Development of Pressure Sensor Based Wearable Pulse Detection Device for Radial Pulse Monitoring
Abstract
:1. Introduction
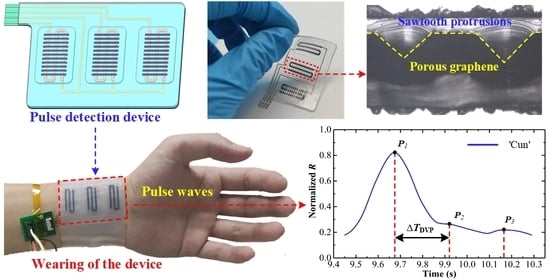
2. Design of Wearable Pulse Detection Device
3. Experimental Setup and Procedure
3.1. Material Preparation and Device Fabrication
3.2. Characterization of Wearable Pulse Detection Device
3.3. Radial Pulse Monitoring Experimental Procedure
4. Results and Discussion
4.1. Characterization Results of Wearable Pulse Detection Device
4.2. Radial Pulse Monitoring Experimental Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, X.; Luo, F.; Yuan, M.; Xie, D.; Shen, L.; Zheng, K.; Wang, Z.; Li, X.; Tao, L.Q. A Dual-functional graphene-based self-alarm health-monitoring e-skin. Adv. Funct. Mater. 2019, 29, 1904706. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y.; Song, C.; Sheng, S.; Yang, L.; Yan, Z.; Hu, D.J.J.; Sun, Q. Wearable alignment-free microfiber-based sensor chip for precise vital signs monitoring and cardiovascular assessment. Adv. Fiber Mater. 2022, 4, 475–486. [Google Scholar] [CrossRef]
- Meng, K.; Xiao, X.; Wei, W.; Chen, G.; Nashalian, A.; Shen, S.; Xiao, X.; Chen, J. Wearable pressure sensors for pulse wave monitoring. Adv. Mater. 2022, 34, e2109357. [Google Scholar] [CrossRef]
- Lin, J.; Fu, R.; Zhong, X.; Yu, P.; Tan, G.; Li, W.; Zhang, H.; Li, Y.; Zhou, L.; Ning, C. Wearable sensors and devices for real-time cardiovascular disease monitoring. Cell Rep. Phys. Sci. 2021, 2, 100541. [Google Scholar] [CrossRef]
- Rodriguez-Labra, J.I.; Kosik, C.; Maddipatla, D.; Narakathu, B.B.; Atashbar, M.Z. Development of a PPG sensor array as a wearable device for monitoring cardiovascular metrics. IEEE Sens. J. 2021, 21, 26320–26327. [Google Scholar] [CrossRef]
- Choi, H.; Sun, J.; Ren, B.; Cha, S.; Lee, J.; Lee, B.-M.; Park, J.-J.; Choi, J.-H.; Park, J.-J. 3D textile structure-induced local strain for a highly amplified piezoresistive performance of carbonized cellulose fabric based pressure sensor for human healthcare monitoring. Chem. Eng. J. 2022, 450, 138193. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, Y.; Wu, Z.; Zhang, Y.; Lin, J.; Chen, T.; Liu, H.; Wang, F.; Sun, L. Wearable multichannel pulse condition monitoring system based on flexible pressure sensor arrays. Microsyst. Nanoeng. 2022, 8, 16. [Google Scholar] [CrossRef]
- Velik, R. An objective review of the technological developments for radial pulse diagnosis in Traditional Chinese Medicine. Eur. J. Integr. Med. 2015, 7, 321–331. [Google Scholar] [CrossRef]
- Geng, X.; Liu, S.; Zhang, Y.; Hou, J.; Zhang, S.; Zhang, J.; Zhang, H. A Noncontact method for locating radial artery above radial styloid process in thermal image. Evid.-Based Complement Altern. Med. 2020, 2020, 4057154. [Google Scholar] [CrossRef]
- Wang, Y.; Miao, F.; An, Q.; Liu, Z.; Chen, C.; Li, Y. Wearable multimodal vital sign monitoring sensor with fully integrated analog front end. IEEE Sens. J. 2022, 22, 13462–13471. [Google Scholar] [CrossRef]
- Long, N.M.H.; Chung, W.-Y. Wearable Wrist Photoplethysmography for optimal monitoring of vital signs: A unified perspective on pulse waveforms. IEEE Photonics J. 2022, 14, 3717717. [Google Scholar] [CrossRef]
- Kang, S.; Pradana Rachim, V.; Baek, J.-H.; Lee, S.Y.; Park, S.-M. A Flexible patch-type strain sensor based on polyaniline for continuous monitoring of pulse waves. IEEE Access 2020, 8, 152105–152115. [Google Scholar] [CrossRef]
- Pan, L.; Han, L.; Liu, H.; Zhao, J.; Dong, Y.; Wang, X. Flexible sensor based on Hair-like microstructured ionic hydrogel with high sensitivity for pulse wave detection. Chem. Eng. J. 2022, 450, 137929. [Google Scholar] [CrossRef]
- Chen, J.; Sun, K.; Zheng, R.; Sun, Y.; Yang, H.; Zhong, Y.; Li, X. Three-dimensional arterial pulse signal acquisition in time domain using flexible pressure-sensor dense arrays. Micromachines 2021, 12, 569. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Lee, Y.; Baek, J.; Kwon, J.; Kim, S.; Lee, S.; Strunk, K.P.; Stehlin, S.; Melzer, C.; Park, S.M.; et al. Spatiotemporal measurement of arterial pulse waves enabled by wearable active-matrix pressure sensor arrays. ACS Nano 2021, 16, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ye, Z.; Shi, F.; Dai, Y.; Yang, L.; Wu, J.; Wang, Y. Reflection-type photoplethysmography pulse sensor based on an integrated optoelectronic chip with a ring structure. Biomed. Opt. Express 2021, 12, 6277–6283. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Gandla, S.; Lim, B.; Kang, S.; Kim, S.; Lee, S.; Kim, S. Alcohol-based highly conductive polymer for conformal nanocoatings on hydrophobic surfaces toward a highly sensitive and stable pressure sensor. NPG Asia Mater. 2020, 12, 65. [Google Scholar] [CrossRef]
- Xia, K.; Wang, C.; Jian, M.; Wang, Q.; Zhang, Y. CVD growth of fingerprint-like patterned 3D graphene film for an ultrasensitive pressure sensor. Nano Res. 2017, 11, 1124–1134. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Liu, Y.; Ma, C.; Cheng, H.-C.; He, Q.; Wu, H.; Wang, C.; Lin, C.-Y.; Huang, Y.; Duan, X. Sensitive pressure sensors based on conductive microstructured air-gap gates and two-dimensional semiconductor transistors. Nat. Electron. 2020, 3, 59–69. [Google Scholar] [CrossRef]
- Park, J.B.; Song, M.S.; Ghosh, R.; Saroj, R.K.; Hwang, Y.; Tchoe, Y.; Oh, H.; Baek, H.; Lim, Y.; Kim, B.; et al. Highly sensitive and flexible pressure sensors using position- and dimension-controlled ZnO nanotube arrays grown on graphene films. NPG Asia Mater. 2021, 13, 57. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Shi, N.; Liu, Z.; Zhang, X.; Wu, M.; Pan, C.; Liu, H.; Li, L.; Wang, Z.L. Transparent and stretchable triboelectric nanogenerator for self-powered tactile sensing. Nano Energy 2019, 59, 302–310. [Google Scholar] [CrossRef]
- Huang, K.-H.; Tan, F.; Wang, T.-D.; Yang, Y.-J. A tactile sensing array integrated with tension sensor for continuously monitoring blood pulse waves. Microelectron. Eng. 2019, 218, 111132. [Google Scholar] [CrossRef]
- Shi, J.; Wang, L.; Dai, Z.; Zhao, L.; Du, M.; Li, H.; Fang, Y. Multiscale hierarchical design of a flexible piezoresistive pressure sensor with high sensitivity and wide linearity range. Small 2018, 14, e1800819. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, Z.; Lv, C.; Wang, Z.; Lu, Z.; Lu, G.; Jia, X.; Wang, C. Electrospun bifunctional MXene-based electronic skins with high performance electromagnetic shielding and pressure sensing. Compos. Sci. Technol. 2022, 221, 109313. [Google Scholar] [CrossRef]
- Zhong, Y.; Tan, X.; Shi, T.; Huang, Y.; Cheng, S.; Chen, C.; Liao, G.; Tang, Z. Tunable wrinkled graphene foams for highly reliable piezoresistive sensor. Sens. Actuator A-Phys. 2018, 281, 141–149. [Google Scholar] [CrossRef]
- Thouti, E.; Nagaraju, A.; Chandran, A.; Prakash, P.V.B.S.S.; Shivanarayanamurthy, P.; Lal, B.; Kumar, P.; Kothari, P.; Panwar, D. Tunable flexible capacitive pressure sensors using arrangement of polydimethylsiloxane micro-pyramids for bio-signal monitoring. Sens. Actuator A-Phys. 2020, 314, 112251. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Hong, J.; Lee, H.; Lee, Y.; Cho, S.; Kim, S.-W.; Kim, J.J.; Kim, S.Y.; Ko, H. Tailoring force sensitivity and selectivity by microstructure engineering of multidirectional electronic skins. NPG Asia Mater. 2018, 10, 163–176. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Pan, P.; Yang, Z.; Wei, J.; Yang, X.; Liu, J.; Zhou, J.; Zhang, X.; Liu, G. 3D printing of a flexible inclined-tip cone array-based pressure sensor. Adv. Mater. Technol. 2021, 7, 2101135. [Google Scholar] [CrossRef]
- Ruth, S.R.A.; Beker, L.; Tran, H.; Feig, V.R.; Matsuhisa, N.; Bao, Z. Rational design of capacitive pressure sensors based on pyramidal microstructures for specialized monitoring of biosignals. Adv. Funct. Mater. 2019, 30, 1903100. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Mei, D.; Zhu, L.; Wang, S.; Fu, X. Highly sensitive and flexible tactile sensor with truncated pyramid-shaped porous graphene/silicone rubber composites for human motion detection. Compos. Sci. Technol. 2022, 217, 109078. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Y.; Mei, D.; Jiang, C. Development of fully flexible tactile pressure sensor with bilayer interlaced bumps for robotic grasping applications. Micromachines 2020, 11, 770. [Google Scholar] [CrossRef]
- Boland, C.S.; Khan, U.; Ryan, G.; Barwich, S.; Charifou, R.; Harvey, A.; Backes, C.; Li, Z.; Ferreira, M.S.; Möbius, M.E.; et al. Sensitive electromechanical sensors using viscoelastic graphene-polymer nanocomposites. Science 2016, 354, 1257–1260. [Google Scholar] [CrossRef]
- Tang, Z.; Jia, S.; Wang, F.; Bian, C.; Chen, Y.; Wang, Y.; Li, B. Highly stretchable core-sheath fibers via wet-spinning for wearable strain sensors. ACS Appl. Mater. Interfaces 2018, 10, 6624–6635. [Google Scholar] [CrossRef]
- Wu, X.; Han, Y.; Zhang, X.; Zhou, Z.; Lu, C. Large-area compliant, low-cost, and versatile pressure-sensing platform based on microcrack-designed carbon Black@Polyurethane sponge for human-machine interfacing. Adv. Funct. Mater. 2016, 26, 6246–6256. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Mizuki, Y.; Tsukagoshi, T.; Takahata, T.; Ichiki, M.; Shimoyama, I. MEMS-based pulse wave sensor utilizing a piezoresistive cantilever. Sensors 2020, 20, 1052. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Zhao, Z.; Tang, Y.; Hao, X.; Wang, X.; Qiu, J. Covalent bonds-integrated graphene foam with superb electromechanical properties as elastic conductor and compressive sensor. Carbon 2019, 147, 206–213. [Google Scholar] [CrossRef]
- Chun, S.; Kim, Y.; Oh, H.S.; Bae, G.; Park, W. A highly sensitive pressure sensor using a double-layered graphene structure for tactile sensing. Nanoscale 2015, 7, 11652–11659. [Google Scholar] [CrossRef]
- Dong, X.; Wei, Y.; Chen, S.; Lin, Y.; Liu, L.; Li, J. A linear and large-range pressure sensor based on a graphene/silver nanowires nanobiocomposites network and a hierarchical structural sponge. Compos. Sci. Technol. 2018, 155, 108–116. [Google Scholar] [CrossRef]
- Zhu, B.; Niu, Z.; Wang, H.; Leow, W.R.; Wang, H.; Li, Y.; Zheng, L.; Wei, J.; Huo, F.; Chen, X. Microstructured graphene arrays for highly sensitive flexible tactile sensors. Small 2014, 10, 3625–3631. [Google Scholar] [CrossRef]
- Ho, D.H.; Sun, Q.; Kim, S.Y.; Han, J.T.; Kim, D.H.; Cho, J.H. Stretchable and multimodal all graphene electronic skin. Adv. Mater. 2016, 28, 2601–2608. [Google Scholar] [CrossRef]
- Wan, Y.; Qiu, Z.; Hong, Y.; Wang, Y.; Zhang, J.; Liu, Q.; Wu, Z.; Guo, C.F. A Highly sensitive flexible capacitive tactile sensor with sparse and high-aspect-ratio microstructures. Adv. Electron. Mater. 2018, 4, 1700586. [Google Scholar] [CrossRef]
- Sharma, S.; Chhetry, A.; Sharifuzzaman, M.; Yoon, H.; Park, J.Y. Wearable capacitive pressure sensor based on mxene composite nanofibrous scaffolds for reliable human physiological signal acquisition. ACS Appl. Mater. Interfaces 2020, 12, 22212–22224. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Li, X.; Lin, Y.; Luo, N.; Long, M.; Zhao, N.; Xu, J.B. Flexible piezoelectric-induced pressure sensors for static measurements based on nanowires/graphene heterostructures. ACS Nano 2017, 11, 4507–4513. [Google Scholar] [CrossRef]
- Nichols, W.W. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am. J. Hypertens. 2005, 18, 3S–10S. [Google Scholar] [CrossRef] [PubMed]





| Ref. | Materials | Sensing Mechanism | Sensitivity | Sensing Range |
|---|---|---|---|---|
| [34] | CB/PU sponge | Piezoresistive | 6.80% kPa−1 | 0–2.2 kPa |
| [35] | Si | Piezoresistive | 0.05% kPa−1 | −10–10 Pa |
| [36] | CCGF | Piezoresistive | 0.36 kPa−1 4.60% kPa−1 | 0–2 kPa 2–5 kPa |
| [37] | Two single-layer graphene | Piezoresistive | −0.24 kPa−1 3.9% kPa−1 | 0.25–0.7 kPa 1–8 kPa |
| [38] | Graphene/Ag | Piezoresistive | 1.6% kPa−1 | 0–40 kPa |
| [39] | Micro-structured graphene | Piezoresistive | −5.53 kPa−1 | 0.0015–1.4 kPa |
| [40] | Graphene | Capacitive | 0.2% kPa−1 | 0–450 kPa |
| [41] | PDMS-AgNWs-CPI | Capacitive | 1.19 kPa−1 7.70% kPa−1 | 0–3 kPa 3–15 kPa |
| [42] | MXene/PVDF-TrFE | Capacitive | 0.51 kPa−1 1.10% kPa−1 | 0–1 kPa 1–167 kPa |
| [26] | PDMS-Ti/Au | Capacitive | 0.16 kPa−1 0.04 kPa−1 | 0–0.75 kPa 0.75–2.5 kPa |
| [43] | ITO-Graphene FET-PDMS | Piezoelectric | 0.94% kPa−1 | 0–1.4 kPa |
| This work | p-GNP-SR | Piezoresistive | 8.65% kPa−1 | 0–12 kPa |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, Z.; Chen, Z.; Mei, D.; Wang, Y. Development of Pressure Sensor Based Wearable Pulse Detection Device for Radial Pulse Monitoring. Micromachines 2022, 13, 1699. https://doi.org/10.3390/mi13101699
Wang S, Zhang Z, Chen Z, Mei D, Wang Y. Development of Pressure Sensor Based Wearable Pulse Detection Device for Radial Pulse Monitoring. Micromachines. 2022; 13(10):1699. https://doi.org/10.3390/mi13101699
Chicago/Turabian StyleWang, Shihang, Zhinan Zhang, Zhijian Chen, Deqing Mei, and Yancheng Wang. 2022. "Development of Pressure Sensor Based Wearable Pulse Detection Device for Radial Pulse Monitoring" Micromachines 13, no. 10: 1699. https://doi.org/10.3390/mi13101699
APA StyleWang, S., Zhang, Z., Chen, Z., Mei, D., & Wang, Y. (2022). Development of Pressure Sensor Based Wearable Pulse Detection Device for Radial Pulse Monitoring. Micromachines, 13(10), 1699. https://doi.org/10.3390/mi13101699