Longitudinal Monitoring of Alpha-Fetoprotein by Dried Blood Spot for Hepatoblastoma Screening in Beckwith–Wiedemann Syndrome
Abstract
:1. Introduction
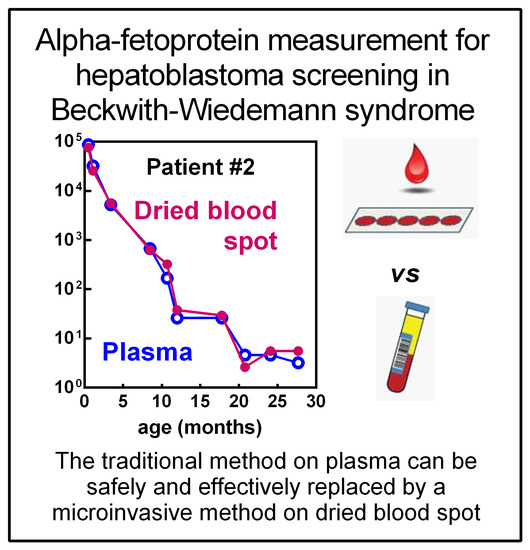
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Study and Screening Protocol
4.3. Laboratory Assays
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Brioude, F.; Kalish, J.M.; Mussa, A.; Foster, A.C.; Bliek, J.; Ferrero, G.B.; Boonen, S.E.; Cole, T.; Baker, R.; Bertoletti, M.; et al. Expert consensus document: Clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: An international consensus statement. Nat. Rev. Endocrinol. 2018, 14, 229–249. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Russo, S.; Larizza, L.; Riccio, A.; Ferrero, G.B. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome: A paradigm for genomic medicine. Clin. Genet. 2016, 89, 403–415. [Google Scholar] [CrossRef]
- Mussa, A.; Russo, S.; De Crescenzo, A.; Freschi, A.; Calzari, L.; Maitz, S.; Macchiaiolo, M.; Molinatto, C.; Baldassarre, G.; Mariani, M.; et al. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome. Eur. J. Hum. Genet. 2016, 24, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Russo, S.; De Crescenzo, A.; Chiesa, N.; Molinatto, C.; Selicorni, A.; Richiardi, L.; Larizza, L.; Silengo, M.C.; Riccio, A.; et al. Prevalence of Beckwith-Wiedemann syndrome in North West of Italy. Am. J. Med. Genet. A 2013, 161A, 2481–2486. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Biesecker, L.G.; Brioude, F.; Deardorff, M.A.; Di Cesare-Merlone, A.; Druley, T.; Ferrero, G.B.; Lapunzina, P.; Larizza, L.; Maas, S.; et al. Nomenclature and definition in asymmetric regional body overgrowth. Am. J. Med. Genet. A 2017. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.M.; Vansenne, F.; Kadouch, D.; Ibrahim, A.; Bliek, J.; Hopman, S.; Mannens, M.M.; Merks, J.H.; Maher, E.R.; Hennekam, R.C. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am. J. Med. Genet. A 2016, 170, 2248–2260. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Molinatto, C.; Baldassarre, G.; Riberi, E.; Russo, S.; Larizza, L.; Riccio, A.; Ferrero, G.B. Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J. Pediatr. 2016, 176, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Birch, J. The epidemiology of hepatoblastoma. Pediatr. Blood Cancer 2012, 59, 776–779. [Google Scholar] [CrossRef]
- Mussa, A.; Duffy, K.A.; Carli, D.; Ferrero, G.B.; Kalish, J.M. Defining an optimal time window to screen for hepatoblastoma in children with Beckwith-Wiedemann syndrome. Pediatr. Blood Cancer 2019, 66, e27492. [Google Scholar] [CrossRef]
- DeBaun, M.R.; Tucker, M.A. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J. Pediatr. 1998, 132, 398–400. [Google Scholar] [CrossRef]
- Mussa, A.; Ferrero, G.B.; Ceoloni, B.; Basso, E.; Chiesa, N.; De Crescenzo, A.; Pepe, E.; Silengo, M.; de Sanctis, L. Neonatal hepatoblastoma in a newborn with severe phenotype of Beckwith-Wiedemann syndrome. Eur. J. Pediatr. 2011, 170, 1407–1411. [Google Scholar] [CrossRef]
- Smith, A.C.; Shuman, C.; Chitayat, D.; Steele, L.; Ray, P.N.; Bourgeois, J.; Weksberg, R. Severe presentation of Beckwith-Wiedemann syndrome associated with high levels of constitutional paternal uniparental disomy for chromosome 11p15. Am. J. Med. Genet. A 2007, 143A, 3010–3015. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Conlin, L.K.; Bhatti, T.R.; Dubbs, H.A.; Harris, M.C.; Izumi, K.; Mostoufi-Moab, S.; Mulchandani, S.; Saitta, S.; States, L.J.; et al. Clinical features of three girls with mosaic genome-wide paternal uniparental isodisomy. Am. J. Med. Genet. A 2013, 161A, 1929–1939. [Google Scholar] [CrossRef]
- Duffy, K.A.; Deardorff, M.A.; Kalish, J.M. The utility of alpha-fetoprotein screening in Beckwith-Wiedemann syndrome. Am. J. Med. Genet. A 2017, 173, 581–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czauderna, P.; Lopez-Terrada, D.; Hiyama, E.; Häberle, B.; Malogolowkin, M.H.; Meyers, R.L. Hepatoblastoma state of the art: Pathology, genetics, risk stratification, and chemotherapy. Curr. Opin. Pediatr. 2014, 26, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Zarate, Y.A.; Mena, R.; Martin, L.J.; Steele, P.; Tinkle, B.T.; Hopkin, R.J. Experience with hemihyperplasia and Beckwith-Wiedemann syndrome surveillance protocol. Am. J. Med. Genet. A 2009, 149A, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Di Candia, S.; Russo, S.; Catania, S.; De Pellegrin, M.; Di Luzio, L.; Ferrari, M.; Tortora, C.; Meazzini, M.C.; Brusati, R.; et al. Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur. J. Med. Genet. 2016, 59, 52–64. [Google Scholar] [CrossRef]
- Tan, T.Y.; Amor, D.J. Tumour surveillance in Beckwith-Wiedemann syndrome and hemihyperplasia: A critical review of the evidence and suggested guidelines for local practice. J. Paediatr. Child. Health 2006, 42, 486–490. [Google Scholar] [CrossRef]
- Bliek, J.; Maas, S.; Alders, M.; Merks, J.H.; Mannens, M. Epigenotype, phenotype, and tumors in patients with isolated hemihyperplasia. J. Pediatr. 2008, 153, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; Venkatramani, R.; Feusner, J.H. Hepatoblastoma in children with Beckwith-Wiedemann syndrome: Does it warrant different treatment? J. Pediatr. Hematol. Oncol. 2014, 36, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Clericuzio, C.L.; Chen, E.; McNeil, D.E.; O'Connor, T.; Zackai, E.H.; Medne, L.; Tomlinson, G.; DeBaun, M. Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J. Pediatr. 2003, 143, 270–272. [Google Scholar] [CrossRef]
- Mussa, A.; Ferrero, G.B. Screening Hepatoblastoma in Beckwith-Wiedemann Syndrome: A Complex Issue. J. Pediatr. Hematol. Oncol. 2015, 37, 627. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Ferrero, G.B. Serum alpha-fetoprotein screening for hepatoblastoma in Beckwith-Wiedemann syndrome. Am. J. Med. Genet. A 2017, 173, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Kalish, J.M.; Deardorff, M.A. Tumor screening in Beckwith-Wiedemann syndrome-To screen or not to screen? Am. J. Med. Genet. A 2016, 170, 2261–2264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blohm, M.E.; Vesterling-Hörner, D.; Calaminus, G.; Göbel, U. Alpha 1-fetoprotein (AFP) reference values in infants up to 2 years of age. Pediatr. Hematol. Oncol. 1998, 15, 135–142. [Google Scholar] [CrossRef]
- Schneider, D.T.; Calaminus, G.; Göbel, U. Diagnostic value of alpha 1-fetoprotein and beta-human chorionic gonadotropin in infancy and childhood. Pediatr. Hematol. Oncol. 2001, 18, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Everman, D.B.; Shuman, C.; Dzolganovski, B.; O’riordan, M.A.; Weksberg, R.; Robin, N.H. Serum alpha-fetoprotein levels in Beckwith-Wiedemann syndrome. J. Pediatr. 2000, 137, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, O.; Cacho, B.; Morales-Espinosa, D.; Ruelas-Villavicencio, A.; Flores-Estrada, D.; Hernández-Pedro, N. The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 2007, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalish, J.M.; Doros, L.; Helman, L.J.; Hennekam, R.C.; Kuiper, R.P.; Maas, S.M.; Maher, E.R.; Nichols, K.E.; Plon, S.E.; Porter, C.C.; et al. Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin. Cancer Res. 2017, 23, e115–e122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, K.A.; Grand, K.L.; Zelley, K.; Kalish, J.M. Tumor Screening in Beckwith-Wiedemann Syndrome: Parental Perspectives. J. Genet. Couns. 2018, 27, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Pagliardini, S.; Pagliardini, V.; Molinatto, C.; Baldassarre, G.; Corrias, A.; Silengo, M.C.; Ferrero, G.B. α-Fetoprotein assay on dried blood spot for hepatoblastoma screening in children with overgrowth-cancer predisposition syndromes. Pediatr. Res. 2014, 76, 544–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, J.I.; Carachi, R.; Gupta, R.; Sim, F.G.; McAllister, E.J.; Weston, R. Plasma alpha fetoprotein reference ranges in infancy: Effect of prematurity. Arch. Dis. Child. 1987, 62, 362–369. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Russo, S.; de Crescenzo, A.; Freschi, A.; Calzari, L.; Maitz, S.; Macchiaiolo, M.; Molinatto, C.; Baldassarre, G.; Mariani, M.; et al. Fetal growth patterns in Beckwith-Wiedemann syndrome. Clin. Genet. 2016, 90, 21–27. [Google Scholar] [CrossRef]
- McDade, T.W. Development and validation of assay protocols for use with dried blood spot samples. Am. J. Hum. Biol. 2014, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Martial, L.C.; Aarnoutse, R.E.; Schreuder, M.F.; Henriet, S.S.; Brüggemann, R.J.; Joore, M.A. Cost Evaluation of Dried Blood Spot Home Sampling as Compared to Conventional Sampling for Therapeutic Drug Monitoring in Children. PLoS ONE 2016, 11, e0167433. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, G.A.; Han, S.; Lee, W.; Chun, S.; Lim, Y.S. Longitudinal Assessment of Three Serum Biomarkers to Detect Very Early Stage Hepatocellular Carcinoma. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Kramer, J.R.; Chen, G.J.; Duan, Z.; Richardson, P.A.; Davila, J.A. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut 2011, 60, 992–997. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mussa, A.; Ciuffreda, V.P.; Sauro, P.; Pagliardini, V.; Pagliardini, S.; Carli, D.; Kalish, J.M.; Fagioli, F.; Pavanello, E.; Ferrero, G.B. Longitudinal Monitoring of Alpha-Fetoprotein by Dried Blood Spot for Hepatoblastoma Screening in Beckwith–Wiedemann Syndrome. Cancers 2019, 11, 86. https://doi.org/10.3390/cancers11010086
Mussa A, Ciuffreda VP, Sauro P, Pagliardini V, Pagliardini S, Carli D, Kalish JM, Fagioli F, Pavanello E, Ferrero GB. Longitudinal Monitoring of Alpha-Fetoprotein by Dried Blood Spot for Hepatoblastoma Screening in Beckwith–Wiedemann Syndrome. Cancers. 2019; 11(1):86. https://doi.org/10.3390/cancers11010086
Chicago/Turabian StyleMussa, Alessandro, Valentina Pia Ciuffreda, Pina Sauro, Veronica Pagliardini, Severo Pagliardini, Diana Carli, Jennifer M. Kalish, Franca Fagioli, Enza Pavanello, and Giovanni Battista Ferrero. 2019. "Longitudinal Monitoring of Alpha-Fetoprotein by Dried Blood Spot for Hepatoblastoma Screening in Beckwith–Wiedemann Syndrome" Cancers 11, no. 1: 86. https://doi.org/10.3390/cancers11010086
APA StyleMussa, A., Ciuffreda, V. P., Sauro, P., Pagliardini, V., Pagliardini, S., Carli, D., Kalish, J. M., Fagioli, F., Pavanello, E., & Ferrero, G. B. (2019). Longitudinal Monitoring of Alpha-Fetoprotein by Dried Blood Spot for Hepatoblastoma Screening in Beckwith–Wiedemann Syndrome. Cancers, 11(1), 86. https://doi.org/10.3390/cancers11010086