. Toxicon 2002, 40, 685–693. [Google Scholar] [CrossRef]
- Spielmeyer, A.; Loeffler, C.R.; Bodi, D. Extraction and LC-MS/MS Analysis of Ciguatoxins: A Semi-Targeted Approach Designed for Fish of Unknown Origin. Toxins 2021, 13, 630. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Miyazaki, K.; Ishihara, Y.; Tatami, A.; Ohnuma, Y.; Kawada, Y.; Komano, K.; Yamashita, S.; Lee, N.; Hirama, M. Total Synthesis of Ciguatoxin and 51-HydroxyCTX3C. J. Am. Chem. Soc. 2006, 128, 9352–9354. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, L.L.; Smith, K.F.; Verma, A.; Murray, S.; Harwood, D.T.; Trnski, T. The dinoflagellate genera Gambierdiscus and Ostreopsis from subtropical Raoul Island and North Meyer Island, Kermadec Islands. N. Z. J. Mar. Freshw. Res. 2017, 51, 490–504. [Google Scholar] [CrossRef]
- Estevez, P.; Sibat, M.; Leao-Martins, J.M.; Tudo, A.; Rambla-Alegre, M.; Aligizaki, K.; Diogène, J.; Gago-Martinez, A.; Hess, P. Use of Mass Spectrometry to Determine the Diversity of Toxins Produced by Gambierdiscus and Fukuyoa Species from Balearic Islands and Crete (Mediterranean Sea) and the Canary Islands (Northeast Atlantic). Toxins 2020, 12, 305. [Google Scholar] [CrossRef]
- Rodríguez, I.; Genta-Jouve, G.; Alfonso, C.; Calabro, K.; Alonso, E.; Sánchez, J.A.; Alfonso, A.; Thomas, O.P.; Botana, L.M. Gambierone, a Ladder-Shaped Polyether from the Dinoflagellate Gambierdiscus belizeanus. Org. Lett. 2015, 17, 2392–2395. [Google Scholar] [CrossRef]
- Boente-Juncal, A.; Álvarez, M.; Antelo, Á.; Rodriguez, I.; Calabro, K.; Vale, C.; Thomas, O.P.; Botana, L.M. Structure Elucidation and Biological Evaluation of Maitotoxin-3, a Homologue of Gambierone, from Gambierdiscus belizeanus. Toxins 2019, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Murray, J.S.; Finch, S.C.; Puddick, J.; Rhodes, L.L.; Harwood, D.T.; van Ginkel, R.; Prinsep, M.R. Acute Toxicity of Gambierone and Quantitative Analysis of Gambierones Produced by Cohabitating Benthic Dinoflagellates. Toxins 2021, 13, 333. [Google Scholar] [CrossRef]
- Caillaud, A.; de la Iglesia, P.; Barber, E.; Eixarch, H.; Mohammad-Noor, N.; Yasumoto, T.; Diogène, J. Monitoring of dissolved ciguatoxin and maitotoxin using solid-phase adsorption toxin tracking devices: Application to Gambierdiscus pacificus in culture. Harmful Algae 2011, 10, 433–446. [Google Scholar] [CrossRef]
- Nagai, H.; Murata, M.; Torigoe, K.; Satake, M.; Yasumoto, T. Gambieric acids, new potent antifungal substances with unprecedented polyether structures from a marine dinoflagellate Gambierdiscus toxicus. J. Org. Chem. 1992, 57, 5448–5453. [Google Scholar] [CrossRef]
- Watanabe, R.; Uchida, H.; Suzuki, T.; Matsushima, R.; Nagae, M.; Toyohara, Y.; Satake, M.; Oshima, Y.; Inoue, A.; Yasumoto, T. Gambieroxide, a novel epoxy polyether compound from the dinoflagellate Gambierdiscus toxicus GTP2 strain. Tetrahedron 2013, 69, 10299–10303. [Google Scholar] [CrossRef]
- Satake, M.; Murata, M.; Yasumoto, T. Gambierol: A new toxic polyether compound isolated from the marine dinoflagellate Gambierdiscus toxicus. J. Am. Chem. Soc. 1993, 115, 361–362. [Google Scholar] [CrossRef]
- Maria Durán-Riveroll, L.; Cembella, A.D.; Okolodkov, Y.B. A Review on the Biodiversity and Biogeography of Toxigenic Benthic Marine Dinoflagellates of the Coasts of Latin America. Front. Mar. Sci. 2019, 6, 148. [Google Scholar] [CrossRef]
- Yasumoto, T.; Bagnis, R.; Vernoux, J.P. Toxicity of the surgeonfishes. II. Properties of the principal water-soluble toxin. Nippon Suisan Gakk. 1976, 42, 359–365. [Google Scholar] [CrossRef]
- Varela, A.T.; Neves, R.A.F.; Nascimento, S.M.; Oliveira, P.J.; Pardal, M.A.; Rodrigues, E.T.; Moreno, A.J. Exposure to marine benthic dinoflagellate toxins may lead to mitochondrial dysfunction. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 240, 108937. [Google Scholar] [CrossRef]
- Pisapia, F.; Sibat, M.; Watanabe, R.; Roullier, C.; Suzuki, T.; Hess, P.; Herrenknecht, C. Characterization of maitotoxin-4 (MTX4) using electrospray positive mode ionization high-resolution mass spectrometry and UV spectroscopy. Rapid Commun. Mass Spectrom. 2020, 34, e8859. [Google Scholar] [CrossRef]
- Martin, V.; Vale, C.; Antelo, A.; Hirama, M.; Yamashita, S.; Vieytes, M.R.; Botana, L.M. Differential Effects of Ciguatoxin and Maitotoxin in Primary Cultures of Cortical Neurons. Chem. Res. Toxicol. 2014, 27, 1387–1400. [Google Scholar] [CrossRef]
- Yon, T.; Sibat, M.; Réveillon, D.; Bertrand, S.; Chinain, M.; Hess, P. Deeper insight into Gambierdiscus polynesiensis toxin production relies on specific optimization of high-performance liquid chromatography-high resolution mass spectrometry. Talanta 2021, 232, 122400. [Google Scholar] [CrossRef]
- Diogène, J.; Reverté, L.; Rambla-Alegre, M.; del Río, V.; de la Iglesia, P.; Campàs, M.; Palacios, O.; Flores, C.; Caixach, J.; Ralijaona, C.; et al. Identification of ciguatoxins in a shark involved in a fatal food poisoning in the Indian Ocean. Sci. Rep. 2017, 7, 8240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morohashi, A.; Satake, M.; Yasumoto, T. The absolute configuration of gambierol, a toxic marine polyether from the dinoflagellate, Gambierdiscus toxicus. Tetrahedron Lett. 1999, 40, 97–100. [Google Scholar] [CrossRef]
- Molgó, J.; Schlumberger, S.; Sasaki, M.; Fuwa, H.; Louzao, M.C.; Botana, L.M.; Servent, D.; Benoit, E. Gambierol Potently Increases Evoked Quantal Transmitter Release and Reverses Pre- and Post -Synaptic Blockade at Vertebrate Neuromuscular Junctions. Neuroscience 2020, 439, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, E.; Abdel-Mottaleb, Y.; Kopljar, I.; Rainier, J.D.; Raes, A.L.; Snyders, D.J.; Tytgat, J. Gambierol, a toxin produced by the dinoflagellate Gambierdiscus toxicus, is a potent blocker of voltage-gated potassium channels. Toxicon 2008, 51, 974–983. [Google Scholar] [CrossRef] [Green Version]
- Roeder, K.; Erler, K.; Kibler, S.; Tester, P.; Van The, H.; Nguyen-Ngoc, L.; Gerdts, G.; Luckas, B. Characteristic profiles of Ciguatera toxins in different strains of Gambierdiscus spp. Toxicon 2010, 56, 731–738. [Google Scholar] [CrossRef]
- Chinain, M.; Darius, H.T.; Ung, A.; Cruchet, P.; Wang, Z.; Ponton, D.; Laurent, D.; Pauillac, S. Growth and toxin production in the ciguatera-causing dinoflagellate Gambierdiscus polynesiensis (Dinophyceae) in culture. Toxicon 2010, 56, 739–750. [Google Scholar] [CrossRef]
- Wu, Z.; Luo, H.; Yu, L.; Lee, W.H.; Li, L.; Mak, Y.L.; Lin, S.; Lam, P.K.S. Characterizing ciguatoxin (CTX)- and Non-CTX-producing strains of Gambierdiscus balechii using comparative transcriptomics. Sci. Total Environ. 2020, 717, 137184. [Google Scholar] [CrossRef]
- Pitz, K.J.; Richlen, M.L.; Fachon, E.; Smith, T.B.; Parsons, M.L.; Anderson, D.M. Development of fluorescence in situ hybridization (FISH) probes to detect and enumerate Gambierdiscus species. Harmful Algae 2021, 101, 101914. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, H.; EI-Shahat, M. Recombinase polymerase amplification as a promising tool in hepatitis C virus diagnosis. World J. Hepatol. 2014, 6, 916–922. [Google Scholar] [CrossRef]
- Gaiani, G.; Leonardo, S.; Tsumuraya, T.; Rambla, M.; Diogène, J.; O’Sullivan, C.; Alcaraz, C.; Campàs, M. Detection of ciguatoxins in fish and algal samples with an electrochemical biosensor. In Proceedings of the 1st International Electronic Conference on Toxins, Online, 14 January 2021. [Google Scholar]
- Gaiani, G.; Toldrà, A.; Andree, K.B.; Rey, M.; Diogène, J.; Alcaraz, C.; O’Sullivan, C.K.; Campàs, M. Detection of Gambierdiscus and Fukuyoa single cells using recombinase polymerase amplification combined with a sandwich hybridization assay. J. Appl. Phycol. 2021, 33, 2273–2282. [Google Scholar] [CrossRef]
- Porcar, M.; Juárez-Pérez, V. PCR-based identification of Bacillus thuringiensis pesticidal crystal genes. FEMS Microbiol. Rev. 2003, 26, 419–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villafana, R.; Ramdass, A.; Rampersad, S. Selection of Fusarium Trichothecene Toxin Genes for Molecular Detection Depends on TRI Gene Cluster Organization and Gene Function. Toxins 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asakura, M.; Samosornsuk, W.; Hinenoya, A.; Misawa, N.; Nishimura, K.; Matsuhisa, A.; Yamasaki, S. Development of a cytolethal distending toxin (cdt) gene-based species-specific multiplex PCR assay for the detection and identification of Campylobacter jejuni, Campylobacter coli and Campylobacter fetus. FEMS Immunol. Med. Microbiol. 2008, 52, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whittle, K.; Gallacher, S. Marine toxins. Br. Med. Bull. 2000, 56, 236–253. [Google Scholar] [CrossRef] [PubMed]
- Juranovic, L.R.; Park, D.L. Foodborne Toxins of Marine Origin: Ciguatera. In Reviews of Environmental Contamination and Toxicology; Ware, G.W., Ed.; Reviews of Environmental Contamination and Toxicology; Springer: New York, NY, USA, 1991; Volume 117, pp. 51–94. ISBN 978-1-4612-7777-4. [Google Scholar]
- Hallegraeff, G.M.; Anderson, D.M.; Cembella, A.D.; Enevoldsen, H.O. Manual on Harmful Marine Microalgae; Hallegraeff, G.M., Anderson, D.M., Cembella, A.D., Eds.; Monographs on Oceanographic Methodology; UNESCO: Paris, France, 2003; ISBN 978-92-3-103871-6. [Google Scholar]
- Martínez, A. Marine Biotoxins. In Springer Handbook of Marine Biotechnology; Kim, S.K., Ed.; Springer Handbooks; Springer: Berlin/Heidelberg, Germany, 2004; pp. 869–904. ISBN 978-3-642-53971-8. [Google Scholar]
- Xu, Y.; He, X.; Lee, W.H.; Chan, L.L.; Lu, D.; Wang, P.; Tao, X.; Li, H.; Yu, K. Ciguatoxin-Producing Dinoflagellate Gambierdiscus in the Beibu Gulf: First Report of Toxic Gambierdiscus in Chinese Waters. Toxins 2021, 13, 643. [Google Scholar] [CrossRef]
- Caillaud, A.; Eixarch, H.; de la Iglesia, P.; Rodriguez, M.; Dominguez, L.; Andree, K.B.; Diogène, J. Towards the standardisation of the neuroblastoma (neuro-2a) cell-based assay for ciguatoxin-like toxicity detection in fish: Application to fish caught in the Canary Islands. Food Addit. Contam. A 2012, 29, 1000–1010. [Google Scholar] [CrossRef]
- Estevez, P.; Castro, D.; Pequeño-Valtierra, A.; Leao, J.; Vilariño, O.; Diogène, J.; Gago-Martínez, A. An Attempt to Characterize the Ciguatoxin Profile in Seriola fasciata Causing Ciguatera Fish Poisoning in Macaronesia. Toxins 2019, 11, 221. [Google Scholar] [CrossRef] [Green Version]
- Leonardo, S.; Gaiani, G.; Tsumuraya, T.; Hirama, M.; Turquet, J.; Sagristà, N.; Rambla-Alegre, M.; Flores, C.; Caixach, J.; Diogène, J.; et al. Addressing the Analytical Challenges for the Detection of Ciguatoxins Using an Electrochemical Biosensor. Anal. Chem. 2020, 92, 4858–4865. [Google Scholar] [CrossRef]
- Hokama, Y.; Banner, A.H.; Boylan, D.B. A radioimmunoassay for the detection of ciguatoxin. Toxicon 1977, 15, 317–325. [Google Scholar] [CrossRef]
- Hokama, Y.; Abad, M.A.; Kimura, L.H. A rapid enzyme-immunoassay for the detection of ciguatoxin in contaminated fish tissues. Toxicon 1983, 21, 817–824. [Google Scholar] [CrossRef]
- Tsumuraya, T.; Fujii, I.; Inoue, M.; Tatami, A.; Miyazaki, K.; Hirama, M. Production of monoclonal antibodies for sandwich immunoassay detection of ciguatoxin 51-hydroxyCTX3C. Toxicon 2006, 48, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Tsumuraya, T.; Fujii, I.; Hirama, M. Production of monoclonal antibodies for sandwich immunoassay detection of Pacific ciguatoxins. Toxicon 2010, 56, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Hokama, Y.; Takenaka, W.E.; Nishimura, K.L.; Ebesu, J.S.M.; Bourke, R.; Sullivan, P.K. A Simple Membrane Immunobead Assay for Detecting Ciguatoxin and Related Polyethers from Human Ciguatera Intoxication and Natural Reef Fishes. J. AOAC Int. 1998, 81, 727–736. [Google Scholar] [CrossRef] [Green Version]
- Campora, C.E.; Dierking, J.; Tamaru, C.S.; Hokama, Y.; Vincent, D. Detection of ciguatoxin in fish tissue using sandwich ELISA and neuroblastoma cell bioassay. J. Clin. Lab. Anal. 2008, 22, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Y.; Zhang, C.; Luan, W. Horseradish peroxidase and antibody labeled gold nanoparticle probe for amplified immunoassay of ciguatoxin in fish samples based on capillary electrophoresis with electrochemical detection. Toxicon 2015, 96, 89–95. [Google Scholar] [CrossRef]
- Hardison, D.R.; Holland, W.C.; McCall, J.R.; Bourdelais, A.J.; Baden, D.G.; Darius, H.T.; Chinain, M.; Tester, P.A.; Shea, D.; Flores Quintana, H.A.; et al. Fluorescent Receptor Binding Assay for Detecting Ciguatoxins in Fish. PLoS ONE 2016, 11, e0153348. [Google Scholar] [CrossRef]
- Caillaud, A.; De la Iglesia, P.; Darius, H.T.; Pauillac, S.; Aligizaki, K.; Fraga, S.; Chinain, M.; Diogène, J. Update on Methodologies Available for Ciguatoxin Determination: Perspectives to Confront the Onset of Ciguatera Fish Poisoning in Europe. Mar. Drugs 2010, 8, 1838–1907. [Google Scholar] [CrossRef]
- Paul, B.; Suzanne, D.; Anne, D. Quantitative Evaluation of Commercially Available Test Kit for Ciguatera in Fish. Food Nutr. Sci. 2011, 2, 594–598. [Google Scholar] [CrossRef] [Green Version]
- Yogi, K.; Oshiro, N.; Inafuku, Y.; Hirama, M.; Yasumoto, T. Detailed LC-MS/MS Analysis of Ciguatoxins Revealing Distinct Regional and Species Characteristics in Fish and Causative Alga from the Pacific. Anal. Chem. 2011, 83, 8886–8891. [Google Scholar] [CrossRef]
- Sibat, M.; Herrenknecht, C.; Darius, H.T.; Roué, M.; Chinain, M.; Hess, P. Detection of pacific ciguatoxins using liquid chromatography coupled to either low or high resolution mass spectrometry (LC-MS/MS). J. Chromatogr. A 2018, 1571, 16–28. [Google Scholar] [CrossRef]
- Oshiro, N.; Tomikawa, T.; Kuniyoshi, K.; Ishikawa, A.; Toyofuku, H.; Kojima, T.; Asakura, H. LC–MS/MS Analysis of Ciguatoxins Revealing the Regional and Species Distinction of Fish in the Tropical Western Pacific. J. Mar. Sci. Eng. 2021, 9, 299. [Google Scholar] [CrossRef]
- Inserra, M.; Lavrukhina, Y.; Jones, A.; Lewis, R.J.; Vetter, I. Ciguatoxin Detection Methods and High-Throughput Assays. In Analysis of Food Toxins and Toxicants; Wong, Y., Lewis, R.J., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; pp. 469–488. ISBN 978-1-118-99268-5. [Google Scholar]
- Tsumuraya, T.; Hirama, M. Rationally Designed Synthetic Haptens to Generate Anti-Ciguatoxin Monoclonal Antibodies, and Development of a Practical Sandwich ELISA to Detect Ciguatoxins. Toxins 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacarizas, J.; Benico, G.; Austero, N.; Azanza, R. Taxonomy and toxin production of Gambierdiscus carpenteri (Dinophyceae) in a tropical marine ecosystem: The first record from the Philippines. Mar. Pollut. Bull. 2018, 137, 430–443. [Google Scholar] [CrossRef] [PubMed]
- Liefer, J.D.; Richlen, M.L.; Smith, T.B.; DeBose, J.L.; Xu, Y.; Anderson, D.M.; Robertson, A. Asynchrony of Gambierdiscus spp. Abundance and Toxicity in the U.S. Virgin Islands: Implications for Monitoring and Management of Ciguatera. Toxins 2021, 13, 413. [Google Scholar] [CrossRef] [PubMed]
- Van Wagoner, R.M.; Satake, M.; Wright, J.L.C. Polyketide biosynthesis in dinoflagellates: What makes it different? Nat. Prod. Rep. 2014, 31, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kohli, G.S.; John, U.; Van Dolah, F.M.; Murray, S.A. Evolutionary distinctiveness of fatty acid and polyketide synthesis in eukaryotes. ISME J. 2016, 10, 1877–1890. [Google Scholar] [CrossRef] [Green Version]
- Vilotijevic, I.; Jamison, T.F. Epoxide-Opening Cascades in the Synthesis of Polycyclic Polyether Natural Products. Angew. Chem. Int. Ed. 2009, 48, 5250–5281. [Google Scholar] [CrossRef] [Green Version]
- Kohli, G.S.; John, U.; Figueroa, R.I.; Rhodes, L.L.; Harwood, D.T.; Groth, M.; Bolch, C.J.S.; Murray, S.A. Polyketide synthesis genes associated with toxin production in two species of Gambierdiscus (Dinophyceae). BMC Genom. 2015, 16, 410. [Google Scholar] [CrossRef] [Green Version]
- Van Dolah, F.M.; Morey, J.S.; Milne, S.; Ung, A.; Anderson, P.E.; Chinain, M. Transcriptomic analysis of polyketide synthases in a highly ciguatoxic dinoflagellate, Gambierdiscus polynesiensis and low toxicity Gambierdiscus pacificus, from French Polynesia. PLoS ONE 2020, 15, e0231400. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, J.K.; Robertson, A.; Kiene, R.P. Effect of Salinity on DMSP Production in Gambierdiscus belizeanus (Dinophyceae). J. Phycol. 2019, 55, 1401–1411. [Google Scholar] [CrossRef]
- Arnold, H.E.; Kerrison, P.; Steinke, M. Interacting effects of ocean acidification and warming on growth and DMS-production in the haptophyte coccolithophore Emiliania huxleyi. Glob. Change Biol. 2013, 19, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, C.; Sun, J. Influence of salinity and nitrogen content on production of dimethylsulfoniopropionate (DMSP) and dimethylsulfide (DMS) by Skeletonema costatum. Chin. J. Oceanol. Limnol. 2011, 29, 378–386. [Google Scholar] [CrossRef]
- McGann, L.E.; Walterson, M.L. Cryoprotection by dimethyl sulfoxide and dimethyl sulfone. Cryobiology 1987, 24, 11–16. [Google Scholar] [CrossRef]
- Sunda, W.; Kieber, D.J.; Kiene, R.P.; Huntsman, S. An antioxidant function for DMSP and DMS in marine algae. Nature 2002, 418, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Ghiaroni, V.; Fuwa, H.; Inoue, M.; Sasaki, M.; Miyazaki, K.; Hirama, M.; Yasumoto, T.; Rossini, G.P.; Scalera, G.; Bigiani, A. Effect of Ciguatoxin 3C on Voltage-Gated Na+ and K+ Currents in Mouse Taste Cells. Chem. Senses 2006, 31, 673–680. [Google Scholar] [CrossRef]
- Hidalgo, J.; Liberona, J.L.; Molgó, J.; Jaimovich, E. Pacific ciguatoxin-1b effect over Na+ and K+ currents, inositol 1,4,5-triphosphate content and intracellular Ca2+ signals in cultured rat myotubes: Effects of Pacific CTX-1b on rat myotubes. Med. J. Aust. 2002, 137, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Lombet, A.; Bidard, J.-N.; Lazdunski, M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987, 219, 355–359. [Google Scholar] [CrossRef] [Green Version]
- Tanyag, B.E.; Perelonia, K.B.S.; Cambia, F.D.; Montojo, U.M. Screening of Ciguatoxins in the Philippines by Animal Assay: Symptoms, Levels, and Distribution in Fish Tissue. TPJF 2021, 28, 87–95. [Google Scholar] [CrossRef]
- Vetter, I.; Touska, F.; Hess, A.; Hinsbey, R.; Sattler, S.; Lampert, A.; Sergejeva, M.; Sharov, A.; Collins, L.S.; Eberhardt, M.; et al. Ciguatoxins activate specific cold pain pathways to elicit burning pain from cooling: How ciguatoxins cause burning pain from cooling. EMBO J. 2012, 31, 3795–3808. [Google Scholar] [CrossRef] [Green Version]
- Pearn, J.H. Chronic fatigue syndrome: Chronic ciguatera poisoning as a differential diagnosis. Med. J. Aust. 1997, 166, 309–310. [Google Scholar] [CrossRef]
- Arulanandam, C.D.; Dharmara, R.; Ragothaman, P.; Vincent, S.G.P. Use of Marine biotoxins to modulate the tyrosine kinase domain of the human epidermal growth factor receptor. ChemRxiv 2021. [Google Scholar] [CrossRef]
- Ryan, J.C.; Morey, J.S.; Bottein, M.-Y.D.; Ramsdell, J.S.; Van Dolah, F.M. Gene expression profiling in brain of mice exposed to the marine neurotoxin ciguatoxin reveals an acute anti-inflammatory, neuroprotective response. BMC Neurosci. 2010, 11, 107. [Google Scholar] [CrossRef] [Green Version]
- Rubiolo, J.; Vale, C.; Boente-Juncal, A.; Hirama, M.; Yamashita, S.; Camiña, M.; Vieytes, M.; Botana, L. Transcriptomic Analysis of Ciguatoxin-Induced Changes in Gene Expression in Primary Cultures of Mice Cortical Neurons. Toxins 2018, 10, 192. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.C.; Wu, Q.; Shoemaker, R.C. Transcriptomic signatures in whole blood of patients who acquire a chronic inflammatory response syndrome (CIRS) following an exposure to the marine toxin ciguatoxin. BMC Med. Genom. 2015, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Guillotin, S.; Delcourt, N. Marine Neurotoxins’ Effects on Environmental and Human Health: An OMICS Overview. Mar. Drugs 2021, 20, 18. [Google Scholar] [CrossRef]
- Ogura, A.; Ohizumi, Y.; Yasumoto, T.; Kasei, M. Calcium-dependent depolarization induced by a marine toxin, maitotoxin, in a neuronal cell. Jpn. J. Pharmacol. 1984, 36, 315. [Google Scholar] [CrossRef]
- Gusovsky, F.; Bitran, J.A.; Yasumoto, T.; Daly, J.W. Mechanism of maitotoxin-stimulated phosphoinositide breakdown in HL-60 cells. J. Pharmacol. Exp. Ther. 1990, 252, 466–473. [Google Scholar]
- Ohizumi, Y.; Yasumoto, T. Contraction and increase in tissue calcium content induced by maitotoxin, the most potent known marine toxin, in intestinal smooth muscle. Br. J. Pharmacol. 1983, 79, 3–5. [Google Scholar] [CrossRef]




| Species | Ciguatoxins (CTXs) | Maitotoxins (MTXs) | Others | References |
|---|---|---|---|---|
| Gambierdiscus australes | CTX1B, P-CTX-3C | MTX, MTX-3 | P-Gambierone analogue, putative gambieroxide | [7,82,83] |
| Gambierdiscus balechii | gambierone | [84] | ||
| Gambierdiscus belizeanus | MTX-3 | [85] | ||
| Gambierdiscus cheloniae | MTX-3 | gambierone | [6,86] | |
| Gambierdiscus excentricus | MTX-4 | [83] | ||
| Gambierdiscus honu | MTX-3 | [6,34] | ||
| Gambierdiscus pacificus | 51-hydroxyCTX-3C, 2,3-dihydroxyCTX-3C | MTX-3 | [6,87] | |
| Gambierdiscus polynesiensis | P-CTX-4A, P-CTX-4B, P-CTX-3C, M-seco-CTX-3C, 49-epiCTX-3C | MTX-1, MTX-3 | [31] | |
| Gambierdiscus toxicus | P-CTX-3C, 2,3-dihydroxy P-CTX-3C, P-CTX-4A/B | Gambieric acids, gambierol, gambieroxide | [88,89,90] |
| Detection Methods | Advantages | Shortcomings | Commercialized Kits |
|---|---|---|---|
| Mouse bioassay | Easy to use | Expensive, lacks specificity, not sensitive enough, and ethical concerns | |
| Mouse neuroblastoma cell-based assay (CBA-N2a) | Automatable | Expensive, time-consuming, requires specific instruments, and lacks specificity [132] | |
| Radioimmunoassay | Sensitive | Expensive, time-consuming, and requires specific instruments | |
| Fluorescent receptor binding assay | Fast | Detection limit is higher than for the CBA-N2a [126] | SeaTox® F-RBA [126] |
| Enzyme immunoassay | Easy to use | Cross-reaction with other polyether compounds [25] | Ciguatect™ [127] |
| Antibody-based immunoassays | Sensitive, field application | Cross-reaction with okadaic acid [121] | |
| Membrane immunobead assay | Specificity | Variation in signal strength [128] | Cigua Check® [128] |
| Enzyme-linked immunosorbent assay (ELISA) | Sensitive, low detection limit | Need laboratory conditions, require anti-CTX antibodies | CTX-ELISATM 1B [133] |
| Capillary electrophoresis-based immunoassay | Faster than ELISA | Need laboratory conditions, require anti-CTX antibodies | |
| Electrochemical immunosensors | Low cost, integrable | ||
| LC–MS/MS | Sensitive, selective | Lack of reference toxins, cannot be used in the field |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.-Z.; Xin, Y.-H.; Wang, M.-H. Gambierdiscus and Its Associated Toxins: A Minireview. Toxins 2022, 14, 485. https://doi.org/10.3390/toxins14070485
Wang D-Z, Xin Y-H, Wang M-H. Gambierdiscus and Its Associated Toxins: A Minireview. Toxins. 2022; 14(7):485. https://doi.org/10.3390/toxins14070485
Chicago/Turabian StyleWang, Da-Zhi, Ye-Hong Xin, and Ming-Hua Wang. 2022. "Gambierdiscus and Its Associated Toxins: A Minireview" Toxins 14, no. 7: 485. https://doi.org/10.3390/toxins14070485
APA StyleWang, D. -Z., Xin, Y. -H., & Wang, M. -H. (2022). Gambierdiscus and Its Associated Toxins: A Minireview. Toxins, 14(7), 485. https://doi.org/10.3390/toxins14070485