Aberrant Sialylation in Cancer: Therapeutic Opportunities
Abstract
:Simple Summary
Abstract
1. Introduction
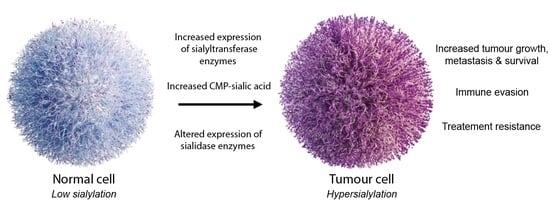
2. Aberrant Sialylation in Cancer
2.1. Sialylated Glycans
| Sialylation Change | Link to Cancer | References |
|---|---|---|
| sialyl Tn (sTn) | sTn is upregulated in numerous epithelial cancers and associated with poor patient outcomes. sTn has been investigated as a circulating biomarker for several cancers, and the Theratope vaccine against sTn has been tested in clinical trials. | [40,41] |
| Sialyl-T (sT) | The sT antigen is upregulated in several tumour types, including breast, ovarian, brain and renal cancers, and is associated with reduced survival times in patients. | [42,43,44,45,46,47]. |
| Selectin ligands | The sialyl Lewis antigens (sLeA and SLeX) are found at high levels in many cancer types and linked to metastasis. sLeA and SLeX are ligands for Selectins and enable cancer cells to leave the bloodstream and colonise other organs. | [48,49,51,52] |
| Siglec ligands | Increased levels of sialylation on cancer cells leads to upregulation of sialylated ligands that are recognised by Siglec receptors on immune cells. Siglec–sialoglycan interactions can modulate immune cell function and promote an immunosuppressive tumour microenvironment (TME). | [53] |
| Polysialic acid (polySia) | Polysialic acid is often upregulated in high-grade tumours, and expression correlates with metastatic disease and poor clinical prognosis. | [3,22,54,55,57,58] |
| Gangliosides | The gangliosides GD2 and GM3 can be upregulated in cancer and are being actively investigated as therapeutic targets. | [59,60,61,62,63,64] |
2.2. Sialyltransferase Enzymes
2.3. Sialidase Enzymes
3. The Functional Role of Sialylation in Cancer
3.1. Metastasis
3.2. Cancer Cell Survival
3.3. Immune Evasion
3.4. Therapy Resistance
4. Therapeutic Strategies to Inhibit Aberrant Sialylation
4.1. Sialyltransferase Inhibition
4.2. Selectin Inhibitors
4.3. Antibody–Sialidase Conjugates
4.4. Anti-Siglec Antibodies
4.5. Vaccines
5. Conclusions
Funding
Conflicts of Interest
References
- Varki, A.; Kornfeld, S. Historical Background and Overview. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 1–20. [Google Scholar]
- Möckl, L. The Emerging Role of the Mammalian Glycocalyx in Functional Membrane Organization and Immune System Regulation. Front. Cell Dev. Biol. 2020, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Gagneux, P.; Hennet, T.; Varki, A. Biological Functions of Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 79–92. [Google Scholar]
- Stanley, P.; Moremen, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. N-Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 103–116. [Google Scholar]
- Brockhausen, I.; Wandall, H.H.; Hagen, K.G.T.; Stanley, P. O-Galnac Glycans. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022; pp. 117–128. [Google Scholar]
- Ferrer, C.M.; Sodi, V.L.; Reginato, M.J. O-GlcNAcylation in Cancer Biology: Linking Metabolism and Signaling. J. Mol. Biol. 2016, 428, 3282–3294. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.M.; Lazar, A.C.; Cortes, D.F.; Kabulski, J.L. Recent advances in the MS analysis of glycoproteins: Theoretical considerations. Electrophoresis 2010, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.; Nasirikenari, M.; Manhardt, C.T.; Ashline, D.J.; Hanneman, A.J.; Reinhold, V.N.; Lau, J.T. Platelets Support Extracellular Sialylation by Supplying the Sugar Donor Substrate. J. Biol. Chem. 2014, 289, 8742–8748. [Google Scholar] [CrossRef]
- Nasirikenari, M.; Veillon, L.; Collins, C.C.; Azadi, P.; Lau, J.T.Y. Remodeling of Marrow Hematopoietic Stem and Progenitor Cells by Non-self ST6Gal-1 Sialyltransferase. J. Biol. Chem. 2014, 289, 7178–7189. [Google Scholar] [CrossRef]
- Hait, N.C.; Maiti, A.; Wu, R.; Andersen, V.L.; Hsu, C.-C.; Wu, Y.; Chapla, D.G.; Takabe, K.; Rusiniak, M.E.; Bshara, W.; et al. Extracellular sialyltransferase st6gal1 in breast tumor cell growth and invasiveness. Cancer Gene Ther. 2022. [Google Scholar] [CrossRef]
- Wu, H.C.; Meezan, E.; Black, P.H.; Robbins, P.W. Comparative studies on the carbohydrate-containing membrane components of normal and virus-transformed mouse fibroblasts. I. Glucosamine-labeling patterns in 3T3, spontaneously transformed 3T3, and SV-40-transformed 3T3 cells. Biochemistry 1969, 8, 2509–2517. [Google Scholar] [CrossRef]
- Häuselmann, I.; Borsig, L. Altered Tumor-Cell Glycosylation Promotes Metastasis. Front. Oncol. 2014, 4, 28. [Google Scholar] [CrossRef]
- Munkley, J.; Elliott, D.J. Hallmarks of glycosylation in cancer. Oncotarget 2016, 7, 35478–35489. [Google Scholar] [CrossRef] [Green Version]
- Bellis, S.L.; Reis, C.A.; Varki, A.; Kannagi, R.; Stanley, P. Glycosylation Changes in Cancer. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor: New York, NY, USA, 2022. [Google Scholar]
- Feizi, T. Carbohydrate antigens in human cancer. Cancer Surv. 1985, 4, 245–269. [Google Scholar] [PubMed]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef]
- Moss, E.L.; Hollingworth, J.; Reynolds, T.M. The role of CA125 in clinical practice. J. Clin. Pathol. 2005, 58, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Gilgunn, S.; Conroy, P.J.; Saldova, R.; Rudd, P.M.; O′Kennedy, R.J. Aberrant PSA glycosylation—A sweet predictor of prostate cancer. Nat. Rev. Urol. 2013, 10, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Mills, I.G.; Elliott, D.J. The role of glycans in the development and progression of prostate cancer. Nat. Rev. Urol. 2016, 13, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Mereiter, S.; Balmana, M.; Campos, D.; Gomes, J.; Reis, C.A. Glycosylation in the Era of Cancer-Targeted Therapy: Where Are We Heading? Cancer Cell 2019, 36, 6–16. [Google Scholar] [CrossRef]
- Rodrigues, E.; Macauley, M. Hypersialylation in Cancer: Modulation of Inflammation and Therapeutic Opportunities. Cancers 2018, 10, 207. [Google Scholar] [CrossRef]
- Pearce, O.M.T.; Läubli, H. Sialic acids in cancer biology and immunity. Glycobiology 2015, 26, 111–128. [Google Scholar] [CrossRef]
- Cohen, M.; Varki, A. The Sialome—Far More Than the Sum of Its Parts. OMICS 2010, 14, 455–464. [Google Scholar] [CrossRef]
- Dobie, C.; Skropeta, D. Insights into the role of sialylation in cancer progression and metastasis. Br. J. Cancer 2020, 124, 76–90. [Google Scholar] [CrossRef]
- Fuster, M.M.; Esko, J.D. The Sweet and Sour of Cancer: Glycans As Novel Therapeutic Targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Pietrobono, S.; Stecca, B. Aberrant Sialylation in Cancer: Biomarker and Potential Target for Therapeutic Intervention? Cancers 2021, 13, 2014. [Google Scholar] [CrossRef] [PubMed]
- Ruhenstroth-Bauer, G.; Fuhrmann, G.F.; Kuebler, W.; Rueff, F.; Munk, K. On the Importance of Neuraminic Acid in the Cell Membrane for the Growth of Malignant Cells. Z Krebsforsch 1962, 65, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Schauer, R. Sialic acids and their role as biological masks. Trends Biochem. Sci. 1985, 10, 357–360. [Google Scholar] [CrossRef]
- Narayanan, S. Sialic acid as a tumour marker. Ann. Clin. Lab. Sci. 1994, 24, 376–385. [Google Scholar]
- Sanford, B.H. An alteration in tumor histocompatibility induced by neuraminidase. Transplantation 1967, 5, 1273–1279. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Bellis, S.L. Regulation of the metastatic cell phenotype by sialylated glycans. Cancer Metastasis Rev. 2012, 31, 501–518. [Google Scholar] [CrossRef]
- Sedlacek, H.H.; Meesmann, H.; Seiler, F.R. Regression of spontaneous mammary tumors in dogs after injection of neuraminidase-treated tumor cells. Int. J. Cancer 1975, 15, 409–416. [Google Scholar] [CrossRef]
- Yogeeswaran, G.; Salk, P.L. Metastatic Potential Is Positively Correlated with Cell Surface Sialylation of Cultured Murine Tumor Cell Lines. Science 1981, 212, 1514–1516. [Google Scholar] [CrossRef]
- Samraj, A.N.; Pearce, O.M.T.; Läubli, H.; Crittenden, A.N.; Bergfeld, A.K.; Banda, K.; Gregg, C.J.; Bingman, A.E.; Secrest, P.; Diaz, S.L.; et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc. Natl. Acad. Sci. USA 2014, 112, 542–547. [Google Scholar] [CrossRef]
- Varki, N.M.; Varki, A. Diversity in cell surface sialic acid presentations: Implications for biology and disease. Lab. Investig. 2007, 87, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Chou, H.-H.; Takematsu, H.; Diaz, S.; Iber, J.; Nickerson, E.; Wright, K.L.; Muchmore, E.A.; Nelson, D.L.; Warren, S.T.; Varki, A. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc. Natl. Acad. Sci. USA 1998, 95, 11751–11756. [Google Scholar] [CrossRef]
- Bardor, M.; Nguyen, D.H.; Diaz, S.; Varki, A. Mechanism of Uptake and Incorporation of the Non-human Sialic Acid N-Glycolylneuraminic Acid into Human Cells. J. Biol. Chem. 2005, 280, 4228–4237. [Google Scholar] [CrossRef]
- Munkley, J.; Scott, E. Targeting Aberrant Sialylation to Treat Cancer. Medicines 2019, 6, 102. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. J. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef]
- Julien, S.; Videira, P.A.; Delannoy, P. Sialyl-tn in cancer: (How) did we miss the target? Biomolecules 2012, 2, 435–466. [Google Scholar] [CrossRef]
- Burchell, J.; Poulsom, R.; Hanby, A.; Whitehouse, C.; Cooper, L.; Clausen, H.; Miles, D.; Taylor-Papadimitriou, J. An 2,3 sialyltransferase (ST3Gal I) is elevated in primary breast carcinomas. Glycobiology 1999, 9, 1307–1311. [Google Scholar] [CrossRef]
- Wu, X.; Zhao, J.; Ruan, Y.; Sun, L.; Xu, C.; Jiang, H. Sialyltransferase ST3GAL1 promotes cell migration, invasion, and TGF-beta1-induced EMT and confers paclitaxel resistance in ovarian cancer. Cell Death Dis. 2018, 9, 1102. [Google Scholar] [CrossRef]
- Chong, Y.K.; Sandanaraj, E.; Koh, L.W.; Thangaveloo, M.; Tan, M.S.; Koh, G.R.; Toh, T.B.; Lim, G.G.; Holbrook, J.D.; Kon, O.L.; et al. ST3GAL1-Associated Transcriptomic Program in Glioblastoma Tumor Growth, Invasion, and Prognosis. J. Natl. Cancer Inst. 2016, 108, djv326. [Google Scholar] [CrossRef]
- Bai, Q.; Liu, L.; Xia, Y.; Long, Q.; Wang, J.; Xu, J.; Guo, J. Prognostic significance of ST3GAL-1 expression in patients with clear cell renal cell carcinoma. BMC Cancer 2015, 15, 880. [Google Scholar] [CrossRef]
- Yeo, H.L.; Fan, T.C.; Lin, R.J.; Yu, J.C.; Liao, G.S.; Chen, E.S.; Ho, M.Y.; Lin, W.D.; Chen, K.; Chen, C.H.; et al. Sialylation of vasorin by ST3Gal1 facilitates TGF-beta1-mediated tumor angiogenesis and progression. Int. J. Cancer 2019, 144, 1996–2007. [Google Scholar] [CrossRef]
- Wen, K.-C.; Sung, P.-L.; Hsieh, S.-L.; Chou, Y.-T.; Lee, O.K.-S.; Wu, C.-W.; Wang, P.-H. α2,3-sialyltransferase type I regulates migration and peritoneal dissemination of ovarian cancer cells. Oncotarget 2017, 8, 29013–29027. [Google Scholar] [CrossRef]
- Natoni, A.; Macauley, M.S.; O’Dwyer, M.E. Targeting Selectins and Their Ligands in Cancer. Front. Oncol. 2016, 6, 93. [Google Scholar] [CrossRef]
- Natoni, A.; Farrell, M.L.; Harris, S.; Falank, C.; Kirkham-McCarthy, L.; Macauley, M.S.; Reagan, M.R.; O’Dwyer, M. Sialyltransferase inhibition leads to inhibition of tumor cell interactions with E-selectin, VCAM1, and MADCAM1, and improves survival in a human multiple myeloma mouse model. Haematologica 2020, 105, 457–467. [Google Scholar] [CrossRef]
- Munkley, J. The glycosylation landscape of pancreatic cancer (Review). Oncol. Lett. 2019, 17, 2569–2575. [Google Scholar] [CrossRef]
- Cazet, A.; Julien, S.; Bobowski, M.; Burchell, J.; Delannoy, P. Tumour-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010, 12, 204. [Google Scholar] [CrossRef]
- Julien, S.; Ivetic, A.; Grigoriadis, A.; QiZe, D.; Burford, B.; Sproviero, D.; Picco, G.; Gillett, C.; Papp, S.L.; Schaffer, L.; et al. Selectin Ligand Sialyl-Lewis x Antigen Drives Metastasis of Hormone-Dependent Breast Cancers. Cancer Res. 2011, 71, 7683–7693. [Google Scholar] [CrossRef]
- van de Wall, S.; Santegoets, K.C.; van Houtum, E.J.; Büll, C.; Adema, G.J. Sialoglycans and Siglecs Can Shape the Tumor Immune Microenvironment. Trends Immunol. 2020, 41, 274–285. [Google Scholar] [CrossRef]
- Falconer, R.; Errington, R.; Shnyder, S.; Smith, P.; Patterson, L. Polysialyltransferase: A New Target in Metastatic Cancer. Curr. Cancer Drug Targets 2012, 12, 925–939. [Google Scholar] [CrossRef]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Inui, K.; Oyanagi, H.; Yamada, T.; et al. Prognostic significance of polysialic acid expression in resected non-small cell lung cancer. Cancer Res. 2001, 61, 1666–1670. [Google Scholar]
- Seidenfaden, R.; Krauter, A.; Schertzinger, F.; Gerardy-Schahn, R.; Hildebrandt, H. Polysialic Acid Directs Tumor Cell Growth by Controlling Heterophilic Neural Cell Adhesion Molecule Interactions. Mol. Cell. Biol. 2003, 23, 5908–5918. [Google Scholar] [CrossRef]
- Al-Saraireh, Y.M.J.; Sutherland, M.; Springett, B.R.; Freiberger, F.; Morais, G.R.; Loadman, P.M.; Errington, R.J.; Smith, P.J.; Fukuda, M.; Gerardy-Schahn, R.; et al. Pharmacological Inhibition of polysialyltransferase ST8SiaII Modulates Tumour Cell Migration. PLoS ONE 2013, 8, e73366. [Google Scholar] [CrossRef] [Green Version]
- Elkashef, S.M.; Allison, S.J.; Sadiq, M.; Basheer, H.A.; Morais, G.R.; Loadman, P.M.; Pors, K.; Falconer, R.A. Polysialic acid sustains cancer cell survival and migratory capacity in a hypoxic environment. Sci. Rep. 2016, 6, 33026. [Google Scholar] [CrossRef]
- Groux-Degroote, S.; Rodriguez-Walker, M.; Dewald, J.H.; Daniotti, J.L.; Delannoy, P. Gangliosides in Cancer Cell Signaling. Prog. Mol. Biol. Transl. Sci. 2018, 156, 197–227. [Google Scholar]
- Nazha, B.; Inal, C.; Owonikoko, T.K. Disialoganglioside GD2 Expression in Solid Tumors and Role as a Target for Cancer Therapy. Front. Oncol. 2020, 10, 1000. [Google Scholar] [CrossRef]
- Majzner, R.G.; Ramakrishna, S.; Yeom, K.W.; Patel, S.; Chinnasamy, H.; Schultz, L.M.; Richards, R.M.; Jiang, L.; Barsan, V.; Mancusi, R.; et al. GD2-CAR T cell therapy for H3K27M-mutated diffuse midline gliomas. Nature 2022, 603, 934–941. [Google Scholar] [CrossRef]
- Ladenstein, R.; Potschger, U.; Valteau-Couanet, D.; Luksch, R.; Castel, V.; Yaniv, I.; Laureys, G.; Brock, P.; Michon, J.M.; Owens, C.; et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1617–1629. [Google Scholar] [CrossRef]
- Zheng, C.; Terreni, M.; Sollogoub, M.; Zhang, Y. Ganglioside GM3 and Its Role in Cancer. Curr. Med. Chem. 2019, 26, 2933–2947. [Google Scholar] [CrossRef]
- Labrada, M.; Dorvignit, D.; Hevia, G.; Rodríguez-Zhurbenko, N.; Hernández, A.M.; Vázquez, A.M.; Fernández, L.E. GM3(Neu5Gc) ganglioside: An evolution fixed neoantigen for cancer immunotherapy. Semin. Oncol. 2018, 45, 41–51. [Google Scholar] [CrossRef]
- Visser, E.A.; Moons, S.J.; Timmermans, S.B.; de Jong, H.; Boltje, T.J.; Büll, C. Sialic acid O-acetylation: From biosynthesis to roles in health and disease. J. Biol. Chem. 2021, 297, 100906. [Google Scholar] [CrossRef]
- Cavdarli, S.; Delannoy, P.; Groux-Degroote, S. O-acetylated Gangliosides as Targets for Cancer Immunotherapy. Cells 2020, 9, 741. [Google Scholar] [CrossRef] [PubMed]
- Mandal, C.; Schwartz-Albiez, R.; Vlasak, R. Functions and Biosynthesis of O-Acetylated Sialic Acids. Top. Curr. Chem. 2012, 366, 1–30. [Google Scholar]
- Harduin-Lepers, A.; Vallejo-Ruiz, V.; Krzewinski-Recchi, M.-A.; Samyn-Petit, B.; Julien, S.; Delannoy, P. The human sialyltransferase family. Biochimie 2001, 83, 727–737. [Google Scholar] [CrossRef]
- Schjoldager, K.T.; Narimatsu, Y.; Joshi, H.J.; Clausen, H. Global view of human protein glycosylation pathways and functions. Nat. Rev. Mol. Cell Biol. 2020, 21, 729–749. [Google Scholar] [CrossRef] [PubMed]
- Szabo, R.; Skropeta, D. Advancement of Sialyltransferase Inhibitors: Therapeutic Challenges and Opportunities. Med. Res. Rev. 2016, 37, 219–270. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Yamaguchi, K. Mammalian sialidases: Physiological and pathological roles in cellular functions. Glycobiology 2012, 22, 880–896. [Google Scholar] [CrossRef] [PubMed]
- Lipničanová, S.; Chmelová, D.; Ondrejovič, M.; Frecer, V.; Miertuš, S. Diversity of sialidases found in the human body—A review. Int. J. Biol. Macromol. 2020, 148, 857–868. [Google Scholar] [CrossRef] [PubMed]
- Forcella, M.; Mozzi, A.; Stefanini, F.M.; Riva, A.; Epistolio, S.; Molinari, F.; Merlo, E.; Monti, E.; Fusi, P.; Frattini, M. Deregulation of sialidases in human normal and tumor tissues. Cancer Biomark. 2018, 21, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.B.; Dorsett, K.A.; Hjelmeland, A.B.; Bellis, S.L. The ST6Gal-I sialyltransferase protects tumor cells against hypoxia by enhancing HIF-1α signaling. J. Biol. Chem. 2018, 293, 5659–5667. [Google Scholar] [CrossRef]
- Dorsett, K.A.; Marciel, M.P.; Hwang, J.; Ankenbauer, E.K.; Bhalerao, N.; Bellis, S.L. Regulation of ST6GAL1 sialyltransferase expression in cancer cells. Glycobiology 2020, 31, 530–539. [Google Scholar] [CrossRef]
- Bull, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor′s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef] [PubMed]
- Munkley, J.; Vodak, D.; Livermore, K.E.; James, K.; Wilson, B.T.; Knight, B.; Mccullagh, P.; Mcgrath, J.; Crundwell, M.; Harries, L.W.; et al. Glycosylation is an Androgen-Regulated Process Essential for Prostate Cancer Cell Viability. eBioMedicine 2016, 8, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Lehoux, S.; Groux-Degroote, S.; Cazet, A.; Dhaenens, C.-M.; Maurage, C.-A.; Caillet-Boudin, M.-L.; Delannoy, P.; Krzewinski-Recchi, M.-A. Transcriptional regulation of the human ST6GAL2 gene in cerebral cortex and neuronal cells. Glycoconj. J. 2009, 27, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Garnham, R.; Scott, E.; Livermore, K.E.; Munkley, J. ST6GAL1: A key player in cancer. Oncol. Lett. 2019, 18, 983–989. [Google Scholar]
- Swindall, A.F.; Londoño-Joshi, A.I.; Schultz, M.J.; Fineberg, N.; Buchsbaum, D.J.; Bellis, S.L. ST6Gal-I Protein Expression Is Upregulated in Human Epithelial Tumors and Correlates with Stem Cell Markers in Normal Tissues and Colon Cancer Cell Lines. Cancer Res. 2013, 73, 2368–2378. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, C.C.; Shyr, Y.M.; Liao, W.Y.; Chen, T.H.; Wang, S.E.; Lu, P.C.; Lin, P.Y.; Chen, Y.B.; Mao, W.Y.; Han, H.Y.; et al. Elevation of beta-galactoside alpha2,6-sialyltransferase 1 in a fructoseresponsive manner promotes pancreatic cancer metastasis. Oncotarget 2017, 8, 7691–7709. [Google Scholar] [CrossRef]
- Wichert, B.; Milde-Langosch, K.; Galatenko, V.; Schmalfeldt, B.; Oliveira-Ferrer, L. Prognostic role of the sialyltransferase ST6GAL1 in ovarian cancer. Glycobiology 2018, 28, 898–903. [Google Scholar] [CrossRef]
- Hou, S.; Hang, Q.; Isaji, T.; Lu, J.; Fukuda, T.; Gu, J. Importance of membrane-proximal N-glycosylation on integrin beta1 in its activation and complex formation. FASEB J. 2016, 30, 4120–4131. [Google Scholar] [CrossRef] [PubMed]
- Seales, E.C.; Jurado, G.A.; Brunson, B.A.; Wakefield, J.K.; Frost, A.R.; Bellis, S.L. Hypersialylation of β1 Integrins, Observed in Colon Adenocarcinoma, May Contribute to Cancer Progression by Up-regulating Cell Motility. Cancer Res. 2005, 65, 4645–4652. [Google Scholar] [CrossRef]
- Liu, Y.; Pan, D.; Bellis, S.L.; Song, Y. Effect of altered glycosylation on the structure of the I-like domain of beta1 integrin: A molecular dynamics study. Proteins 2008, 73, 989–1000. [Google Scholar] [CrossRef]
- Chiang, C.-H.; Wang, C.-H.; Chang, H.-C.; More, S.V.; Li, W.-S.; Hung, W.-C. A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J. Cell. Physiol. 2010, 223, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Bellis, S.L. Variant glycosylation: An underappreciated regulatory mechanism for $beta;1 integrins. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1663, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Britain, C.M.; Holdbrooks, A.T.; Anderson, J.C.; Willey, C.D.; Bellis, S.L. Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J. Ovarian Res. 2018, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.C.; Beggs, R.R.; Ankenbauer, K.E.; Hwang, J.; Ma, V.P.-Y.; Salaita, K.; Bellis, S.L.; Mattheyses, A.L. ST6Gal-I–mediated sialylation of the epidermal growth factor receptor modulates cell mechanics and enhances invasion. J. Biol. Chem. 2022, 298, 101726. [Google Scholar] [CrossRef]
- Liu, N.; Zhu, M.; Linhai, Y.; Song, Y.; Gui, X.; Tan, G.; Li, J.; Liu, Y.; Deng, Z.; Chen, X.; et al. Increasing HER2 α2,6 sialylation facilitates gastric cancer progression and resistance via the Akt and ERK pathways. Oncol. Rep. 2018, 40, 2997–3005. [Google Scholar] [CrossRef]
- Holdbrooks, A.T.; Britain, C.M.; Bellis, S.L. ST6Gal-I sialyltransferase promotes tumor necrosis factor (TNF)-mediated cancer cell survival via sialylation of the TNF receptor 1 (TNFR1) death receptor. J. Biol. Chem. 2018, 293, 1610–1622. [Google Scholar] [CrossRef]
- Swindall, A.F.; Bellis, S.L. Sialylation of the Fas Death Receptor by ST6Gal-I Provides Protection against Fas-mediated Apoptosis in Colon Carcinoma Cells. J. Biol. Chem. 2011, 286, 22982–22990. [Google Scholar] [CrossRef]
- Lu, J.; Isaji, T.; Im, S.; Fukuda, T.; Hashii, N.; Takakura, D.; Kawasaki, N.; Gu, J. Beta-Galactoside alpha2,6-sialyltranferase 1 promotes transforming growth factor-beta-mediated epithelial-mesenchymal transition. J. Biol. Chem. 2014, 289, 34627–34641. [Google Scholar] [CrossRef] [PubMed]
- Schultz, M.J.; Holdbrooks, A.T.; Chakraborty, A.; Grizzle, W.E.; Landen, C.N.; Buchsbaum, D.J.; Conner, M.G.; Arend, R.C.; Yoon, K.J.; Klug, C.A.; et al. The Tumor-Associated Glycosyltransferase ST6Gal-I Regulates Stem Cell Transcription Factors and Confers a Cancer Stem Cell Phenotype. Cancer Res. 2016, 76, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Perdicchio, M.; Ilarregui, J.M.; Verstege, M.I.; Cornelissen, L.A.M.; Schetters, S.T.T.; Engels, S.; Ambrosini, M.; Kalay, H.; Veninga, H.; Haan, J.M.M.D.; et al. Sialic acid-modified antigens impose tolerance via inhibition of T-cell proliferation and de novo induction of regulatory T cells. Proc. Natl. Acad. Sci. USA 2016, 113, 3329–3334. [Google Scholar] [CrossRef]
- Wang, L.; Li, S.; Yu, X.; Han, Y.; Wu, Y.; Wang, S.; Chen, X.; Zhang, J.; Wang, S. Alpha2,6-Sialylation promotes immune escape in hepatocarcinoma cells by regulating T cell functions and CD147/MMP signaling. J. Physiol. Biochem. 2019, 75, 199–207. [Google Scholar] [CrossRef]
- Irons, E.E.; Lau, J.T.Y. Systemic ST6Gal-1 Is a Pro-survival Factor for Murine Transitional B Cells. Front. Immunol. 2018, 9, 2150. [Google Scholar] [CrossRef] [PubMed]
- Irons, E.E.; Punch, P.R.; Lau, J.T.Y. Blood-Borne ST6GAL1 Regulates Immunoglobulin Production in B Cells. Front. Immunol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Irons, E.E.; Lee-Sundlov, M.M.; Zhu, Y.; Neelamegham, S.; Hoffmeister, K.M.; Lau, J.T. B cells suppress medullary granulopoiesis by an extracellular glycosylation-dependent mechanism. eLife 2019, 8, e47328. [Google Scholar] [CrossRef]
- Jones, M.B.; Oswald, D.M.; Joshi, S.; Whiteheart, S.W.; Orlando, R.; Cobb, B.A. B-cell-independent sialylation of IgG. Proc. Natl. Acad. Sci. USA 2016, 113, 7207–7212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myojin, Y.; Kodama, T.; Maesaka, K.; Motooka, D.; Sato, Y.; Tanaka, S.; Abe, Y.; Ohkawa, K.; Mita, E.; Hayashi, Y.; et al. ST6GAL1 Is a Novel Serum Biomarker for Lenvatinib-Susceptible FGF19-Driven Hepatocellular Carcinoma. Clin. Cancer Res. 2021, 27, 1150–1161. [Google Scholar] [CrossRef]
- Zhang, H.; Freitas, D.; Kim, H.S.; Fabijanic, K.; Li, Z.; Chen, H.; Mark, M.T.; Molina, H.; Martin, A.B.; Bojmar, L.; et al. Identification of distinct nanoparticles and subsets of extracellular vesicles by asymmetric flow field-flow fractionation. Nat. Cell Biol. 2018, 20, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Nasirikenari, M.; Lugade, A.A.; Neelamegham, S.; Gao, Z.; Moremen, K.W.; Bogner, P.N.; Thanavala, Y.; Lau, J.T.Y. Recombinant Sialyltransferase Infusion Mitigates Infection-Driven Acute Lung Inflammation. Front. Immunol. 2019, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Picco, G.; Julien, S.; Brockhausen, I.; Beatson, R.; Antonopoulos, A.; Haslam, S.; Mandel, U.; Dell, A.; Pinder, S.; Taylor-Papadimitriou, J.; et al. Over-expression of ST3Gal-I promotes mammary tumorigenesis. Glycobiology 2010, 20, 1241–1250. [Google Scholar] [CrossRef]
- Fan, T.-C.; Yeo, H.L.; Hsu, H.-M.; Yu, J.-C.; Ho, M.-Y.; Lin, W.-D.; Chang, N.-C.; Yu, J.; Yu, A.L. Reciprocal feedback regulation of ST3GAL1 and GFRA1 signaling in breast cancer cells. Cancer Lett. 2018, 434, 184–195. [Google Scholar] [CrossRef]
- Sproviero, D.; Julien, S.; Burford, B.; Taylor-Papadimitriou, J.; Burchell, J.M. Cyclooxygenase-2 enzyme induces the expression of the alpha-2,3-sialyltransferase-3 (ST3Gal-I) in breast cancer. J. Biol. Chem. 2012, 287, 44490–44497. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-D.; Fan, T.-C.; Hung, J.-T.; Yeo, H.-L.; Wang, S.-H.; Kuo, C.-W.; Khoo, K.-H.; Pai, L.-M.; Yu, J.; Yu, A.L. Sialylation of CD55 by ST3GAL1 Facilitates Immune Evasion in Cancer. Cancer Immunol. Res. 2021, 9, 113–122. [Google Scholar] [CrossRef]
- Pérez-Garay, M.; Arteta, B.; Pagès, L.; de Llorens, R.; de Bolòs, C.; Vidal-Vanaclocha, F.; Peracaula, R. α2,3-Sialyltransferase ST3Gal III Modulates Pancreatic Cancer Cell Motility and Adhesion In Vitro and Enhances Its Metastatic Potential In Vivo. PLoS ONE 2010, 5, e12524. [Google Scholar] [CrossRef]
- Rodriguez, E.; Boelaars, K.; Brown, K.; Li, R.J.E.; Kruijssen, L.; Bruijns, S.C.M.; van Ee, T.; Schetters, S.T.T.; Crommentuijn, M.H.W.; van der Horst, J.C.; et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat. Commun. 2021, 12, 1270. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamashita, S.-I.; Endoh, M.; Yamato, T.; Hoshi, S.; Ohyama, C.; Watanabe, R.; Ito, A.; Satoh, M.; Wada, T.; et al. Clinical significance of ST3Gal IV expression in human renal cell carcinoma. Oncol. Rep. 2002, 9, 1251–1255. [Google Scholar] [CrossRef] [PubMed]
- Glavey, S.V.; Manier, S.; Natoni, A.; Sacco, A.; Moschetta, M.; Reagan, M.R.; Murillo, L.S.; Sahin, I.; Wu, P.; Mishima, Y.; et al. The sialyltransferase ST3GAL6 influences homing and survival in multiple myeloma. Blood 2014, 124, 1765–1776. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.S.; Harduin-Lepers, A.; Magalhaes, A.; Machado, E.; Mendes, N.; Costa, L.T.; Matthiesen, R.; Almeida, R.; Costa, J.; Reis, C.A. Differential expression of alpha-2,3-sialyltransferases and alpha-1,3/4-fucosyltransferases regulates the levels of sialyl Lewis a and sialyl Lewis x in gastrointestinal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, X.; Dong, W.; Xu, Z.; Jian, Y.; Xu, C.; Zhang, L.; Wei, A.; Yu, X.; Wang, S.; et al. ST3Gal IV Mediates the Growth and Proliferation of Cervical Cancer Cells In Vitro and In Vivo Via the Notch/p21/CDKs Pathway. Front. Oncol. 2020, 10, 540332. [Google Scholar] [CrossRef]
- Gomes, C.; Osorio, H.; Pinto, M.T.; Campos, D.; Oliveira, M.J.; Reis, C.A. Expression of ST3GAL4 leads to SLe(x) expression and induces c-Met activation and an invasive phenotype in gastric carcinoma cells. PLoS ONE 2013, 8, e66737. [Google Scholar] [CrossRef]
- Laubli, H.; Borsig, L. Selectins promote tumor metastasis. Semin. Cancer Biol. 2010, 20, 169–177. [Google Scholar] [CrossRef]
- Marcos, N.T.; Pinho, S.; Grandela, C.; Cruz, A.; Samyn-Petit, B.; Harduin-Lepers, A.; Almeida, R.; Silva, F.; Morais, V.; Costa, J.; et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialyl-Tn antigen. Cancer Res. 2004, 64, 7050–7057. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, F.C.; Figueiredo, P.; Lacerda, M. Simple mucin-type carbohydrate antigens (T, sialosyl-T, Tn and sialosyl-Tn) in breast carcinogenesis. Virchows Arch. 1995, 427, 251–258. [Google Scholar] [CrossRef]
- Victorzon, M.; Nordling, S.; Nilsson, O.; Roberts, P.J.; Haglund, C. Sialyl Tn antigen is an independent predictor of outcome in patients with gastric cancer. Int. J. Cancer 1996, 65, 295–300. [Google Scholar] [CrossRef]
- Leivonen, M.; Nordling, S.; Lundin, J.; von Boguslawski, K.; Haglund, C. STn and Prognosis in Breast Cancer. Oncology 2001, 61, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Pereira, S.; Lima, J.; Peixoto, A.; Fernandes, E.; Neves, D.; Neves, M.; Gaiteiro, C.; Tavares, A.; Gil Da Costa, R.M.; et al. Abnormal Protein Glycosylation and Activated PI3K/Akt/mTOR Pathway: Role in Bladder Cancer Prognosis and Targeted Therapeutics. PLoS ONE 2015, 10, e0141253. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Hirohashi, Y.; Murai, A.; Nishidate, T.; Okita, K.; Wang, L.; Ikehara, Y.; Satoyoshi, T.; Usui, A.; Kubo, T.; et al. ST6GALNAC1 plays important roles in enhancing cancer stem phenotypes of colorectal cancer via the Akt pathway. Oncotarget 2017, 8, 112550–112564. [Google Scholar] [CrossRef]
- Julien, S.; Adriaenssens, E.; Ottenberg, K.; Furlan, A.; Courtand, G.; Vercoutter-Edouart, A.-S.; Hanisch, F.-G.; Delannoy, P.; Le Bourhis, X. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology 2005, 16, 54–64. [Google Scholar] [CrossRef]
- Wang, W.-Y.; Cao, Y.-X.; Zhou, X.; Wei, B.; Zhan, L.; Sun, S.-Y. Stimulative role of ST6GALNAC1 in proliferation, migration and invasion of ovarian cancer stem cells via the Akt signaling pathway. Cancer Cell Int. 2019, 19, 86. [Google Scholar] [CrossRef]
- Ozaki, H.; Matsuzaki, H.; Ando, H.; Kaji, H.; Nakanishi, H.; Ikehara, Y.; Narimatsu, H. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin. Exp. Metastasis 2012, 29, 229–238. [Google Scholar] [CrossRef]
- Kvorjak, M.; Ahmed, Y.; Miller, M.L.; Sriram, R.; Coronnello, C.; Hashash, J.G.; Hartman, D.J.; Telmer, C.A.; Miskov-Zivanov, N.; Finn, O.J.; et al. Cross-talk between Colon Cells and Macrophages Increases ST6GALNAC1 and MUC1-sTn Expression in Ulcerative Colitis and Colitis-Associated Colon Cancer. Cancer Immunol. Res. 2020, 8, 167–178. [Google Scholar] [CrossRef]
- Wang, J.; Sun, J.; Liu, L.N.; Flies, D.B.; Nie, X.; Toki, M.; Zhang, J.; Song, C.; Zarr, M.; Zhou, X.; et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat. Med. 2019, 25, 656–666. [Google Scholar] [CrossRef] [PubMed]
- Angata, T.; Tabuchi, Y.; Nakamura, K.; Nakamura, M. Siglec-15: An immune system Siglec conserved throughout vertebrate evolution. Glycobiology 2007, 17, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Nason, R.; Sun, L.; Van Coillie, J.; Sørensen, D.M.; Moons, S.J.; Yang, Z.; Arbitman, S.; Fernandes, S.M.; Furukawa, S.; et al. Probing the binding specificities of human Siglecs by cell-based glycan arrays. Proc. Natl. Acad. Sci. USA 2021, 118, e2026102118. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Marcos, N.T.; Ferreira, B.; Carvalho, A.S.; Oliveira, M.J.; Santos-Silva, F.; Harduin-Lepers, A.; Reis, C.A. Biological significance of cancer-associated sialyl-Tn antigen: Modulation of malignant phenotype in gastric carcinoma cells. Cancer Lett. 2007, 249, 157–170. [Google Scholar] [CrossRef]
- Schneider, F.; Kemmner, W.; Haensch, W.; Franke, G.; Gretschel, S.; Karsten, U.; Schlag, P.M. Overexpression of sialyltransferase CMP-sialic acid: Galbeta1,3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001, 61, 4605–4611. [Google Scholar]
- Miao, X.; Zhao, Y. ST6GalNAcII mediates tumor invasion through PI3K/Akt/NF-kappaB signaling pathway in follicular thyroid carcinoma. Oncol. Rep. 2016, 35, 2131–2140. [Google Scholar] [CrossRef] [Green Version]
- Murugaesu, N.; Iravani, M.; van Weverwijk, A.; Ivetic, A.; Johnson, D.A.; Antonopoulos, A.; Fearns, A.; Jamal-Hanjani, M.; Sims, D.; Fenwick, K.; et al. An In Vivo Functional Screen Identifies ST6GalNAc2 Sialyltransferase as a Breast Cancer Metastasis Suppressor. Cancer Discov. 2014, 4, 304–317. [Google Scholar] [CrossRef]
- Angata, K.; Suzuki, M.; McAuliffe, J.; Ding, Y.; Hindsgaul, O.; Fukuda, M. Differential biosynthesis of polysialic acid on neural cell adhesion molecule (NCAM) and oligosaccharide acceptors by three distinct alpha 2,8-sialyltransferases, ST8Sia IV (PST), ST8Sia II (STX), and ST8Sia III. J. Biol. Chem. 2000, 275, 18594–18601. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Mollicone, R.; Delannoy, P.; Oriol, R. The animal sialyltransferases and sialyltransferase-related genes: A Phylogenetic approach. Glycobiology 2005, 15, 805–817. [Google Scholar] [CrossRef]
- Harduin-Lepers, A.; Petit, D.; Mollicone, R.; Delannoy, P.; Petit, J.-M.; Oriol, R. Evolutionary history of the alpha2,8-sialyltransferase (ST8Sia) gene family: Tandem duplications in early deuterostomes explain most of the diversity found in the vertebrate ST8Sia genes. BMC Evol. Biol. 2008, 8, 258. [Google Scholar] [CrossRef]
- Jarahian, M.; Marofi, F.; Maashi, M.S.; Ghaebi, M.; Khezri, A.; Berger, M.R. Re-Expression of Poly/Oligo-Sialylated Adhesion Molecules on the Surface of Tumor Cells Disrupts Their Interaction with Immune-Effector Cells and Contributes to Pathophysiological Immune Escape. Cancers 2021, 13, 5203. [Google Scholar] [CrossRef] [PubMed]
- Scheidegger, E.P.; Sternberg, L.R.; Roth, J.; Lowe, J.B. A Human STX cDNA Confers Polysialic Acid Expression in Mammalian Cells. J. Biol. Chem. 1995, 270, 22685–22688. [Google Scholar] [CrossRef]
- Tanaka, F.; Otake, Y.; Nakagawa, T.; Kawano, Y.; Miyahara, R.; Li, M.; Yanagihara, K.; Nakayama, J.; Fujimoto, I.; Ikenaka, K.; et al. Expression of polysialic acid and STX, a human polysialyltransferase, is correlated with tumor progression in non-small cell lung cancer. Cancer Res. 2000, 60, 3072–3080. [Google Scholar] [PubMed]
- Suzuki, M.; Suzuki, M.; Nakayama, J.; Suzuki, A.; Angata, K.; Chen, S.; Sakai, K.; Hagihara, K.; Yamaguchi, Y.; Fukuda, M. Polysialic acid facilitates tumor invasion by glioma cells. Glycobiology 2005, 15, 887–894. [Google Scholar] [CrossRef]
- Gong, L.; Zhou, X.; Yang, J.; Jiang, Y.; Yang, H. Effects of the regulation of polysialyltransferase ST8SiaII on the invasiveness and metastasis of small cell lung cancer cells. Oncol. Rep. 2016, 37, 131–1388. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Dong, W.; Su, Z.; Zhao, L.; Miao, Y.; Li, N.; Zhou, H.; Jia, L. Functional roles of sialylation in breast cancer progression through miR-26a/26b targeting ST8SIA4. Cell Death Dis. 2016, 7, e2561. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Wu, Y.; Hu, J.; Shan, Y.; Ma, J.; Ma, H.; Qi, X.; Jia, L. Long noncoding RNA HOTAIR promotes renal cell carcinoma malignancy through alpha-2, 8-sialyltransferase 4 by sponging microRNA-124. Cell Prolif. 2018, 51, e12507. [Google Scholar] [CrossRef]
- Ma, H.; Zhou, H.; Song, X.; Shi, S.; Zhang, J.; Jia, L. Modification of sialylation is associated with multidrug resistance in human acute myeloid leukemia. Oncogene 2014, 34, 726–740. [Google Scholar] [CrossRef]
- Ma, W.; Zhao, X.; Liang, L.; Wang, G.; Li, Y.; Miao, X.; Zhao, Y. miR-146a and miR-146b promote proliferation, migration and invasion of follicular thyroid carcinoma via inhibition of ST8SIA4. Oncotarget 2017, 8, 28028–28041. [Google Scholar] [CrossRef]
- Baeza-Kallee, N.; Berges, R.; Souberan, A.; Colin, C.; Denicolai, E.; Appay, R.; Tchoghandjian, A.; Figarella-Branger, D. Glycolipids Recognized by A2B5 Antibody Promote Proliferation, Migration, and Clonogenicity in Glioblastoma Cells. Cancers 2019, 11, 1267. [Google Scholar] [CrossRef]
- Nguyen, K.; Yan, Y.; Yuan, B.; Dasgupta, A.; Sun, J.; Mu, H.; Do, K.A.; Ueno, N.T.; Andreeff, M.; Battula, V.L. ST8SIA1 Regulates Tumor Growth and Metastasis in TNBC by Activating the FAK-AKT-mTOR Signaling Pathway. Mol. Cancer Ther. 2018, 17, 2689–2701. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, Y.; Zhang, P.; Momota, H.; Kato, A.; Hashimoto, N.; Ohmi, Y.; Bhuiyan, R.H.; Farhana, Y.; Natsume, A.; Wakabayashi, T.; et al. Lack of GD3 synthase (St8sia1) attenuates malignant properties of gliomas in genetically engineered mouse model. Cancer Sci. 2021, 112, 3756–3768. [Google Scholar] [CrossRef] [PubMed]
- Penrose, H.; Cable, C.; Heller, S.; Ungerleider, N.; Nakhoul, H.; Baddoo, M.; Hartono, A.B.; Lee, S.; Burow, M.E.; Flemington, E.F.; et al. Loss of Forkhead Box O3 Facilitates Inflammatory Colon Cancer: Transcriptome Profiling of the Immune Landscape and Novel Targets. Cell. Mol. Gastroenterol. Hepatol. 2018, 7, 391–408. [Google Scholar] [CrossRef]
- Takashima, S.; Ishida, H.K.; Inazu, T.; Ando, T.; Ishida, H.; Kiso, M.; Tsuji, S.; Tsujimoto, M. Molecular cloning and expression of a sixth type of alpha 2,8-sialyltransferase (ST8Sia VI) that sialylates O-glycans. J. Biol. Chem. 2002, 277, 24030–24038. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Crotts, S.B.; Shapiro, M.J.; Rajcula, M.; McCue, S.; Liu, X.; Khazaie, K.; Dong, H.; Shapiro, V.S. ST8Sia6 Promotes Tumor Growth in Mice by Inhibiting Immune Responses. Cancer Immunol. Res. 2021, 9, 952–966. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, T.; Takahashi, K.; Hata, K.; Shiozaki, K.; Yamaguchi, K. Sialidase significance for cancer progression. Glycoconj. J. 2012, 29, 567–577. [Google Scholar] [CrossRef]
- Uemura, T.; Shiozaki, K.; Yamaguchi, K.; Miyazaki, S.; Satomi, S.; Kato, K.; Sakuraba, H.; Miyagi, T. Contribution of sialidase NEU1 to suppression of metastasis of human colon cancer cells through desialylation of integrin beta4. Oncogene 2009, 28, 1218–1229. [Google Scholar] [CrossRef]
- Zhou, X.; Zhai, Y.; Liu, C.; Yang, G.; Guo, J.; Li, G.; Sun, C.; Qi, X.; Li, X.; Guan, F. Sialidase NEU1 suppresses progression of human bladder cancer cells by inhibiting fibronectin-integrin alpha5beta1 interaction and Akt signaling pathway. Cell Commun. Signal. 2020, 18, 44. [Google Scholar] [CrossRef]
- Ren, L.R.; Zhang, L.P.; Huang, S.Y.; Zhu, Y.F.; Li, W.J.; Fang, S.Y.; Shen, L.; Gao, Y.L. Effects of sialidase NEU1 siRNA on proliferation, apoptosis, and invasion in human ovarian cancer. Mol. Cell Biochem. 2016, 411, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Gilmour, A.M.; Abdulkhalek, S.; Cheng, T.S.; Alghamdi, F.; Jayanth, P.; O’Shea, L.K.; Geen, O.; Arvizu, L.A.; Szewczuk, M.R. A novel epidermal growth factor receptor-signaling platform and its targeted translation in pancreatic cancer. Cell. Signal. 2013, 25, 2587–2603. [Google Scholar] [CrossRef]
- Kakugawa, Y.; Wada, T.; Yamaguchi, K.; Yamanami, H.; Ouchi, K.; Sato, I.; Miyagi, T. Up-regulation of plasma membrane-associated ganglioside sialidase (Neu3) in human colon cancer and its involvement in apoptosis suppression. Proc. Natl. Acad. Sci. USA 2002, 99, 10718–10723. [Google Scholar] [CrossRef] [PubMed]
- Ueno, S.; Saito, S.; Wada, T.; Yamaguchi, K.; Satoh, M.; Arai, Y.; Miyagi, T. Plasma Membrane-associated Sialidase Is Up-regulated in Renal Cell Carcinoma and Promotes Interleukin-6-induced Apoptosis Suppression and Cell Motility. J. Biol. Chem. 2006, 281, 7756–7764. [Google Scholar] [CrossRef]
- Nomura, H.; Tamada, Y.; Miyagi, T.; Suzuki, A.; Taira, M.; Suzuki, N.; Susumu, N.; Irimura, T.; Aoki, D. Expression of NEU3 (Plasma Membrane-Associated Sialidase) in Clear Cell Adenocarcinoma of the Ovary: Its Relationship with T Factor of pTNM Classification. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2006, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, S.; Sato, I.; Wada, T.; Yamaguchi, K.; Li, Y.; Li, D.; Zhao, X.; Ueno, S.; Aoki, H.; Tochigi, T.; et al. Plasma membrane-associated sialidase (NEU3) regulates progression of prostate cancer to androgen-independent growth through modulation of androgen receptor signaling. Cell Death Differ. 2011, 19, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Hata, K.; Tochigi, T.; Sato, I.; Kawamura, S.; Shiozaki, K.; Wada, T.; Takahashi, K.; Moriya, S.; Yamaguchi, K.; Hosono, M.; et al. Increased sialidase activity in serum of cancer patients: Identification of sialidase and inhibitor activities in human serum. Cancer Sci. 2015, 106, 383–389. [Google Scholar] [CrossRef]
- Yamanami, H.; Shiozaki, K.; Wada, T.; Yamaguchi, K.; Uemura, T.; Kakugawa, Y.; Hujiya, T.; Miyagi, T. Down-regulation of sialidase NEU4 may contribute to invasive properties of human colon cancers. Cancer Sci. 2007, 98, 299–307. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the Origin of Cancer Metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Ganesh, K.; Massagué, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Almaraz, R.T.; Tian, Y.; Bhattarcharya, R.; Tan, E.; Chen, S.-H.; Dallas, M.R.; Chen, L.; Zhang, Z.; Zhang, H.; Konstantopoulos, K.; et al. Metabolic Flux Increases Glycoprotein Sialylation: Implications for Cell Adhesion and Cancer Metastasis. Mol. Cell. Proteom. 2012, 11, M112-017558. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, L.; Shen, S.; Wu, S.; Burdick, M.M. Effect of alpha 2,6 sialylation on integrin-mediated adhesion of breast cancer cells to fibronectin and collagen IV. Life Sci. 2016, 149, 138–145. [Google Scholar] [CrossRef]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2018, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Britain, C.M.; Bhalerao, N.; Silva, A.D.; Chakraborty, A.; Buchsbaum, D.J.; Crowley, M.R.; Crossman, D.K.; Edwards, Y.J.; Bellis, S.L. Glycosyltransferase ST6Gal-I promotes the epithelial to mesenchymal transition in pancreatic cancer cells. J. Biol. Chem. 2021, 296, 100034. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Hong, S.; Dong, L.; Cheng, B.; Lin, L.; Zhao, B.; Chen, Y.G.; Chen, X. Dynamic Sialylation in Transforming Growth Factor-beta (TGF-beta)-induced Epithelial to Mesenchymal Transition. J. Biol. Chem. 2015, 290, 12000–12013. [Google Scholar] [CrossRef]
- Hakomori, S. Glycosylation defining cancer malignancy: New wine in an old bottle. Proc. Natl. Acad. Sci. USA 2002, 99, 10231–10233. [Google Scholar] [CrossRef]
- Coupland, L.; Parish, C.R. Platelets, Selectins, and the Control of Tumor Metastasis. Semin. Oncol. 2014, 41, 422–434. [Google Scholar] [CrossRef]
- Läubli, H.; Borsig, L. Selectins as Mediators of Lung Metastasis. Cancer Microenviron. 2010, 3, 97–105. [Google Scholar] [CrossRef]
- Rosen, S.D.; Bertozzi, C.R. The selectins and their ligands. Curr. Opin. Cell Biol. 1994, 6, 663–673. [Google Scholar] [CrossRef]
- Barbier, V.; Erbani, J.; Fiveash, C.; Davies, J.M.; Tay, J.; Tallack, M.R.; Lowe, J.; Magnani, J.L.; Pattabiraman, D.R.; Perkins, A.; et al. Endothelial E-selectin inhibition improves acute myeloid leukaemia therapy by disrupting vascular niche-mediated chemoresistance. Nat. Commun. 2020, 11, 2042. [Google Scholar] [CrossRef]
- Muz, B.; Abdelghafer, A.; Markovic, M.; Yavner, J.; Melam, A.; Salama, N.N.; Azab, A.K. Targeting E-Selectin to Tackle Cancer Using Uproleselan. Cancers 2021, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Sipkins, D.A.; Wei, X.; Wu, J.W.; Runnels, J.M.; Côté, D.; Means, T.K.; Luster, A.D.; Scadden, D.T.; Lin, C.P. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature 2005, 435, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Price, T.T.; Burness, M.L.; Sivan, A.; Warner, M.J.; Cheng, R.; Lee, C.H.; Olivere, L.; Comatas, K.; Magnani, J.; Lyerly, H.K.; et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci. Transl. Med. 2016, 8, 340ra73. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Mondal, N.; Greco, T.M.; Wei, Y.; Spadazzi, C.; Lin, S.-C.; Zheng, H.; Cheung, C.; Magnani, J.L.; Lin, S.-H.; et al. Bone vascular niche E-selectin induces mesenchymal–epithelial transition and Wnt activation in cancer cells to promote bone metastasis. Nat. Cell Biol. 2019, 21, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Lichtenstein, R.; Rabinovich, A.G. Glycobiology of cell death: When glycans and lectins govern cell fate. Cell Death Differ. 2013, 20, 976–986. [Google Scholar] [CrossRef]
- Zhuo, Y.; Chammas, R.; Bellis, S.L. Sialylation of beta1 integrins blocks cell adhesion to galectin-3 and protects cells against galectin-3-induced apoptosis. J. Biol. Chem. 2008, 283, 22177–22185. [Google Scholar]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Scott, E.; Elliott, D.J.; Munkley, J. Tumour associated glycans: A route to boost immunotherapy? Clin. Chim. Acta 2020, 502, 167–173. [Google Scholar] [CrossRef]
- Rodríguez, E.; Schetters, S.T.T.; Van Kooyk, Y. The tumour glyco-code as a novel immune checkpoint for immunotherapy. Nat. Rev. Immunol. 2018, 18, 204–211. [Google Scholar] [CrossRef]
- Cohen, M.; Elkabets, M.; Perlmutter, M.; Porgador, A.; Voronov, E.; Apte, R.N.; Lichtenstein, R. Sialylation of 3-Methylcholanthrene–Induced Fibrosarcoma Determines Antitumor Immune Responses during Immunoediting. J. Immunol. 2010, 185, 5869–5878. [Google Scholar]
- Macauley, M.S.; Crocker, P.R.; Paulson, J.C. Siglec-mediated regulation of immune cell function in disease. Nat. Rev. Immunol. 2014, 14, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, J.; Rodríguez, E.; Van Kooyk, Y. Modulation of Immune Tolerance via Siglec-Sialic Acid Interactions. Front. Immunol. 2018, 9, 2807. [Google Scholar] [PubMed]
- Smith, B.A.H.; Bertozzi, C.R. The clinical impact of glycobiology: Targeting selectins, Siglecs and mammalian glycans. Nat. Rev. Drug Discov. 2021, 20, 217–243. [Google Scholar] [CrossRef] [PubMed]
- Beatson, R.; Tajadura-Ortega, V.; Achkova, D.; Picco, G.; Tsourouktsoglou, T.-D.; Klausing, S.; Hillier, M.; Maher, D.A.J.; Noll, S.K.T.; Crocker, P.R.; et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat. Immunol. 2016, 17, 1273–1281. [Google Scholar] [CrossRef]
- Beatson, R.; Graham, R.; Freile, F.G.; Cozzetto, D.; Kannambath, S.; Pfeifer, E.; Woodman, N.; Owen, J.; Nuamah, R.; Mandel, U.; et al. Cancer-associated hypersialylated MUC1 drives the differentiation of human monocytes into macrophages with a pathogenic phenotype. Commun. Biol. 2020, 3, 644. [Google Scholar] [CrossRef]
- Barkal, A.A.; Brewer, R.E.; Markovic, M.; Kowarsky, M.; Barkal, S.A.; Zaro, B.; Krishnan, V.; Hatakeyama, J.; Dorigo, O.; Barkal, L.J.; et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature 2019, 572, 392–396. [Google Scholar] [CrossRef]
- Hudak, J.E.; Canham, S.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2013, 10, 69–75. [Google Scholar] [CrossRef]
- Daly, J.; Carlsten, M.; O’Dwyer, M. Sugar Free: Novel Immunotherapeutic Approaches Targeting Siglecs and Sialic Acids to Enhance Natural Killer Cell Cytotoxicity Against Cancer. Front. Immunol. 2019, 10, 1047. [Google Scholar] [CrossRef] [Green Version]
- Engblom, C.; Pfirschke, C.; Zilionis, R.; Martins, J.D.S.; Bos, S.A.; Courties, G.; Rickelt, S.; Severe, N.; Baryawno, N.; Faget, J.; et al. Osteoblasts Remotely Supply Lung Tumors with Cancer-Promoting Siglecf(High) Neutrophils. Science 2017, 358, 6367. [Google Scholar] [CrossRef]
- Ikehara, Y.; Ikehara, S.K.; Paulson, J.C. Negative regulation of T cell receptor signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem. 2004, 279, 43117–43125. [Google Scholar] [CrossRef]
- Vuchkovska, A.; Glanville, D.G.; Scurti, G.M.; Nishimura, M.I.; White, P.; Ulijasz, A.T.; Iwashima, M. Siglec-5 is an inhibitory immune checkpoint molecule for human T cells. Immunology 2022, 166, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Läubli, H.; Varki, A. Sialic Acid-Binding Immunoglobulin-Like Lectins (Siglecs) Detect Self-Associated Molecular Patterns to Regulate Immune Responses. Cell Mol. Life Sci. 2020, 77, 593–605. [Google Scholar] [CrossRef] [PubMed]
- O′Sullivan, J.A.; Wei, Y.; Carroll, D.J.; Moreno-Vinasco, L.; Cao, Y.; Zhang, F.; Lee, J.J.; Zhu, Z.; Bochner, B.S. Frontline Science: Characterization of a novel mouse strain expressing human Siglec-8 only on eosinophils. J. Leukoc. Biol. 2018, 104, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Landig, C.S.; Siddiqui, S.; Secundino, I.; Olson, J.; Varki, N.; Nizet, V.; Varki, A. Paired Siglec receptors generate opposite inflammatory responses to a human-specific pathogen. EMBO J. 2017, 36, 751–760. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yu, K.-R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.-H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef]
- Bull, C.; Boltje, T.J.; Balneger, N.; Weischer, S.M.; Wassink, M.; van Gemst, J.J.; Bloemendal, V.R.; Boon, L.; van der Vlag, J.; Heise, T.; et al. Sialic Acid Blockade Suppresses Tumor Growth by Enhancing T-cell–Mediated Tumor Immunity. Cancer Res. 2018, 78, 3574–3588. [Google Scholar] [CrossRef]
- Fedarko, N.; Fohr, B.; Robey, P.; Young, M.F.; Fisher, L.W. Factor H Binding to Bone Sialoprotein and Osteopontin Enables Tumor Cell Evasion of Complement-mediated Attack. J. Biol. Chem. 2000, 275, 16666–16672. [Google Scholar] [CrossRef]
- Schultz, M.J.; Swindall, A.F.; Wright, J.W.; Sztul, E.S.; Landen, C.N.; Bellis, S.L. ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J. Ovarian Res. 2013, 6, 25. [Google Scholar] [CrossRef] [Green Version]
- Ou, L.; He, X.; Liu, N.; Song, Y.; Li, J.; Gao, L.; Huang, X.; Deng, Z.; Wang, X.; Lin, S. Sialylation of FGFR1 by ST6Gal-I overexpression contributes to ovarian cancer cell migration and chemoresistance. Mol. Med. Rep. 2020, 21, 1449–1460. [Google Scholar] [CrossRef]
- Chen, X.; Wang, L.; Zhao, Y.; Yuan, S.; Wu, Q.; Zhu, X.; Niang, B.; Wang, S.; Zhang, J. ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 2016, 7, 51955–51964. [Google Scholar] [CrossRef]
- Patel, K.D.; De, M.; Jethva, D.D.; Rathod, B.S.; Patel, P.S. Alterations in Sialylation Patterns are Significantly Associated with Imatinib Mesylate Resistance in Chronic Myeloid Leukemia. Arch. Med. Res. 2021, 53, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.N.; Junqueira, M.S.; Francisco, G.; Vilanova, M.; Magalhães, A.; Baruffi, M.D.; Chammas, R.; Harris, A.L.; Reis, C.A.; Bernardes, E.S. O-glycan sialylation alters galectin-3 subcellular localization and decreases chemotherapy sensitivity in gastric cancer. Oncotarget 2016, 7, 83570–83587. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-Y.; Liu, Y.-C.; Chen, N.-Y.; Tsai, C.-F.; Wang, Y.-T.; Chen, Y.-J.; Hsu, T.-L.; Yang, P.-C.; Wong, C.-H. Effect of sialylation on EGFR phosphorylation and resistance to tyrosine kinase inhibition. Proc. Natl. Acad. Sci. USA 2015, 112, 6955–6960. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Lee, H.-J.; Bae, S.; Lee, Y.-S. Protein Sialylation by Sialyltransferase Involves Radiation Resistance. Mol. Cancer Res. 2008, 6, 1316–1325. [Google Scholar] [CrossRef]
- Lee, M.; Lee, H.J.; Seo, W.D.; Park, K.H.; Lee, Y.S. Sialylation of integrin beta1 is involved in radiation-induced adhesion and migration in human colon cancer cells. Int. J. Radiat Oncol. Biol. Phys. 2010, 76, 1528–1536. [Google Scholar] [CrossRef]
- Park, J.-J.; Lee, M. Increasing the α 2, 6 Sialylation of Glycoproteins May Contribute to Metastatic Spread and Therapeutic Resistance in Colorectal Cancer. Gut Liver 2013, 7, 629–641. [Google Scholar] [CrossRef]
- Punch, P.R.; Irons, E.E.; Manhardt, C.T.; Marathe, H.; Lau, J.T.Y. The sialyltransferase ST6GAL1 protects against radiation-induced gastrointestinal damage. Glycobiology 2020, 30, 446–453. [Google Scholar] [CrossRef]
- Duarte, H.O.; Rodrigues, J.G.; Gomes, C.; Hensbergen, P.J.; Ederveen, A.L.H.; de Ru, A.H.; Mereiter, S.; Polónia, A.; Fernandes, E.; Ferreira, J.A.; et al. ST6Gal1 targets the ectodomain of ErbB2 in a site-specific manner and regulates gastric cancer cell sensitivity to trastuzumab. Oncogene 2021, 40, 3719–3733. [Google Scholar] [CrossRef]
- Harvey, B.; Thomas, P. Inhibition of CMP-Sialic Acid Transport in Human Liver and Colorectal Cancer Cell Lines by a Sialic Acid Nucleoside Conjugate (KI-8110). Biochem. Biophys. Res. Commun. 1993, 190, 571–575. [Google Scholar] [CrossRef]
- Perdicchio, M.; Cornelissen, L.A.M.; Streng-Ouwehand, I.; Engels, S.; Verstege, M.I.; Boon, L.; Geerts, D.; van Kooyk, Y.; Unger, W.W.J. Tumor sialylation impedes T cell mediated anti-tumor responses while promoting tumor associated-regulatory T cells. Oncotarget 2016, 7, 8771–8782. [Google Scholar] [CrossRef]
- Rillahan, C.D.; Antonopoulos, A.; Lefort, C.T.; Sonon, R.; Azadi, P.; Ley, K.; Dell, A.; Haslam, S.M.; Paulson, J.C. Global metabolic inhibitors of sialyl- and fucosyltransferases remodel the glycome. Nat. Chem. Biol. 2012, 8, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; Wassink, M.; de Graaf, A.M.; van Delft, F.L.; den Brok, M.H.; Adema, G.J. Targeting aberrant sialylation in cancer cells using a fluorinated sialic acid analog impairs adhesion, migration, and in vivo tumor growth. Mol. Cancer Ther. 2013, 12, 1935–1946. [Google Scholar] [CrossRef] [PubMed]
- Macauley, M.S.; Arlian, B.M.; Rillahan, C.D.; Pang, P.-C.; Bortell, N.; Marcondes, M.C.G.; Haslam, S.M.; Dell, A.; Paulson, J.C. Systemic Blockade of Sialylation in Mice with a Global Inhibitor of Sialyltransferases. J. Biol. Chem. 2014, 289, 35149–35158. [Google Scholar] [CrossRef] [PubMed]
- Bull, C.; Boltje, T.J.; van Dinther, E.A.W.; Peters, T.; de Graaf, A.M.A.; Leusen, J.H.W.; Kreutz, M.; Figdor, C.G.; Brok, M.H.D.; Adema, G.J. Targeted Delivery of a Sialic Acid-Blocking Glycomimetic to Cancer Cells Inhibits Metastatic Spread. ACS Nano 2015, 9, 733–745. [Google Scholar] [CrossRef]
- Heise, T.; Pijnenborg, J.F.A.; Büll, C.; van Hilten, N.; Kers-Rebel, E.D.; Balneger, N.; Elferink, H.; Adema, G.J.; Boltje, T.J. Potent Metabolic Sialylation Inhibitors Based on C-5-Modified Fluorinated Sialic Acids. J. Med. Chem. 2018, 62, 1014–1021. [Google Scholar] [CrossRef]
- Huang, W.; Sun, L.; Wang, B.; Ma, Y.; Yao, D.; Han, W.; Wang, L. Ginsenosides, potent inhibitors of sialyltransferase. Z. Für Nat. C 2019, 75, 41–49. [Google Scholar] [CrossRef]
- Chang, W.-W.; Yu, C.-Y.; Lin, T.-W.; Wang, P.-H.; Tsai, Y.-C. Soyasaponin I decreases the expression of α2,3-linked sialic acid on the cell surface and suppresses the metastatic potential of B16F10 melanoma cells. Biochem. Biophys. Res. Commun. 2006, 341, 614–619. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lin, T.-W.; Chang, W.-W.; Wu, C.-Y.; Lo, W.-H.; Wang, P.-H.; Tsai, Y.-C. Soyasaponin-I-modified invasive behavior of cancer by changing cell surface sialic acids. Gynecol. Oncol. 2005, 96, 415–422. [Google Scholar] [CrossRef]
- Hidari, K.I.; Oyama, K.-I.; Ito, G.; Nakayama, M.; Inai, M.; Goto, S.; Kanai, Y.; Watanabe, K.-I.; Yoshida, K.; Furuta, T.; et al. Identification and characterization of flavonoids as sialyltransferase inhibitors. Biochem. Biophys. Res. Commun. 2009, 382, 609–613. [Google Scholar] [CrossRef]
- Chang, K.-H.; Lee, L.; Chen, J.; Li, W.-S. Lithocholic Acid Analogues, New and Potent α-2,3-Sialyltransferase Inhibitors. Chem. Commun. 2006, 37, 629–631. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Tang, Y.-A.; Huang, S.-M.; Juan, H.-F.; Wu, L.-W.; Sun, Y.-C.; Wang, S.-C.; Wu, K.-W.; Balraj, G.; Chang, T.-T.; et al. A Novel Sialyltransferase Inhibitor Suppresses FAK/Paxillin Signaling and Cancer Angiogenesis and Metastasis Pathways. Cancer Res. 2011, 71, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Brown, S.J.; Register, A.C.; Rosen, H.; Paulson, J.C. High-Throughput Screening for Inhibitors of Sialyl- and Fucosyltransferases. Angew. Chem. Int. Ed. 2011, 50, 12534–12537. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Niu, Y.; Xiong, D.-C.; Cao, X.; Ye, X.-S. Highly Substituted Cyclopentane–CMP Conjugates as Potent Sialyltransferase Inhibitors. J. Med. Chem. 2015, 58, 7972–7990. [Google Scholar] [CrossRef] [PubMed]
- Bowles, W.H.D.; Gloster, T.M. Sialidase and Sialyltransferase Inhibitors: Targeting Pathogenicity and Disease. Front. Mol. Biosci. 2021, 8, 705133. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, B.; Benz, J.; Greif, M.; Engel, A.M.; Sobek, H.; Rudolph, M.G. The structure of human α-2,6-sialyltransferase reveals the binding mode of complex glycans. Acta Crystallogr. Sect. D Biol. Crystallogr. 2013, 69, 1826–1838. [Google Scholar] [CrossRef]
- Moremen, K.W.; Ramiah, A.; Stuart, M.; Steel, J.; Meng, L.; Forouhar, F.; Moniz, A.H.; Gahlay, G.; Gao, Z.; Chapla, D.; et al. Expression system for structural and functional studies of human glycosylation enzymes. Nat. Chem. Biol. 2017, 14, 156–162. [Google Scholar] [CrossRef]
- Volkers, G.; Worrall, L.J.; Kwan, D.H.; Yu, C.-C.; Baumann, L.; Lameignere, E.; Wasney, A.G.; Scott, E.N.; Wakarchuk, W.; Foster, L.J.; et al. Structure of human ST8SiaIII sialyltransferase provides insight into cell-surface polysialylation. Nat. Struct. Mol. Biol. 2015, 22, 627–635. [Google Scholar] [CrossRef]
- Montgomery, A.; Dobie, C.; Szabo, R.; Hallam, L.; Ranson, M.; Yu, H.; Skropeta, D. Design, synthesis and evaluation of carbamate-linked uridyl-based inhibitors of human ST6Gal I. Bioorganic Med. Chem. 2020, 28, 115561. [Google Scholar] [CrossRef]
- Ataga, K.I.; Kutlar, A.; Kanter, J.; Liles, D.; Cancado, R.; Friedrisch, J.; Guthrie, T.H.; Knight-Madden, J.; Alvarez, O.A.; Gordeuk, V.R.; et al. Crizanlizumab for the Prevention of Pain Crises in Sickle Cell Disease. N. Engl. J. Med. 2017, 376, 429–439. [Google Scholar] [CrossRef]
- Muz, B.; Azab, F.; de la Puente, P.; Rollins, S.; Alvarez, R.; Kawar, Z.; Azab, A.K. Inhibition of P-Selectin and PSGL-1 Using Humanized Monoclonal Antibodies Increases the Sensitivity of Multiple Myeloma Cells to Bortezomib. BioMed Res. Int. 2015, 2015, 417586. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Jonas, B.A.; Becker, P.S.; O’Dwyer, M.; Advani, A.S.; Marlton, P.; Magnani, J.; Thackray, H.M.; Liesveld, J. GMI-1271, a novel E-selectin antagonist, combined with induction chemotherapy in elderly patients with untreated AML. J. Clin. Oncol. 2017, 35, 2560. [Google Scholar] [CrossRef]
- DeAngelo, D.J.; Jonas, A.B.; Liesveld, J.L.; Bixby, D.L.; Advani, A.S.; Marlton, P.; O′Dwyer, E.M.; Fogler, W.E.; Magnani, J.L.; Chen, M.M.; et al. High E-Selectin Ligand Expression Contributes to Chemotherapy-Resistance in Poor Risk Relapsed and Refractory (R/R) Acute Myeloid Leukemia (AML) Patients and Can be Overcome with the Addition of Uproleselan. Blood 2019, 134, 2690. [Google Scholar] [CrossRef]
- Ferber, S.; Tiram, G.; Sousa-Herves, A.; Eldar-Boock, A.; Krivitsky, A.; Scomparin, A.; Yeini, E.; Ofek, P.; Ben-Shushan, D.; Vossen, L.I.; et al. Co-targeting the tumor endothelium and P-selectin-expressing glioblastoma cells leads to a remarkable therapeutic outcome. eLife 2017, 6, e25281. [Google Scholar] [CrossRef]
- Bhat, R.; García, I.; Aznar, E.; Arnaiz, B.; Martínez-Bisbal, M.C.; Liz-Marzán, L.M.; Penadés, S.; Martínez-Máñez, R. Lectin-gated and glycan functionalized mesoporous silica nanocontainers for targeting cancer cells overexpressing Lewis X antigen. Nanoscale 2017, 10, 239–249. [Google Scholar] [CrossRef]
- Shodai, T.; Suzuki, J.; Kudo, S.; Itoh, S.; Terada, M.; Fujita, S.; Shimazu, H.; Tsuji, T. Inhibition of P-selectin-mediated cell adhesion by a sulfated derivative of sialic acid. Biochem. Biophys. Res. Commun. 2003, 312, 787–793. [Google Scholar] [CrossRef]
- Morita, Y.; Kamal, M.; Kang, S.-A.; Zhang, R.; Lokesh, G.L.; Thiviyanathan, V.; Hasan, N.; Woo, S.; Zhao, D.; Leslie, M.; et al. E-selectin Targeting PEGylated-thioaptamer Prevents Breast Cancer Metastases. Mol. Ther.-Nucleic Acids 2016, 5, e399. [Google Scholar] [CrossRef]
- Chen, W.C.; Completo, G.C.; Sigal, D.S.; Crocker, P.R.; Saven, A.; Paulson, J.C. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood 2010, 115, 4778–4786. [Google Scholar] [CrossRef]
- Jandus, C.; Boligan, K.F.; Chijioke, O.; Liu, H.; Dahlhaus, M.; Demoulins, T.; Schneider, C.; Wehrli, M.; Hunger, R.E.; Baerlocher, G.M.; et al. Interactions between Siglec-7/9 receptors and ligands influence NK cell-dependent tumor immunosurveillance. J. Clin. Investig. 2014, 124, 1810–1820. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, E.; Macauley, M.S. Targeted self-destruction. Nat. Chem. Biol. 2020, 16, 1281–1283. [Google Scholar] [CrossRef]
- Xiao, H.; Woods, E.C.; Vukojicic, P.; Bertozzi, C.R. Precision glycocalyx editing as a strategy for cancer immunotherapy. Proc. Natl. Acad. Sci. USA 2016, 113, 10304–10309. [Google Scholar] [CrossRef]
- Gray, M.A.; Stanczak, M.A.; Mantuano, N.R.; Xiao, H.; Pijnenborg, J.F.A.; Malaker, S.A.; Miller, C.L.; Weidenbacher, P.A.; Tanzo, J.T.; Ahn, G.; et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376–1384. [Google Scholar] [CrossRef]
- Läubli, H.; Pearce, O.M.T.; Schwarz, F.; Siddiqui, S.S.; Deng, L.; Stanczak, M.A.; Deng, L.; Verhagen, A.; Secrest, P.; Lusk, C.; et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14211–14216. [Google Scholar] [CrossRef] [PubMed]
- Bénac, O.; Gaudin, M.; Ors, M.; Le Roy, A.; Blanc, H.R.; Soulas, C.; Chanteux, S.; Rossi, B.; Gauthier, L.; Paturel, C.; et al. Abstract 2713: Preclinical development of first-in-class antibodies targeting Siglec-9 immune checkpoint for cancer immunotherapy. Cancer Res. 2018, 78, 2713. [Google Scholar] [CrossRef]
- He, F.; Wang, N.; Li, J.; He, L.; Yang, Z.; Lu, J.; Xiong, G.; Yu, C.; Wang, S. High affinity monoclonal antibody targeting Siglec-15 for cancer immunotherapy. J. Clin. Transl. Res. 2021, 7, 739–749. [Google Scholar]
- Cao, G.; Xiao, Z.; Yin, Z. Normalization cancer immunotherapy: Blocking Siglec-15! Signal Transduct. Target Ther. 2019, 4, 10. [Google Scholar] [CrossRef]
- Kobayashi, H.; Terao, T.; Kawashima, Y. Serum sialyl Tn as an independent predictor of poor prognosis in patients with epithelial ovarian cancer. J. Clin. Oncol. 1992, 10, 95–101. [Google Scholar] [CrossRef]
- Ibrahim, N.K.; Murray, J.L. Clinical Development of the STn-KLH Vaccine (Theratope®). Clin. Breast Cancer 2003, 3, S139–S143. [Google Scholar] [CrossRef]
- Holmberg, A.L.; Sandmaier, B.M. Theratope® vaccine (STn-KLH). Expert Opin. Biol. Ther. 2001, 1, 881–891. [Google Scholar] [CrossRef]
- MacLean, G.D.; Reddish, M.; Koganty, R.R.; Wong, T.; Gandhi, S.; Smolenski, M.; Samuel, J.; Nabholtzl, J.M.; Longenecker, B.M. Immunization of breast cancer patients using a synthetic sialyl-Tn glycoconjugate plus Detox adjuvant. Cancer Immunol. Immunother. 1993, 36, 215–222. [Google Scholar] [CrossRef]
- MacLean, G.D.; Reddish, M.A.; Koganty, R.R.; Longenecker, B.M. Antibodies against Mucin-Associated Sialyl-Tn Epitopes Correlate with Survival of Metastatic Adenocarcinoma Patients Undergoing Active Specific Immunotherapy with Synthetic STn Vaccine. J. Immunother. Emphas. Tumor. Immunol. 1996, 19, 59–68. [Google Scholar] [CrossRef]
- Miles, D.; Roché, H.; Martin, M.; Perren, T.J.; Cameron, D.A.; Glaspy, J.; Dodwell, D.; Parker, J.; Mayordomo, J.; Tres, A.; et al. Phase III Multicenter Clinical Trial of the Sialyl-TN (STn)-Keyhole Limpet Hemocyanin (KLH) Vaccine for Metastatic Breast Cancer. Oncologist 2011, 16, 1092–1100. [Google Scholar] [CrossRef]
- O′Cearbhaill, R.E.; Ragupathi, G.; Zhu, J.; Wan, Q.; Mironov, S.; Yang, G.; Spassova, M.K.; Iasonos, A.; Kravetz, S.; Ouerfelli, O.; et al. A Phase I Study of Unimolecular Pentavalent (Globo-H-GM2-sTn-TF-Tn) Immunization of Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer in First Remission. Cancers 2016, 8, 46. [Google Scholar] [CrossRef]
- Ragupathi, G.; Damani, P.; Srivastava, G.; Srivastava, O.; Sucheck, S.J.; Ichikawa, Y.; Livingston, P.O. Synthesis of sialyl Lewis(a) (sLe (a), CA19-9) and construction of an immunogenic sLe(a) vaccine. Cancer Immunol. Immunother. 2009, 58, 1397–1405. [Google Scholar] [CrossRef] [Green Version]



| Approach | Progress | References |
|---|---|---|
| Sialyltransferase inhibitors | Intra-tumoural injection of 3Fax-Neu5Ac suppresses tumour growth in multiple cancer models by promoting T-cell mediated immunity. Targeted delivery of P-3FAX-Neu5Ac using nanoparticles can prevent metastasis in a mouse lung cancer model. However, 3Fax-Neu5Ac has been shown to produce liver and kidney dysfunction in mice. C-5 carbamate sialyltransferase inhibitors that reach higher concentrations within the cell and induce prolonged inhibition of sialylation have been developed but are yet to be tested in vivo. | [168,218,219,220] |
| Selectin inhibitors | Blocking antibodies for P-Selectin have been developed. The E-selectin inhibitor Uproleselan (GMI-1271) has shown promise for treating acute myeloid leukaemia (AML) in combination with chemotherapy, and may also re-sensitise multiple myeloma to therapy. | [234,235] NCT02306291 [175,235,236,237] NCT03616470 |
| Antibody–sialidase conjugates | A sialidase conjugated to a HER2 antibody (tratuzumab) can de-sialylate cancer cells, remove Siglec ligands and prolong survival times in mice. A HER2-sialidase conjugate is currently in Phase I/II clinical trials in combination with traditional immune checkpoint blockade for patients with non-small cell lung cancer, colorectal cancer, melanoma, pancreatic cancer, and ovarian cancer. | [188,193,245,246] NCT05259696 |
| Anti-Siglec antibodies | Anti-Siglec 9 antibodies are in preclinical development. Anti-Siglec 7 antibodies have been shown to promote NK mediated killing. The anti-Siglec-15 blocking antibody (NC318) is being tested in clinical trials. | [192,247,248,249,250] NCT03665285 |
| Vaccines | The Theratope sTn-KLH vaccine can promote the generation of anti-sTn antibodies, but a phase III trial showed no benefit for metastatic breast cancer patients. A unimolecular pentavalent vaccine containing vaccine portions of Globo-H, GM2, sTn, TF and the Tn antigen has been tested in patients with ovarian, fallopian tube or peritoneal cancer, and produced increased antibody titres to these antigens in a phase I clinical trial. A KLH conjugate vaccine has also been produced for sLeA and tested in metastatic breast cancer patients. | NCT00003638 [256] NCT01248273 NCT00470574 [257,258] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munkley, J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers 2022, 14, 4248. https://doi.org/10.3390/cancers14174248
Munkley J. Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers. 2022; 14(17):4248. https://doi.org/10.3390/cancers14174248
Chicago/Turabian StyleMunkley, Jennifer. 2022. "Aberrant Sialylation in Cancer: Therapeutic Opportunities" Cancers 14, no. 17: 4248. https://doi.org/10.3390/cancers14174248
APA StyleMunkley, J. (2022). Aberrant Sialylation in Cancer: Therapeutic Opportunities. Cancers, 14(17), 4248. https://doi.org/10.3390/cancers14174248