Degradation of Swainsonine by the NADP-Dependent Alcohol Dehydrogenase A1R6C3 in Arthrobacter sp. HW08
Abstract
:1. Introduction
2. Results
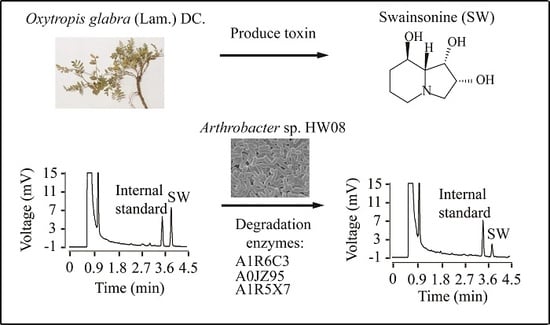
2.1. Degradation of SW by Strain HW08
2.2. LC-ESI-MS/MS Analysis of Strain HW08
2.3. Bioinformatics Analysis of SW Degradation-Relevant Differentially Expressed Proteins
2.4. Screening and Validation of SW-Degrading Genes
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Bacterial Culture and Gas Chromatographic Analysis
5.2. LC-ESI-MS/MS Analysis
5.3. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes Analysis
5.4. Gene Cloning and Expression
5.5. Real Time RT-PCR Analysis
5.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| BCA | bicinchoninic acid |
| CGMCC | China General Microbiological Culture Collection Center |
| FASP | filter-aided sample preparation |
| FID | flame ionization detector |
| GC | gas chromatographic |
| GO | Gene ontology |
| iBAQ | intensity-based absolute quantification |
| IPTG | isopropyl β-d-thiogalactoside |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LB | Luria-Bertani |
| LC-ESI-MS/MS | liquid chromatography-tandem mass spectrometry |
| LFQ | label-free quantitative |
| me-Gal | methyl α-d-galactopyranoside |
| MS | mass spectrometry |
| MSM | mineral salts medium |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
References
- Wu, C.; Wang, W.; Liu, X.; Ma, F.; Cao, D.; Yang, X.; Wang, S.; Geng, P.; Lu, H.; Zhao, B. Pathogenesis and preventive treatment for animal disease due to locoweed poisoning. Environ. Toxicol. Pharmacol. 2014, 37, 336–347. [Google Scholar]
- Obeidat, B.S.; Strickland, J.R.; Vogt, M.L.; Taylor, J.B.; Krehbiel, C.R.; Remmenga, M.D.; Clayshulte-Ashley, A.K.; Whittet, K.M.; Hallford, D.M.; Hernandez, J.A. Effects of locoweed on serum swainsonine and selected serum constituents in sheep during acute and subacute oral/intraruminal exposure. J. Anim. Sci. 2005, 83, 466–477. [Google Scholar] [PubMed]
- Yu, Y.; Zhao, Q.; Wang, J.; Wang, Y.; Song, Y.; Geng, G.; Li, Q. Swainsonine-producing fungal endophytes from major locoweed species in china. Toxicon 2010, 56, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, B.L.; James, L.F.; Panter, K.E.; Gardner, D.R.; Ralphs, M.H.; Pfister, J.A. Tissue swainsonine clearance in sheep chronically poisoned with locoweed (Oxytropis sericea). J. Anim. Sci. 1998, 76, 1140–1144. [Google Scholar] [PubMed]
- Wang, Y.; Li, Y.; Hu, Y.; Li, J.; Yang, G.; Kang, D.; Li, H.; Wang, J. Potential degradation of swainsonine by intracellular enzymes of Arthrobacter sp. HW08. Toxins (Basel) 2013, 5, 2161–2171. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, L.; Zhang, Q.; Zhang, W.; An, L.; Zhang, Y.; Tong, D.; Zhao, B.; Chen, S.; Zhao, S. Exposure to swainsonine impairs adult neurogenesis and spatial learning and memory. Toxicol. Lett. 2014, 232, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Wang, S.S.; Wang, W.L.; Zhang, L.; Zhao, B.Y. Effect of swainsonine in Oxytropis kansuensis on golgi α-mannosidase II expression in the brain tissues of sprague-dawley rats. J. Agric. Food Chem. 2014, 62, 7407–7412. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, W.; Zhao, X.; Ding, L.; Yu, G.; Dong, F.; Du, Q.; Xu, X.; Tong, D. Swainsonine differentially affects steroidogenesis and viability in caprine luteal cells in vitro. Theriogenology 2013, 80, 41–49. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, K.; Sterling, T.; Ivey, S. Herbicidal control of locoweed. Int. Symp. Poisonous Plants 2005, 6, 6–10. [Google Scholar]
- Ralphs, M.H.; Monaco, T.A. Seeding cool-season grasses to suppress white locoweed (Oxytropis sericea) reestablishment and increase forage production. Weed Technol. 2007, 21, 661–669. [Google Scholar] [CrossRef]
- Tong, D.W.; Mu, P.H.; Dong, Q.; Zhao, B.Y.; Liu, W.M.; Zhao, J.; Li, L.; Zhou, T.; Wang, J.Y.; Sui, G.D. Immunological evaluation of SW-HSA conjugate on goats. Coll. Surf. B Biointerfaces 2007, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, Y.C.; Wang, J.H.; Yu, Y.T.; Song, Y.M.; Yang, G.D.; Geng, G.X. Isolation and characterization of Arthrobacter sp. HW08 capable of biodegrading swainsonine. Afr. J. Microbiol. Res. 2010, 4, 1635–1638. [Google Scholar]
- Abraham, D.J.; Sidebothom, R.; Winchester, B.G.; Dorling, P.R.; Dell, A. Swainsonine affects the processing of glycoproteins in vivo. FEBS Lett. 1983, 163, 110–113. [Google Scholar] [CrossRef]
- Kang, M.S.; Elbein, A.D. Mechanism of inhibition of jack bean α-mannosidase by swainsonine. Plant Physiol. 1983, 71, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Stegelmeier, B.L.; Edgar, J.A.; Colegate, S.M.; Gardner, D.R.; Schoch, T.K.; Coulombe, R.A.; Molyneux, R.J. Pyrrolizidine alkaloid plants, metabolism and toxicity. J. Nat. Toxins 1999, 8, 95–116. [Google Scholar] [PubMed]
- Zhang, D.C.; Schumann, P.; Liu, H.C.; Xin, Y.H.; Zhou, Y.G.; Schinner, F.; Margesin, R. Arthrobacter alpinus sp. Nov., a psychrophilic bacterium isolated from alpine soil. Int. J. Syst. Evolut. Microbiol. 2010, 60, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Mongodin, E.F.; Shapir, N.; Daugherty, S.C.; DeBoy, R.T.; Emerson, J.B.; Shvartzbeyn, A.; Radune, D.; Vamathevan, J.; Riggs, F.; Grinberg, V.; et al. Secrets of soil survival revealed by the genome sequence of Arthrobacter aurescens TC1. PLoS Genet. 2006, 2. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, N.K.; Pakshirajan, K.; Ghosh, P.K. Biodegradation of 4-bromophenol by Arthrobacter chlorophenolicus A6 in batch shake flasks and in a continuously operated packed bed reactor. Biodegradation 2014, 25, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Pipke, R.; Amrhein, N. Isolation and characterization of a mutant of Arthrobacter sp. Strain GLP-1 which utilizes the herbicide glyphosate as its sole source of phosphorus and nitrogen. Appl. Environ. Microbiol. 1988, 54, 2868–2870. [Google Scholar] [PubMed]
- Li, S.S.; Zhang, D.; Yan, W. Enhanced biodegradation of methyl tert-butyl-ether by a microbial consortium. Curr. Microbiol. 2014, 68, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Chong, N.M.; Chang, H.W. Plasmid as a measure of microbial degradation capacity for 2,4-dichlorophenoxyacetic acid. Bioresour. Technol. 2009, 100, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Baitsch, D.; Sandu, C.; Brandsch, R.; Igloi, G.L. Gene cluster on pAO1 of Arthrobacter nicotinovorans involved in degradation of the plant alkaloid nicotine: Cloning, purification, and characterization of 2,6-dihydroxypyridine 3-hydroxylase. J. Bacteriol. 2001, 183, 5262–5267. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.K.; Jain, R.K. Pathway for degradation of 2-chloro-4-nitrophenol in Arthrobacter sp. SJCon. Curr. Microbiol. 2011, 63, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Sabourin, C.L.; Carpenter, J.C.; Leib, T.K.; Spivack, J.L. Biodegradation of dimethylsilanediol in soils. Appl. Environ. Microbiol. 1996, 62, 4352–4360. [Google Scholar] [PubMed]
- Casellas, M.; Grifoll, M.; Bayona, J.M.; Solanas, A.M. New metabolites in the degradation of fluorene by Arthrobacter sp. Strain F101. Appl. Environ. Microbiol. 1997, 63, 819–826. [Google Scholar] [PubMed]
- Wu, X.; Liang, R.; Dai, Q.; Jin, D.; Wang, Y.; Chao, W. Complete degradation of di-n-octyl phthalate by biochemical cooperation between Gordonia sp. Strain JDC-2 and Arthrobacter sp. Strain JDC-32 isolated from activated sludge. J. Hazard. Mater. 2010, 176, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Johana, H.; Spain, J.C.; Hughes, J.B. Growth of Arthrobacter sp. Strain JBH1 on nitroglycerin as the sole source of carbon and nitrogen. Appl. Environ. Microbiol. 2010, 76, 1689–1691. [Google Scholar]
- Strong, L.C.; Rosendahl, C.; Johnson, G.; Sadowsky, M.J.; Wackett, L.P. Arthrobacter aurescens TC1 metabolizes diverse s-triazine ring compounds. Appl. Environ. Microbiol. 2002, 68, 5973–5980. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Jauregui, O.; Casanova, E.; Trillas, I. Simultaneous quantitative LC-ESI-MS/MS analyses of salicylic acid and jasmonic acid in crude extracts of Cucumis sativus under biotic stress. Phytochemistry 2006, 67, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Johnston, M. Purification and properties of a secondary alcohol dehydrogenase from the parasitic protozoan tritrichomonas foetus. J. Biol. Chem. 1985, 260, 8038–8043. [Google Scholar] [PubMed]
- Valencia, E.; Rosell, A.; Larroy, C.; Farres, J.; Biosca, J.A.; Fita, I.; Pares, X.; Ochoa, W.F. Crystallization and preliminary X-ray analysis of NADP(H)-dependent alcohol dehydrogenases from Saccharomyces cerevisiae and Rana perezi. Acta Crystallogr. D Biol. Crystallogr. 2003, 59, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dong, Y.; Zhang, J.; Zhang, A.; Wang, L.; Feng, L. Two novel metal-independent long-chain alkyl alcohol dehydrogenases from Geobacillus thermodenitrificans NG80-2. Microbiology 2009, 155, 2078–2085. [Google Scholar] [CrossRef] [PubMed]
- Radianingtyas, H.; Wright, P.C. Alcohol dehydrogenases from thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol. Rev. 2003, 27, 593–616. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.C.; Wang, J.H.; Liu, Z.B.; Yang, G.D.; Geng, G.X. Ultrasound-assisted solvent extraction of swainsonine from Oxytropis ochrocephala bunge. J. Med. Plants Res. 2011, 5, 890–894. [Google Scholar]
- Akio, U.; Yukiya, I.; Isao, Y.; Hidetoshi, O. Isolation and characterization of bacteria from soil contaminated with diesel oil and the possible use of these in autochthonous bioaugmentation. World J. Microbiol. Biotechnol. 2007, 23, 1739–1745. [Google Scholar]
- Zhao, X.H.; He, X.; Wang, J.N.; Song, Y.M.; Geng, G.X.; Wang, J.H. Biodegradation of swainsonine by acinetobacter calcoaceticus strain YLZZ-1 and its isolation and identification. Biodegradation 2009, 20, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.H.; Zhao, X.H.; Li, Q.F.; Wang, J.H.; Geng, G.X. Determination of swainsonine in astragalus locoweed by internal standard gas chromatography. J. Northwest A F Univ. (Nat. Sci. Ed.) 2008, 36, 17–21. [Google Scholar]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Schwanhausser, B.; Busse, D.; Li, N.; Dittmar, G.; Schuchhardt, J.; Wolf, J.; Chen, W.; Selbach, M. Global quantification of mammalian gene expression control. Nature 2011, 473, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Luber, C.A.; Cox, J.; Lauterbach, H.; Fancke, B.; Selbach, M.; Tschopp, J.; Akira, S.; Wiegand, M.; Hochrein, H.; O’Keeffe, M.; et al. Quantitative proteomics reveals subset-specific viral recognition in dendritic cells. Immunity 2010, 32, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009, 1, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S.; Sato, Y.; Furumichi, M.; Tanabe, M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012, 40, D109–D114. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of∆ relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]





| Pathway | Number of Matched Pathways | Pathway | Number of Matched Pathways |
|---|---|---|---|
| Biotin metabolism | 1 | Phenylpropanoid biosynthesis | 1 |
| Pyruvate metabolism | 2 | Aminoacyl-tRNA biosynthesis | 2 |
| Glycolysis/Gluconeogenesis | 3 | Butanoate metabolism | 2 |
| Arginine and proline metabolism | 2 | Benzoate degradation | 1 |
| Fatty acid degradation | 1 | Purine metabolism | 4 |
| Glycine, serine and threonine metabolism | 2 | Pantothenate and CoA biosynthesis | 1 |
| Ubiquinone and other terpenoid-quinone biosynthesis | 1 | Glutathione metabolism | 1 |
| Styrene degradation | 1 | Caprolactam degradation | 1 |
| Ethylbenzene degradation | 1 | T cell receptor signaling pathway | 1 |
| Tyrosine metabolism | 1 | Fatty acid elongation | 1 |
| One carbon pool by folate | 3 | Fatty acid biosynthesis | 2 |
| Biosynthesis of unsaturated fatty acids | 2 | Alanine, aspartate and glutamate metabolism | 1 |
| Geraniol degradation | 1 | Valine, leucine, and isoleucine degradation | 2 |
| Thiamine metabolism | 1 | beta-Alanine metabolism | 1 |
| Nicotinate and nicotinamide metabolism | 1 | Glyoxylate and dicarboxylate metabolism | 1 |
| Citrate cycle (TCA cycle) | 2 | Glycerolipid metabolism | 1 |
| Pyrimidine metabolism | 1 | alpha-Linolenic acid metabolism | 1 |
| Phenylalanine, tyrosine and tryptophan biosynthesis | 2 | Carbon fixation pathways in prokaryotes | 1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhai, A.; Zhang, Y.; Qiu, K.; Wang, J.; Li, Q. Degradation of Swainsonine by the NADP-Dependent Alcohol Dehydrogenase A1R6C3 in Arthrobacter sp. HW08. Toxins 2016, 8, 145. https://doi.org/10.3390/toxins8050145
Wang Y, Zhai A, Zhang Y, Qiu K, Wang J, Li Q. Degradation of Swainsonine by the NADP-Dependent Alcohol Dehydrogenase A1R6C3 in Arthrobacter sp. HW08. Toxins. 2016; 8(5):145. https://doi.org/10.3390/toxins8050145
Chicago/Turabian StyleWang, Yan, A’guan Zhai, Yanqi Zhang, Kai Qiu, Jianhua Wang, and Qinfan Li. 2016. "Degradation of Swainsonine by the NADP-Dependent Alcohol Dehydrogenase A1R6C3 in Arthrobacter sp. HW08" Toxins 8, no. 5: 145. https://doi.org/10.3390/toxins8050145
APA StyleWang, Y., Zhai, A., Zhang, Y., Qiu, K., Wang, J., & Li, Q. (2016). Degradation of Swainsonine by the NADP-Dependent Alcohol Dehydrogenase A1R6C3 in Arthrobacter sp. HW08. Toxins, 8(5), 145. https://doi.org/10.3390/toxins8050145