FDG-PET/CT for the Management of Post-Chemotherapy Residual Mass in Hodgkin lymphoma
Abstract
:Simple Summary
Abstract
1. Introduction
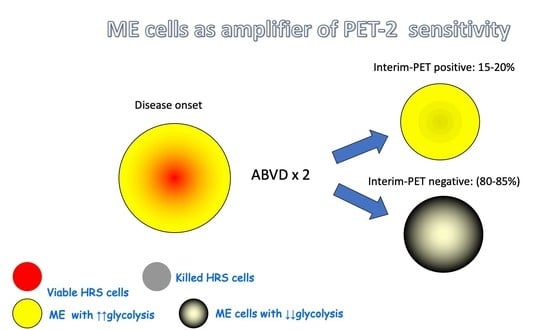
2. Residual Mass after ABVD Chemotherapy
3. Residual Mass after BEACOPPescalated Chemotherapy in Advanced Hodgkin lymphoma
4. Interpretation Criteria for PET/CT at the End of Therapy in Patients with a CT-Detected Residual Mass
5. Texture Analysis—Radiomics in Hodgkin lymphoma
6. Residual Mass after Immune Checkpoint Inhibitors Therapy
7. PET/MRI for Lymphoma Staging and Restaging
8. Discussion
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Canellos, G.P. Residual mass in lymphoma may not be residual disease. J. Clin. Oncol. 1988, 6, 931–933. [Google Scholar] [CrossRef]
- Radford, J.A.; Cowan, R.; Flanagan, M.; Dunn, G.; Crowther, D.; Johnson, R.J.; Eddleston, B. The significance of residual mediastinal abnormality on the chest radiograph following treatment for Hodgkin’s disease. J. Clin. Oncol. 1988, 6, 940–946. [Google Scholar] [CrossRef]
- Naumann, R.; Vaic, A.; Beuthien-Baumann, B.; Bredow, J.; Kropp, J.; Kittner, T.; Franke, W.G.; Ehninger, G. Prognostic value of posi-tron emission tomography in the evaluation of post-treatment residual mass in patients with Hodgkin’s disease and non-Hodgkin’s lymphoma. Br. J. Haematol. 2001, 115, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Gallamini, A.; Hutchings, M.; Borra, A. Functional Imaging in Hodgkin lymphoma. In Hodgkin lymphoma; Engert, A., Younes, A., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 107–130. [Google Scholar]
- Jerusalem, G.; Beguin, Y.; Fassotte, M.F.; Najjar, F.; Paulus, P.; Rigo, P.; Fillet, G. Whole-Body Positron Emission Tomography Using 18F-Fluorodeoxyglucose for Posttreatment Evaluation in Hodgkin’s Disease and Non-Hodgkin’s Lymphoma Has Higher Di-agnostic and Prognostic Value Than Classical Computed Tomography Scan Imaging. Blood 1999, 94, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Zaucha, J.M.; Chauvie, S.; Zaucha, R.; Biggii, A.; Gallamini, A. The role of PET/CT in the modern treatment of Hodgkin lymphoma. Cancer Treat. Rev. 2019, 77, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Warburg, O.; Poesner, K.; Negelein, E. Ueber den stoffwechsel der tumoren. Biol. Chem. 1924, 152, 319–344. [Google Scholar]
- Mathas, S.; Hartmann, S.; Küppers, R. Hodgkin lymphoma: Pathology and biology. Semin. Hematol. 2016, 53, 139–147. [Google Scholar] [CrossRef]
- Ma, Y.; Visser, L.; Roelofsen, H.; De Vries, M.; Diepstra, A.; Van Imhoff, G.; Van Der Wal, T.; Luinge, M.; Alvarez-Llamas, G.; Vos, H.; et al. Proteomics analysis of Hodgkin lymphoma: Identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood 2008, 111, 2339–2346. [Google Scholar] [CrossRef]
- Gallamini, A. Positron emission tomography scanning: A new paradigm for the management of Hodgkin’s lymphoma. Haematologica 2010, 95, 1046–1048. [Google Scholar] [CrossRef]
- Terasawa, T.; Nihashi, T.; Hotta, T.; Nagai, H. 18F-FDG PET for Posttherapy Assessment of Hodgkin’s Disease and Aggressive Non-Hodgkin’s Lymphoma: A Systematic Review. J. Nucl. Med. 2007, 49, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D.; Horning, S.J.; Coiffier, B.; Shipp, M.A.; Fisher, R.I.; Connors, J.M.; Lister, T.A.; Vose, J.; Grillo-López, A.; Hagenbeek, A.; et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J. Clin. Oncol. 1999, 17, 1244–1253. [Google Scholar] [CrossRef]
- Brepoels, L.; Stroobants, S.; De Wever, W.; Spaepen, K.; Vandenberghe, P.; Thomas, J.; Uyttebroeck, A.; Mortelmans, L.; De Wolf-Peeters, C.; Verhoef, G. Hodgkin lymphoma: Response assessment by Revised International Workshop Criteria. Leuk. Lymphoma 2007, 48, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Cheson, B.D.; Fisher, R.I.; Barrington, S.F.; Cavalli, F.; Schwartz, L.H.; Zucca, E.; Lister, T.A. Recommendations for initial evalua-tion, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014, 32, 3059–3068. [Google Scholar] [CrossRef] [PubMed]
- Engert, A.; Haverkamp, H.; Kobe, C.; Markova, J.; Renner, C.; Ho, A.; Zijlstra, J.; Král, Z.; Fuchs, M.; Hallek, M.; et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): A randomised, open-label, phase 3 non-inferiority trial. Lancet 2012, 379, 1791–1799. [Google Scholar] [CrossRef]
- Savage, K.J.; Connors, J.M.; Villa, D.R.; Hapgood, G.; Gerrie, A.S.; Shenkier, T.N.; Scott, D.W.; Gascoyne, R.D.; Benard, F.; Morris, J.; et al. Advanced Stage Classical Hodgkin lymphoma Patients with a Negative PET-Scan Following Treatment with ABVD Have Excellent Outcomes without the Need for Consolidative Radiotherapy Regardless of Disease Bulk at Presentation. Blood 2015, 126, 579. [Google Scholar] [CrossRef]
- Picardi, M.; De Renzo, A.; Pane, F.; Nicolai, E.; Pacelli, R.; Salvatore, M.; Rotoli, B. Randomized comparison of consolidation radiationversusobservation in bulky Hodgkin’s lymphoma with post-chemotherapy negative positron emission tomography scans. Leuk. Lymphoma 2007, 48, 1721–1727. [Google Scholar] [CrossRef]
- Gallamini, A.; Tarella, C.; Viviani, S.; Rossi, A.; Patti, C.; Mulé, A.; Picardi, M.; Romano, A.; Cantonetti, M.; La Nasa, G.; et al. Early Chemotherapy Intensification with Escalated BEACOPP in Patients with Advanced-Stage Hodgkin lymphoma with a Positive Interim Positron Emission Tomography/Computed Tomography Scan After Two ABVD Cycles: Long-Term Results of the GITIL/FIL HD 0607 Trial. J. Clin. Oncol. 2018, 36, 454–462. [Google Scholar] [CrossRef]
- Meignan, M.; Gallamini, A.; Haioun, C. Report on the First International Workshop on interim-PET scan in lymphoma. Leuk. Lymphoma 2009, 50, 1257–1260. [Google Scholar] [CrossRef]
- Gallamini, A.; Rossi, A.; Patti, C.; Picardi, M.; Romano, A.; Cantonetti, M.; Oppi, S.; Viviani, S.; Bolis, S.; Trentin, L.; et al. Consolidation Radiotherapy Could Be Safely Omitted in Advanced Hodgkin lymphoma with Large Nodal Mass in Complete Metabolic Response After ABVD: Final Analysis of the Randomized GITIL/FIL HD0607 Trial. J. Clin. Oncol. 2020, 38, 3905–3913. [Google Scholar] [CrossRef]
- Borchmann, P.; Plütschow, A.; Kobe, C.; Greil, R.; Meissner, J.; Topp, M.S.; Ostermann, H.; Dierlamm, J.; Mohm, J.; Thiemer, J.; et al. PET-guided omission of radiotherapy in early-stage unfavourable Hodgkin lymphoma (GHSG HD17): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 223–234. [Google Scholar] [CrossRef]
- Diehl, V.; Franklin, J.; Pfreundschuh, M.; Lathan, B.; Paulus, U.; Hasenclever, D.; Tesch, H.; Herrmann, R.; Dörken, B.; Müller-Hermelink, H.-K.; et al. Standard and Increased-Dose BEACOPP Chemotherapy Compared with COPP-ABVD for Ad-vanced Hodgkin’s Disease. N. Engl. J. Med. 2003, 348, 2386–2395. [Google Scholar] [CrossRef] [Green Version]
- Borchmann, P.; Haverkamp, H.; Diehl, V.; Cerny, T.; Markova, J.; Ho, A.D.; Eich, H.-T.; Mueller-Hermelink, H.K.; Kanz, L.; Greil, R.; et al. Eight Cycles of Escalated-Dose BEACOPP Compared with Four Cycles of Escalated-Dose BEACOPP Followed by Four Cycles of Baseline-Dose BEACOPP with or without Radiotherapy in Patients with Advanced-Stage Hodgkin’s Lymphoma: Final Analysis of the HD12 Trial of the German Hodgkin Study Group. J. Clin. Oncol. 2011, 29, 4234–4242. [Google Scholar] [CrossRef]
- Weihrauch, M.R.; Re, D.; Scheidhauer, K.; Ansén, S.; Dietlein, M.; Bischoff, S.; Bohlen, H.; Wolf, J.; Schicha, H.; Diehl, V. Thorac-ic positron emission tomography using 18F-fluorodeoxyglucose for the evaluation of residual mediastinal Hodgkin disease. Blood 2001, 98, 2930–2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrington, S.; Kirkwood, A.A.; Franceschetto, A.; Fulham, M.; Roberts, T.H.; Almquist, H.; Brun, E.; Hjorthaug, K.; Viney, Z.N.; Pike, L.; et al. PET-CT for staging and early response: Results from the Response-Adapted Therapy in Advanced Hodgkin lymphoma study. Blood 2016, 127, 1531–1538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juweid, M.E.; Stroobants, S.; Hoekstra, O.S.; Mottaghy, F.M.; Dietlein, M.; Guermazi, A.; Wiseman, G.A.; Kostakoglu, L.; Scheidhauer, K.; Buck, A.; et al. Use of positron emission tomography for response assessment of lymphoma: Consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J. Clin. Oncol. 2007, 25, 571–578. [Google Scholar] [CrossRef]
- Borchmann, P.; Goergen, H.; Kobe, C.; Lohri, A.; Greil, R.; Eichenauer, D.A.; Zijlstra, J.M.; Markova, J.; Meissner, J.; Feuring-Buske, M.; et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): Final results of an open-label, international, randomised phase 3 trial by the German Hodgkin Study Group. Lancet 2017, 390, 2790–2802. [Google Scholar] [CrossRef]
- Kobe, C.; Goergen, H.; Baues, C.; Kuhnert, G.; Voltin, C.-A.; Zijlstra, J.; Hoekstra, O.; Mettler, J.; Drzezga, A.; Engert, A.; et al. Outcome-based interpretation of early interim PET in advanced-stage Hodgkin lymphoma. Blood 2018, 132, 2273–2279. [Google Scholar] [CrossRef] [Green Version]
- Kobe, C.; (Department on Nuclear Medicine, University Hospital of Cologne, Kerpener Str. 62, Cologne, Germany). Recommendation of GHSG for Consolidative Radiotherapy of Residual Mass in Patients with Advanced Hodgkin lymphoma after Effective BEACOPPesc Treatment. Personal communication, 2021. [Google Scholar]
- Barrington, S.F.; Mikhaeel, N.G.; Kostakoglu, L.; Meignan, M.; Hutchings, M.; Müeller, S.P.; Schwartz, L.H.; Zucca, E.; Fisher, R.I.; Trotman, J.; et al. Role of Imaging in the Staging and Response Assessment of Lymphoma: Consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J. Clin. Oncol. 2014, 32, 3048–3058. [Google Scholar] [CrossRef]
- Picardi, M.; Fonti, R.; Della Pepa, R.; Giordano, C.; Pugliese, N.; Nicolai, E.; Salvatore, M.; Mainolfi, C.; Venetucci, P.; Rascato, M.; et al. 2-deoxy-2[F-18] fluoro-d-glucose positron emission tomography Deauville scale and core-needle biopsy to determine successful management after six doxorubicin, bleomycin, vinblastine and dacarbazine cycles in advanced-stage Hodgkin lymphoma. Eur. J. Cancer 2020, 132, 85–97. [Google Scholar] [CrossRef]
- Woll, J.P.P.; Vicente, A.M.G.; Rubio, M.P.T.; Muñoz, A.M.P.; Londoño, G.J.; Martín, A.L.; Primo, C.C.; Castejón, A.M.S. Quantitative and qualitative evaluation of the interim PET/CT in lymphoma treatment in the prediction of complete metabolic response. Rev. Esp. Med. Nucl. Imagen. Mol. 2013, 32, 70–76. [Google Scholar] [CrossRef]
- Isik, E.G.; Kuyumcu, S.; Kebudi, R.; Sanli, Y.; Karakas, Z.; Cakir, F.B.; Unal, S.N. Prediction of outcome in pediatric Hodgkin lymphoma based on interpretation of (18)FDG-PET/CT according to ΔSUV(max), Deauville 5-point scale and IHP criteria. Ann. Nucl. Med. 2017, 31, 660–668. [Google Scholar] [CrossRef]
- Grant, C.; Dunleavy, K.; Eberle, F.C.; Pittaluga, S.; Wilson, W.H.; Jaffe, E.S. Primary Mediastinal Large B-Cell Lymphoma, Classic Hodgkin lymphoma Presenting in the Mediastinum, and Mediastinal Gray Zone Lymphoma: What is the Oncologist to do? Curr. Hematol. Malign. Rep. 2011, 6, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Ceriani, L.; Milan, L.; Martelli, M.; Ferreri, A.J.M.; Cascione, L.; Zinzani, P.L.; Di Rocco, A.; Conconi, A.; Stathis, A.; Cavalli, F.; et al. Metabolic heterogeneity on baseline 18FDG-PET/CT scan is a predictor of outcome in primary mediastinal B-cell lymphoma. Blood 2018, 132, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glatstein, E.; Kaplan, H.S.; Guernsey, J.M.; Rosenberg, S.A. The value of laparotomy and splenectomy in the staging of Hodgkin’s disease. Cancer 1969, 24, 709–718. [Google Scholar] [CrossRef]
- Durmo, R.; Cottereau, A.S.; Rebaud, L.; Nioche, C.; Ruffini, A.; Fioroni, F.; Meignan, M.; Buvat, I.; Merli, F.; Versari, A.; et al. Prognostic role of lesion dissemination feature (dmax) calculated on baseline pet/ct in hodgkin lymphoma. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Trichelair, P. How machine learning can enhance clinical development. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Dercle, L.; Mokrane, F.-Z.; De Colella, J.M.S.; Stamatoullas, A.; Morschhauser, F.; Brice, P.; Ghesquières, H.; Casasnovas, R.-O.; Chen, A.; Manson, G.; et al. Unconventional immune-related phenomena observed using 18F-FDG PET/CT in Hodgkin lymphoma treated with anti PD-1 monoclonal antibodies. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1391–1392. [Google Scholar] [CrossRef] [Green Version]
- Cheson, B.D.; Ansell, S.; Schwartz, L.; Gordon, L.I.; Advani, R.; Jacene, H.A.; Hoos, A.; Barrington, S.; Armand, P. Refinement of the Lugano Classification lymphoma response criteria in the era of immunomodulatory therapy. Blood 2016, 128, 2489–2496. [Google Scholar] [CrossRef] [Green Version]
- Bair, S.M.; Strelec, L.E.; Feldman, T.A.; Ahmed, G.; Armand, P.; Shah, N.N.; Singavi, A.N.; Reddy, N.; Khan, N.; Andreadis, C.; et al. Outcomes and Toxicities of Programmed Death-1 (PD-1) Inhibitors in Hodgkin lymphoma Patients in the United States: A Real-World, Multicenter Retrospective Analysis. Oncologist 2019, 24, 955–962. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Su, H.; Song, Y.; Jiang, W.; Sun, X.; Qian, W.; Zhang, W.; Gao, Y.; Jin, Z.; Zhou, J.; et al. Safety and activity of sintilimab in patients with relapsed or refractory classical Hodgkin lymphoma (ORIENT-1): A multicentre, single-arm, phase 2 trial. Lancet Haematol. 2019, 6, e12–e19. [Google Scholar] [CrossRef]
- Chen, R.; Zinzani, P.L.; Lee, H.J.; Armand, P.; Johnson, N.A.; Brice, P.; Radford, J.; Ribrag, V.; Molin, D.; Vassilakopoulos, T.P.; et al. Pembrolizumab in relapsed or refractory Hodgkin lymphoma: 2-year follow-up of KEYNOTE-087. Blood 2019, 134, 1144–1153. [Google Scholar] [CrossRef] [Green Version]
- Kuruvilla, J.; Ramchandren, R.; Santoro, A.; Paszkiewicz-Kozik, E.; Gasiorowski, R.; Johnson, N.A.; Fogliatto, L.M.; Goncalves, I.; de Oliveira, J.S.R.; Buccheri, V.; et al. Pembrolizumab versus brentuximab vedotin in relapsed or refractory classical Hodgkin lymphoma (KEYNOTE-204): An interim analysis of a multicentre, randomised, open-label, phase 3 study. Lancet Oncol. 2021, 22, 512–524. [Google Scholar] [CrossRef]
- Ramchandren, R.; Domingo-Domènech, E.; Rueda, A.; Trněný, M.; Feldman, T.A.; Lee, H.J.; Provencio, M.; Sillaber, C.; Cohen, J.B.; Savage, K.J.; et al. Nivolumab for Newly Diagnosed Advanced-Stage Classic Hodgkin lymphoma: Safety and Efficacy in the Phase II CheckMate 205 Study. J. Clin. Oncol. 2019, 37, 1997–2007. [Google Scholar] [CrossRef]
- Mokrane, F.-Z.; Chen, A.; Schwartz, L.H.; Morschhauser, F.; Stamatoullas, A.; Schiano de Colella, J.-M.; Vercellino, L.; Casasnovas, O.; Chauchet, A.; Delmer, A.; et al. Performance of CT Compared with 18F-FDG PET in Predicting the Efficacy of Nivolumab in Relapsed or Refractory Hodgkin lymphoma. Radiology 2020, 295, 651–661. [Google Scholar] [CrossRef]
- Merryman, R.W.; Redd, R.A.; Nishihori, T.; Chavez, J.; Nieto, Y.; Darrah, J.M.; Rao, U.; Byrne, M.T.; Bond, D.A.; Maddocks, K.J.; et al. Autologous stem cell transplantation after anti-PD-1 therapy for multiply relapsed or refractory Hodgkin lymphoma. Blood Adv. 2021, 5, 1648–1659. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.J.; Advani, R.H.; Bartlett, N.L.; Vose, M.J.M.; Ramchandren, R.; Feldman, T.A.; LaCasce, M.A.S.; Christian, B.A.; Ansell, S.M.; Moskowitz, C.H.; et al. Brentuximab Vedotin and Nivolumab for Relapsed or Refractory Classic Hodgkin lymphoma: Long-Term Follow-up Results from the Single-Arm Phase 1/2 Study. Blood 2019, 134, 238. [Google Scholar] [CrossRef]
- Alizadeh, A. The architecture of liquid biopsy research for lymphoma monitoring. Hematol. Oncol. 2021, 39. [Google Scholar] [CrossRef]
- Afaq, A.; Fraioli, F.; Sidhu, H.; Wan, S.; Punwani, S.; Chen, S.-H.; Akin, O.; Linch, D.; Ardeshna, K.; Lambert, J.; et al. Comparison of PET/MRI with PET/CT in the Evaluation of Disease Status in Lymphoma. Clin. Nucl. Med. 2017, 42, e1–e7. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, W.; Catana, C.; Abramson, J.S.; Arabasz, G.; McDermott, S.; Catalano, O.; Muse, V.; Blake, M.A.; Barnes, J.; Shelly, M.; et al. Hybrid FDG-PET/MR compared to FDG-PET/CT in adult lymphoma patients. Abdom. Radiol. 2016, 41, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Padhani, A.R.; Liu, G.; Mu-Koh, D.; Chenevert, T.L.; Thoeny, H.C.; Takahara, T.; Dzik-Jurasz, A.; Ross, B.D.; Van Cauteren, M.; Collins, D.; et al. Diffusion-Weighted Magnetic Resonance Imaging as a Cancer Biomarker: Consensus and Recommendations. Neoplasia 2009, 11, 102–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horger, M.; Claussen, C.; Kramer, U.; Fenchel, M.; Lichy, M.; Kaufmann, S. Very early indicators of response to systemic therapy in lymphoma patients based on alterations in water diffusivity—A preliminary experience in 20 patients undergoing whole-body diffusion-weighted imaging. Eur. J. Radiol. 2014, 83, 1655–1664. [Google Scholar] [CrossRef]
- Sasaki, M.; Yamada, K.; Watanabe, Y.; Matsui, M.; Ida, M.; Fujiwara, S.; Shibata, E. Variability in Absolute Apparent Diffusion Coefficient Values across Different Platforms May Be Substantial: A Multivendor, Multi-institutional Comparison Study. Radiology 2008, 249, 624–630. [Google Scholar] [CrossRef]
- Brancato, V.; Aiello, M.; Della Pepa, R.; Basso, L.; Garbino, N.; Nicolai, E.; Picardi, M.; Salvatore, M.; Cavaliere, C. Automatic Prediction and Assessment of Treatment Response in Patients with Hodgkin’s Lymphoma Using a Whole-Body DW-MRI Based Approach. Diagnostics 2020, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Dubreuil, J.; Salles, G.; Bozzetto, J.; Tordo, J.; Djaïleb, L.; Berriolo-Riedinger, A.; Leenhardt, J.; Giammarile, F.; Meignan, M.; Skanjeti, A. Usual and unusual pitfalls of 18F-FDG-PET/CT in lymphoma after treatment. Nucl. Med. Commun. 2017, 38, 563–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jerushalmi, J.; Frenkel, A.; Bar-Shalom, R.; Khoury, J.; Israel, O. Physiologic Thymic Uptake of 18F-FDG in Children and Young Adults: A PET/CT Evaluation of Incidence, Patterns, and Relationship to Treatment. J. Nucl. Med. 2009, 50, 849–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paydas, S. Pulmonary sarcoidosis induced by the anti-PD-1 monoclonal antibody pembrolizumab or post-immunotherapy granulomatous reaction: Which is more appropriate terminology? Ann. Oncol. 2016, 27, 1650–1651. [Google Scholar] [CrossRef]
- Montaudié, H.; Pradelli, J.; Passeron, T.; Lacour, J.-P.; Leroy, S. Pulmonary sarcoid-like granulomatosis induced by nivolumab. Br. J. Dermatol. 2017, 176, 1060–1063. [Google Scholar] [CrossRef]
- Zinzani, P.L.; Tani, M.; Trisolini, R.; Fanti, S.; Stefoni, V.; Alifano, M.; Castellucci, P.; Musuraca, G.; Dalpiaz, G.; Alinari, L.; et al. Histological verification of positive positron emission tomography findings in the follow-up of patients with mediastinal lymphoma. Haematologica 2007, 92, 771–777. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, H.; Pourdehnad, M.; Lambright, E.S.; Yamamoto, A.J.; Lanuti, M.; Li, P.; Mozley, P.D.; Rossman, M.D.; Albelda, S.M.; Alavi, A. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J. Nucl. Med. 2001, 42, 1412–1417. [Google Scholar]
- Borra, A.; Morbelli, S.; Zwarthoed, C.; Bianchi, A.; Bergesio, F.; Chauvie, S.; Zaucha, J.; Taszner, M.; Malkowski, B.; Biggi, A.; et al. Dual-point FDG-PET/CT for treatment response assessment in Hodgkin lymphoma, when an FDG-avid lesion persists after treatment. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 176–184. [Google Scholar] [PubMed]
- Spina, V.; Bruscaggin, A.; Cuccaro, A.; Martini, M.; Di Trani, M.; Forestieri, G.; Manzoni, M.; Condoluci, A.; Arribas, A.; Terzi-Di Bergamo, L.; et al. Circulating tumor DNA reveals genetics, clonal evolution, and residual disease in classical Hodgkin lym-phoma. Blood 2018, 131, 2413–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallamini, A.; Kurlapski, M.; Zaucha, J.M. FDG-PET/CT for the Management of Post-Chemotherapy Residual Mass in Hodgkin lymphoma. Cancers 2021, 13, 3952. https://doi.org/10.3390/cancers13163952
Gallamini A, Kurlapski M, Zaucha JM. FDG-PET/CT for the Management of Post-Chemotherapy Residual Mass in Hodgkin lymphoma. Cancers. 2021; 13(16):3952. https://doi.org/10.3390/cancers13163952
Chicago/Turabian StyleGallamini, Andrea, Michał Kurlapski, and Jan Maciej Zaucha. 2021. "FDG-PET/CT for the Management of Post-Chemotherapy Residual Mass in Hodgkin lymphoma" Cancers 13, no. 16: 3952. https://doi.org/10.3390/cancers13163952
APA StyleGallamini, A., Kurlapski, M., & Zaucha, J. M. (2021). FDG-PET/CT for the Management of Post-Chemotherapy Residual Mass in Hodgkin lymphoma. Cancers, 13(16), 3952. https://doi.org/10.3390/cancers13163952