The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice
Abstract
:1. Introduction
2. Results
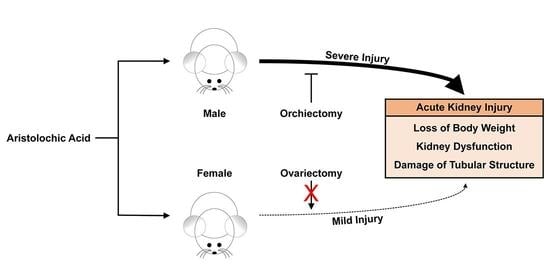
2.1. Female Mice Are More Resistant to AA-Induced Nephrotoxicity
2.2. Ovariectomy Has No Effect on AA-Induced Nephrotoxicity in Female Mice
2.3. Orchiectomy Reduces Susceptibility to AA-Induced Nephrotoxicity in Male Mice
3. Discussion
4. Materials and Methods
4.1. Animal Preparation
4.2. Body Weight
4.3. Kidney Function
4.4. Tubular Injury Score
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butler, J. Bodies That Matter: On the Discursive Limits of Sex, 1st ed.; Routledge: Abingdon-on-Thames, UK, 2011; p. 219. [Google Scholar]
- Miguel-Aliaga, I. Let’s talk about (biological) sex. Nat. Rev. Mol. Cell Biol. 2022, 23, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.L.; Harwood, D.; Bender, L.; Shrestha, L.; Brands, M.W.; Morwitzer, M.J.; Kennard, S.; Antonova, G.; Belin de Chantemele, E.J. Lack of Suppression of Aldosterone Production Leads to Salt-Sensitive Hypertension in Female but Not Male Balb/C Mice. Hypertension 2018, 72, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Brady, T.M.; Roem, J.; Cox, C.; Schneider, M.F.; Wilson, A.C.; Furth, S.L.; Warady, B.A.; Mitsnefes, M. Adiposity, Sex, and Cardiovascular Disease Risk in Children with CKD: A Longitudinal Study of Youth Enrolled in the Chronic Kidney Disease in Children (CKiD) Study. Am. J. Kidney Dis. 2020, 76, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Park, K.M.; Kim, J.I.; Ahn, Y.; Bonventre, A.J.; Bonventre, J.V. Testosterone is responsible for enhanced susceptibility of males to ischemic renal injury. J. Biol. Chem. 2004, 279, 52282–52292. [Google Scholar] [CrossRef]
- Morrow, E.H. The evolution of sex differences in disease. Biol. Sex. Differ. 2015, 6, 5. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Female sex reduces the risk of hospital-associated acute kidney injury: A meta-analysis. BMC Nephrol. 2018, 19, 314. [Google Scholar] [CrossRef]
- Vanherweghem, J.L.; Depierreux, M.; Tielemans, C.; Abramowicz, D.; Dratwa, M.; Jadoul, M.; Richard, C.; Vandervelde, D.; Verbeelen, D.; Vanhaelen-Fastre, R.; et al. Rapidly progressive interstitial renal fibrosis in young women: Association with slimming regimen including Chinese herbs. Lancet 1993, 341, 387–391. [Google Scholar] [CrossRef]
- Grollman, A.P.; Shibutani, S.; Moriya, M.; Miller, F.; Wu, L.; Moll, U.; Suzuki, N.; Fernandes, A.; Rosenquist, T.; Medverec, Z.; et al. Aristolochic acid and the etiology of endemic (Balkan) nephropathy. Proc. Natl. Acad. Sci. USA 2007, 104, 12129–12134. [Google Scholar] [CrossRef]
- Cosyns, J.P.; Jadoul, M.; Squifflet, J.P.; De Plaen, J.F.; Ferluga, D.; van Ypersele de Strihou, C. Chinese herbs nephropathy: A clue to Balkan endemic nephropathy? Kidney Int. 1994, 45, 1680–1688. [Google Scholar] [CrossRef]
- Yang, L.; Su, T.; Li, X.M.; Wang, X.; Cai, S.Q.; Meng, L.Q.; Zou, W.Z.; Wang, H.Y. Aristolochic acid nephropathy: Variation in presentation and prognosis. Nephrol. Dial. Transplant. 2012, 27, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Ban, T.H.; Min, J.W.; Seo, C.; Kim, D.R.; Lee, Y.H.; Chung, B.H.; Jeong, K.H.; Lee, J.W.; Kim, B.S.; Lee, S.H.; et al. Update of aristolochic acid nephropathy in Korea. Korean J. Intern Med. 2018, 33, 961–969. [Google Scholar] [CrossRef]
- Chen, D.; Tang, Z.; Luo, C.; Chen, H.; Liu, Z. Clinical and pathological spectrums of aristolochic acid nephropathy. Clin. Nephrol. 2012, 78, 54–60. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; Dagher, P.C. Tubular cross talk in acute kidney injury: A story of sense and sensibility. Am. J. Physiol. Ren. Physiol. 2015, 308, F1317–F1323. [Google Scholar] [CrossRef]
- Kim, J.; Padanilam, B.J. Renal denervation prevents long-term sequelae of ischemic renal injury. Kidney Int. 2015, 87, 350–358. [Google Scholar] [CrossRef]
- Kim, J.; Devalaraja-Narashimha, K.; Padanilam, B.J. TIGAR regulates glycolysis in ischemic kidney proximal tubules. Am. J. Physiol. Ren. Physiol. 2015, 308, F298–F308. [Google Scholar] [CrossRef]
- Heemskerk, S.; Pickkers, P.; Bouw, M.P.; Draisma, A.; van der Hoeven, J.G.; Peters, W.H.; Smits, P.; Russel, F.G.; Masereeuw, R. Upregulation of renal inducible nitric oxide synthase during human endotoxemia and sepsis is associated with proximal tubule injury. Clin. J. Am. Soc. Nephrol. 2006, 1, 853–862. [Google Scholar] [CrossRef]
- Yang, L.; Besschetnova, T.Y.; Brooks, C.R.; Shah, J.V.; Bonventre, J.V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 2010, 16, 535–543. [Google Scholar] [CrossRef]
- Atanasova, S.Y.; von Ahsen, N.; Toncheva, D.I.; Dimitrov, T.G.; Oellerich, M.; Armstrong, V.W. Genetic polymorphisms of cytochrome P450 among patients with Balkan endemic nephropathy (BEN). Clin. Biochem. 2005, 38, 223–228. [Google Scholar] [CrossRef]
- Nie, W.; Lv, Y.; Yan, L.; Chen, X.; Lv, H. Prediction and Characterisation of the System Effects of Aristolochic Acid: A Novel Joint Network Analysis towards Therapeutic and Toxicological Mechanisms. Sci. Rep. 2015, 5, 17646. [Google Scholar] [CrossRef]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995–1043. [Google Scholar] [CrossRef]
- Shi, M.; Ma, L.; Zhou, L.; Fu, P. Renal Protective Effects of 17beta-Estradiol on Mice with Acute Aristolochic Acid Nephropathy. Molecules 2016, 21, 1391. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, J.P.; Chapman, L.M.; Kretschmer, X.C.; Baldwin, W.S. Gender-specific induction of cytochrome P450s in nonylphenol-treated FVB/NJ mice. Toxicol. Appl. Pharmacol. 2006, 216, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Alevizaki, M.; Saltiki, K.; Cimponeriu, A.; Kanakakis, I.; Xita, N.; Alevizaki, C.C.; Georgiou, I.; Sarika, H.L. Severity of cardiovascular disease in postmenopausal women: Associations with common estrogen receptor alpha polymorphic variants. Eur. J. Endocrinol. 2007, 156, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Cassis, L.A.; Eghbali, M.; Reue, K.; Sandberg, K. Sex Hormones and Sex Chromosomes Cause Sex Differences in the Development of Cardiovascular Diseases. Arter. Thromb. Vasc. Biol. 2017, 37, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kil, I.S.; Seok, Y.M.; Yang, E.S.; Kim, D.K.; Lim, D.G.; Park, J.W.; Bonventre, J.V.; Park, K.M. Orchiectomy attenuates post-ischemic oxidative stress and ischemia/reperfusion injury in mice. A role for manganese superoxide dismutase. J. Biol. Chem. 2006, 281, 20349–20356. [Google Scholar] [CrossRef]
- Neugarten, J.; Golestaneh, L. Sex Differences in Acute Kidney Injury. Semin. Nephrol. 2022, 42, 208–218. [Google Scholar] [CrossRef]
- Mengs, U. Acute toxicity of aristolochic acid in rodents. Arch. Toxicol. 1987, 59, 328–331. [Google Scholar] [CrossRef]
- Gordon, C.J. Thermophysiological responses to hyperthermic drugs: Extrapolating from rodent to human. Prog. Brain Res. 2007, 162, 63–79. [Google Scholar] [CrossRef]
- Ekwall, B.; Clemedson, C.; Crafoord, B.; Ekwall, B.; Hallander, S.; Walum, E.; Bondesson, I. MEIC Evaluation of Acute Systemic Toxicity: Part V. Rodent and Human Toxicity Data for the 50 Reference Chemicals. Altern. Lab. Anim. 1998, 26 (Suppl. 2), 571–616. [Google Scholar]
- Moon, D.; Padanilam, B.J.; Jang, H.S.; Kim, J. 2-Mercaptoethanol protects against DNA double-strand breaks after kidney ischemia and reperfusion injury through GPX4 upregulation. Pharmacol. Rep. 2022, 74, 1041–1053. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.-L.; Padanilam, B.J.; Kim, J. The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice. Toxins 2023, 15, 118. https://doi.org/10.3390/toxins15020118
Li W-L, Padanilam BJ, Kim J. The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice. Toxins. 2023; 15(2):118. https://doi.org/10.3390/toxins15020118
Chicago/Turabian StyleLi, Wei-Long, Babu J. Padanilam, and Jinu Kim. 2023. "The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice" Toxins 15, no. 2: 118. https://doi.org/10.3390/toxins15020118
APA StyleLi, W. -L., Padanilam, B. J., & Kim, J. (2023). The Presence of Testis Determines Aristolochic Acid-Induced Nephrotoxicity in Mice. Toxins, 15(2), 118. https://doi.org/10.3390/toxins15020118