Impact of Shape Irregularity in Medial Sphenoid Wing Meningiomas on Postoperative Cranial Nerve Functioning, Proliferation, and Progression-Free Survival
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
2.2. Data Recording
2.3. Surgical Procedure and Follow-Up Regime
2.4. Immunohistochemistry
2.5. Statistics of Institutional Data
2.6. Systematic Review
2.6.1. Search Workflow
2.6.2. Selection Criteria
2.6.3. Data Collection, Data Extraction, and Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Patient Characteristics in Regularly and Irregularly Shaped Medial Sphenoid Wing Meningiomas
3.3. Progression-Free Survival Outcomes in Regularly and Irregularly Shaped Medial Sphenoid Wing Meningiomas
3.4. Association between Shape and MIB-1 Index
3.5. Association between Shape and New Cranial Nerve Morbidity after Medial Sphenoid Wing Meningioma Surgery
3.6. Association between Shape and Postoperative Functioning of Preoperatively Exisiting Cranial Nerve Deficits
3.7. Systematic Review
3.7.1. Literature Search Results of Meningioma Shape and MIB-1 Index
3.7.2. Association between Shape and MIB-1 Index in the Pooled Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23 (Suppl. 2), iii1–iii105. [Google Scholar] [CrossRef] [PubMed]
- O’Rahilly, R.; Müller, F. The meninges in human development. J. Neuropathol. Exp. Neurol. 1986, 45, 588–608. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Vasudevan, H.N.; Seo, K.; Villanueva-Meyer, J.E.; Choudhury, A.; Liu, S.J.; Pekmezci, M.; Findakly, S.; Hilz, S.; Lastella, S.; et al. Multiplatform genomic profiling and magnetic resonance imaging identify mechanisms underlying intratumor heterogeneity in meningioma. Nat. Commun. 2020, 11, 4803. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Rutkowski, M.J.; Chen, C.J.; Shangari, G.; Kane, A.J.; Parsa, A.T.; Berger, M.S.; McDermott, M.W.; Hénaux, P.-L.; Bretonnier, M.; et al. Modern surgical outcomes following surgery for sphenoid wing meningiomas. J. Neurosurg. 2013, 119, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Güdük, M.; Özduman, K.; Pamir, M.N. Sphenoid Wing Meningiomas: Surgical Outcomes in a Series of 141 Cases and Proposal of a Scoring System Predicting Extent of Resection. World Neurosurg. 2019, 125, e48–e59. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Holoubi, A.; Rifai, A.; Fox, J.L. Microsurgical removal of suprasellar meningiomas. Neurosurgery 1985, 16, 364–372. [Google Scholar] [CrossRef]
- Dolenc, V. Microsurgical removal of large sphenoidal bone meningiomas. Acta Neurochir. Suppl. 1979, 28, 391–396. [Google Scholar]
- Lee, J.H.; Sade, B.; Park, B.J.A. surgical technique for the removal of clinoidal meningiomas. Neurosurgery 2006, 59 (Suppl. S1), ONS108–ONS114. [Google Scholar] [CrossRef]
- McDermott, M.W.; Durity, F.A.; Rootman, J.; Woodhurst, W.B. Combined frontotemporal-orbitozygomatic approach for tumors of the sphenoid wing and orbit. Neurosurgery 1990, 26, 107–116. [Google Scholar] [CrossRef]
- Mathiesen, T.; Lindquist, C.; Kihlström, L.; Karlsson, B. Recurrence of cranial base meningiomas. Neurosurgery 1996, 39, 2–7; discussion 8–9. [Google Scholar] [CrossRef] [PubMed]
- Bonnal, J.; Thibaut, A.; Brotchi, J.; Born, J. Invading meningiomas of the sphenoid ridge. J. Neurosurg. 1980, 53, 587–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heye, S.; Maleux, G.; Van Loon, J.; Wilms, G. Symptomatic stenosis of the cavernous portion of the internal carotid artery due to an irresectable medial sphenoid wing meningioma: Treatment by endovascular stent placement. AJNR Am. J. Neuroradiol. 2006, 27, 1532–1534. [Google Scholar] [PubMed]
- Masalha, W.; Heiland, D.H.; Steiert, C.; Krüger, M.T.; Schnell, D.; Heiland, P.; Bissolo, M.; Grosu, A.L.; Schnell, O.; Beck, J.; et al. Management of Medial Sphenoid Wing Meningioma Involving the Cavernous Sinus: A Single-Center Series of 105 Cases. Cancers 2022, 14, 2201. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Roser, F.; Jacobs, C.; Vorkapic, P.; Samii, M. Medial sphenoid wing meningiomas: Clinical outcome and recurrence rate. Neurosurgery 2006, 58, 626–639. [Google Scholar] [CrossRef]
- Chaichana, K.L.; Jackson, C.; Patel, A.; Miller, N.R.; Subramanian, P.; Lim, M.; Gallia, G.; Olivi, A.; Weingart, J.; Brem, H.; et al. Predictors of visual outcome following surgical resection of medial sphenoid wing meningiomas. J. Neurol. Surg. B Skull Base 2012, 73, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Kendall, B.; Pullicino, P. Comparison of consistency of meningiomas and CT appearances. Neuroradiology 1979, 18, 173–176. [Google Scholar] [CrossRef]
- Little, K.M.; Friedman, A.H.; Sampson, J.H.; Wanibuchi, M.; Fukushima, T. Surgical management of petroclival meningiomas: Defining resection goals based on risk of neurological morbidity and tumor recurrence rates in 137 patients. Neurosurgery 2005, 56, 546–559. [Google Scholar]
- Sauvigny, T.; Ricklefs, F.L.; Hoffmann, L.; Schwarz, R.; Westphal, M.; Schmidt, N.O. Features of tumor texture influence surgery and outcome in intracranial meningioma. Neurooncol. Adv. 2020, 2, vdaa113. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Toma, M.; Hölzel, M.; Güresir, E. Increased MIB-1 Labeling Index Is Associated with Abducens Nerve Morbidity in Primary Sporadic Petroclival Meningioma Surgery: Beyond Location and Approach. Curr. Oncol. 2022, 29, 5026–5041. [Google Scholar] [CrossRef]
- Popadic, B.; Scheichel, F.; Pinggera, D.; Weber, M.; Ungersboeck, K.; Kitzwoegerer, M.; Roetzer, T.; Oberndorfer, S.; Sherif, C.; Freyschlag, C.F.; et al. The meningioma surface factor: A novel approach to quantify shape irregularity on preoperative imaging and its correlation with WHO grade. J. Neurosurg. 2021, 136, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Al-Mefty, O. Operative Atlas of Meningiomas; Lippincott-Raven: Philadelphia, PA, USA, 1998. [Google Scholar]
- Antinheimo, J.; Haapasalo, H.; Haltia, M.; Tatagiba, M.; Thomas, S.; Brandis, A.; Sainio, M.; Carpen, O.; Samii, M.; Jääskeläinen, J. Proliferation potential and histological features in neurofibromatosis 2-associated and sporadic meningiomas. J. Neurosurg. 1997, 87, 610–614. [Google Scholar] [CrossRef] [PubMed]
- Goldbrunner, R.; Minniti, G.; Preusser, M.; Jenkinson, M.D.; Sallabanda, K.; Houdart, E.; von Deimling, A.; Stavrinou, P.; Lefranc, F.; Lund-Johansen, M.; et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016, 17, e383–e391. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Chotai, S.; Chen, M.; Jin, S.; Qi, S.T.; Pan, J. Preoperative radiologic classification of convexity meningioma to predict the survival and aggressive meningioma behavior. PLoS ONE 2015, 10, e0118908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadell, H. Volume, shape, and roundness of quartz particles. J. Geol. 1935, 43, 250–280. [Google Scholar] [CrossRef]
- Fedorov, A.; Beichel, R.; Kalpathy-Cramer, J.; Finet, J.; Fillion-Robin, J.C.; Pujol, S.; Bauer, C.; Jennings, D.; Fennessy, F.; Sonka, M.; et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn. Reason. Imaging 2012, 30, 1323–1341. [Google Scholar] [CrossRef] [Green Version]
- Pichon, E.; Tannenbaum, A.; Kikinis, R. A statistically based flow for image segmentation. Med. Image Anal. 2004, 8, 267–274. [Google Scholar] [CrossRef]
- Kim, B.W.; Kim, M.S.; Kim, S.W.; Chang, C.H.; Kim, O.L. Peritumoral brain edema in meningiomas: Correlation of radiologic and pathologic features. J. Korean Neurosurg. Soc. 2011, 49, 26–30. [Google Scholar] [CrossRef]
- Zada, G.; Yashar, P.; Robison, A.; Winer, J.; Khalessi, A.; Mack, W.J.; Giannotta, S.L. A proposed grading system for standardizing tumor consistency of intracranial meningiomas. Neurosurg. Focus 2013, 35, E1. [Google Scholar] [CrossRef] [Green Version]
- Friconnet, G.; Espindola Ala, V.H.; Janot, K.; Brinjikji, W.; Bogey, C.; Lemnos, L.; Salle, H.; Saleme, S.; Mounayer, C.; Rouchaud, A. MRI predictive score of pial vascularization of supratentorial intracranial meningioma. Eur. Radiol. 2019, 29, 3516–3522. [Google Scholar] [CrossRef]
- Lemée, J.M.; Corniola, M.V.; Meling, T.R. Benefits of re-do surgery for recurrent intracranial meningiomas. Sci. Rep. 2020, 10, 303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashiba, T.; Hashimoto, N.; Maruno, M.; Izumoto, S.; Suzuki, T.; Kagawa, N.; Yoshimine, T. Scoring radiologic characteristics to predict proliferative potential in meningiomas. Brain Tumor Pathol. 2006, 23, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Nakasu, S.; Nakajima, M.; Matsumura, K.; Nakasu, Y.; Handa, J. Meningioma: Proliferating potential and clinicoradiological features. Neurosurgery 1995, 37, 1049–1055. [Google Scholar] [CrossRef]
- Liu, H.; Zhou, J.; Li, W.; Liu, G. Comparative analysis of the magnetic resonance imaging features between anaplastic meningioma and atypical meningioma. J. Craniofac. Surg. 2016, 27, e229–e233. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Xu, Y.; Liu, S.; Xue, F.; Wang, B.; Qin, S.; Sun, X.; He, J. Predicting the grade of meningiomas by clinical-radiological features: A comparison of precontrast and postcontrast MRI. Front. Oncol. 2022, 12, 1053089. [Google Scholar] [CrossRef] [PubMed]
- Yan, P.F.; Yan, L.; Hu, T.T.; Xiao, D.D.; Zhang, Z.; Zhao, H.Y.; Feng, J. The Potential Value of Preoperative MRI Texture and Shape Analysis in Grading Meningiomas: A Preliminary Investigation. Transl. Oncol. 2017, 10, 570–577. [Google Scholar] [CrossRef]
- Sanghani, P.; Ti, A.B.; Kam King, N.K.; Ren, H. Evaluation of tumor shape features for overall survival prognosis in glioblastoma multiforme patients. Surg. Oncol. 2019, 29, 178–183. [Google Scholar] [CrossRef]
- Morin, O.; Chen, W.C.; Nassiri, F.; Susko, M.; Magill, S.T.; Vasudevan, H.N.; Wu, A.; Vallières, M.; Gennatas, E.D.; Valdes, G.; et al. Integrated models incorporating radiologic and radiomic features predict meningioma grade, local failure, and overall survival. Neurooncol. Adv. 2019, 1, vdz011. [Google Scholar] [CrossRef] [Green Version]
- Spille, D.C.; Sporns, P.B.; Hess, K.; Stummer, W.; Brokinkel, B. Prediction of High-Grade Histology and Recurrence in Meningiomas Using Routine Preoperative Magnetic Resonance Imaging: A Systematic Review. World Neurosurg. 2019, 128, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Musigmann, M.; Akkurt, B.H.; Krähling, H.; Brokinkel, B.; Henssen, D.J.H.A.; Sartoretti, T.; Nacul, N.G.; Stummer, W.; Heindel, W.; Mannil, M. Assessing preoperative risk of STR in skull meningiomas using MR radiomics and machine learning. Sci. Rep. 2022, 12, 14043. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.M.; Benjamin, V. Medial sphenoid ridge meningiomas: Classification, microsurgical anatomy, operative nuances, and long-term surgical outcome in 35 consecutive patients. Neurosurgery 2008, 62 (Suppl. S1), 38–50; discussion 50. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.C.; Pereira, C.E.; Gonçalves, M.; Zanon, N. Extended Pterional Approach for Medial Sphenoid Wing Meningioma: A Series of 47 Patients. J. Neurol. Surg. B Skull Base 2020, 81, 107–113. [Google Scholar] [CrossRef]
- Behari, S.; Giri, P.J.; Shukla, D.; Jain, V.K.; Banerji, D. Surgical strategies for giant medial sphenoid wing meningiomas: A new scoring system for predicting extent of resection. Acta Neurochir. 2008, 150, 865–877; discussion 877. [Google Scholar] [CrossRef]
- Wach, J.; Lampmann, T.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Cases-Cunillera, S.; Hölzel, M.; Toma, M.; Güresir, E. Proliferative Potential, and Inflammatory Tumor Microenvironment in Meningioma Correlate with Neurological Function at Presentation and Anatomical Location-From Convexity to Skull Base and Spine. Cancers 2022, 14, 1033. [Google Scholar] [CrossRef]
- Liu, N.; Song, S.Y.; Jiang, J.B.; Wang, T.J.; Yan, C.X. The prognostic role of Ki-67/MIB-1 in meningioma: A systematic review with meta-analysis. Medicine 2020, 99, e18644. [Google Scholar] [CrossRef]
- Maiuri, F.; Mariniello, G.; de Divitiis, O.; Esposito, F.; Guadagno, E.; Teodonno, G.; Barbato, M.; Del Basso De Caro, M. Progesterone Receptor Expression in Meningiomas: Pathological and Prognostic Implications. Front. Oncol. 2021, 11, 611218. [Google Scholar] [CrossRef]
- Kato, Y.; Nishihara, H.; Mohri, H.; Kanno, H.; Kobayashi, H.; Kimura, T.; Tanino, M.; Terasaka, S.; Tanaka, S. Clinicopathological evaluation of cyclooxygenase-2 expression in meningioma: Immunohistochemical analysis of 76 cases of low and high-grade meningioma. Brain Tumor Pathol. 2014, 31, 23–30. [Google Scholar] [CrossRef] [Green Version]
- Wach, J.; Lampmann, T.; Güresir, Á.; Schuss, P.; Vatter, H.; Herrlinger, U.; Becker, A.; Hölzel, M.; Toma, M.; Güresir, E. FORGE: A Novel Scoring System to Predict the MIB-1 Labeling Index in Intracranial Meningiomas. Cancers 2021, 13, 3643. [Google Scholar] [CrossRef]
- Todo, T.; Adams, E.F.; Rafferty, B.; Fahlbusch, R.; Dingermann, T.; Werner, H. Secretion of interleukin-6 by human meningioma cells: Possible autocrine inhibitory regulation of neoplastic cell growth. J. Neurosurg. 1994, 81, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Kasuya, H.; Kubo, O.; Tanaka, M.; Amano, K.; Kato, K.; Hori, T. Clinical and radiological features related to the growth potential of meninigioma. Neurosurg. Rev. 2006, 29, 293–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuno, A.; Fujimaki, T.; Sasaki, T.; Nagashima, T.; Ide, T.; Asai, A.; Matsuura, R.; Utsunomiya, H.; Kirino, T. Clinical and histopathological analysis of proliferative potentials of recurrent and non-recurrent meningiomas. Acta Neuropathol. 1996, 91, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Ragel, B.T.; Jensen, R.L.; Gillespie, D.L.; Prescott, S.M.; Couldwell, W.T. Celecoxib inhibits meningioma tumor growth in a mouse xenograft model. Cancer 2007, 109, 588–597. [Google Scholar] [CrossRef]
- Wach, J.; Güresir, Á.; Vatter, H.; Herrlinger, U.; Becker, A.; Toma, M.; Hölzel, M.; Güresir, E. Low-Dose Acetylsalicylic Acid Treatment in Non-Skull-Base Meningiomas: Impact on Tumor Proliferation and Seizure Burden. Cancers 2022, 14, 4285. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Boström, J.; Meyer-Puttlitz, B.; Wolter, M.; Blaschke, B.; Weber, R.G.; Lichter, P.; Ichimura, K.; Collins, V.P.; Reifenberger, G. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am. J. Pathol. 2001, 159, 661–669. [Google Scholar] [CrossRef] [Green Version]
- Goutagny, S.; Yang, H.W.; Zucman-Rossi, J.; Chan, J.; Dreyfuss, J.M.; Park, P.J.; Black, P.M.; Giovannini, M.; Carroll, R.S.; Kalamarides, M. Genomic profiling reveals alternative genetic pathways of meningioma malignant progression dependent on the underlying NF2 status. Clin. Cancer Res. 2010, 16, 4155–4164. [Google Scholar] [CrossRef] [Green Version]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Mirian, C.; Duun-Henriksen, A.K.; Juratli, T.; Sahm, F.; Spiegl-Kreinecker, S.; Peyre, M.; Biczok, A.; Tonn, J.C.; Goutagny, S.; Bertero, L.; et al. Poor prognosis associated with TERT gene alterations in meningioma is independent of the WHO classification: An individual patient data meta-analysis. J. Neurol. Neurosurg. Psychiatry 2020, 91, 378–387. [Google Scholar] [CrossRef]
- Ruttledge, M.H.; Sarrazin, J.; Rangaratnam, S.; Phelan, C.M.; Twist, E.; Merel, P.; Delattre, O.; Thomas, G.; Nordenskjöld, M.; Collins, V.P.; et al. Evidence for the complete inactivation of the NF2 gene in the majority of sporadic meningiomas. Nat. Genet. 1994, 6, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Teranishi, Y.; Okano, A.; Miyawaki, S.; Ohara, K.; Ishigami, D.; Hongo, H.; Dofuku, S.; Takami, H.; Mitsui, J.; Ikemura, M.; et al. Clinical significance of NF2 alteration in grade I meningiomas revisited; prognostic impact integrated with extent of resection, tumour location, and Ki-67 index. Acta Neuropathol. Commun. 2022, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Topsakal, C.; Al-Mefty, O.; Bulsara, K.R.; Williford, V.S. Intraoperative monitoring of lower cranial nerves in skull base surgery: Technical report and review of 123 monitored cases. Neurosurg. Rev. 2008, 31, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Magill, S.T.; Sughrue, M.E.; Rodriguez, L.R.; McDermott, M.W. Middle fossa meningiomas. Handb. Clin. Neurol. 2020, 170, 65–67. [Google Scholar]
- Fisher, F.L.; Zamanipoor Najafabadi, A.H.; van der Meer, P.B.; Boele, F.W.; Peerdeman, S.M.; Peul, W.C.; Taphoorn, M.J.B.; Dirven, L.; van Furth, W.R. Long-term health-related quality of life and neurocognitive functioning after treatment in skull base meningioma patients. J. Neurosurg. 2021, 136, 1077–1089. [Google Scholar] [CrossRef]
- Bi, W.L.; Dunn, I.F. Current and emerging principles in surgery for meningioma. Chin. Clin. Oncol. 2017, 6 (Suppl. S1), S7. [Google Scholar] [CrossRef]
- Schneider, M.; Borger, V.; Güresir, Á.; Becker, A.; Vatter, H.; Schuss, P.; Güresir, E. High Mib-1-score correlates with new cranial nerve deficits after surgery for frontal skull base meningioma. Neurosurg. Rev. 2021, 44, 381–387. [Google Scholar] [CrossRef]
- Giammalva, G.R.; Brunasso, L.; Paolini, F.; Costanzo, R.; Bonosi, L.; Benigno, U.E.; Ferini, G.; Sava, S.; Colarossi, C.; Umana, G.E.; et al. The Long and Winding Road: An Overview of the Immunological Landscape of Intracranial Meningiomas. Cancers 2022, 14, 3639. [Google Scholar] [CrossRef]
- Domingues, P.; González-Tablas, M.; Otero, Á.; Pascual, D.; Miranda, D.; Ruiz, L.; Sousa, P.; Ciudad, J.; Gonçalves, J.M.; Lopes, M.C.; et al. Tumor infiltrating immune cells in gliomas and meningiomas. Brain Behav. Immun. 2016, 53, 1–15. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Bergdall, V.K.; Tober, K.L.; Hill, K.J.; Mitra, S.; Flavahan, N.A.; Oberyszyn, T.M. The impact of cyclooxygenase-2 mediated inflammation on scarless fetal wound healing. Am. J. Pathol. 2004, 165, 753–761. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Li, J.; Zhu, Y.; Tang, H.; He, X.; Xu, Y. The neuroprotective effects of aspirin following crush injury to rat sciatic nerve. Int. J. Clin. Exp. Med. 2015, 8, 18185–18190. [Google Scholar] [PubMed]
- Ko, C.C.; Zhang, Y.; Chen, J.H.; Chang, K.T.; Chen, T.Y.; Lim, S.W.; Wu, T.C.; Su, M.Y. Pre-operative MRI Radiomics for the Prediction of Progression and Recurrence in Meningiomas. Front. Neurol. 2021, 12, 636235. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, A.; Marwah, R.; Smith, J.; Nguyen, D.; Bhatti, A.; Lim, C.P.; Lasocki, A. Machine learning imaging applications in the differentiation of true tumour progression from treatment-related effects in brain tumours: A systematic review and meta-analysis. J. Med. Imaging Radiat. Oncol. 2022, 66, 781–797. [Google Scholar] [CrossRef] [PubMed]
- Coons, S.W.; Johnson, P.C. Regional heterogeneity in the proliferative activity of human gliomas as measured by the Ki-67 labeling index. J. Neuropathol. Exp. Neurol. 1993, 52, 609–618. [Google Scholar] [CrossRef]






| Characteristics | n = 74 |
|---|---|
| Median age (years, ±SD) | 59.0 ± 14.1 |
| Female sex | 48 (64.9%) |
| Median preoperative KPS (IQR) | 80.0 (70.0, 80.0) |
| Preoperative visual deterioration (CN II) | 26 (35.1%) |
| Preoperative CN III dysfunction | 7 (9.5%) |
| Preoperative CN IV dysfunction | 10 (13.5%) |
| Preoperative CN VI dysfunction | 8 (10.8%) |
| ASA classification | |
| I | 8 (10.8%) |
| II | 52 (70.3%) |
| III | 13 (17.6%) |
| IV | 1 (1.4%) |
| Cavernous sinus infiltration | 16 (21.6%) |
| Vascular encasement | 33 (44.6%) |
| Calcification | 6 (8.1%) |
| Cystic appearance | 6 (8.1%) |
| Peritumoral flow voids | 24 (32.0%) |
| Disruption of arachnoid layer | 26 (35.1%) |
| Brain edema | 27 (36.5%) |
| Surface area, (mean ± SD), mm2 | 3881.8 ± 3519.7 |
| Tumor volume, (mean ± SD), cm3 | 23.4 ± 28.8 |
| Brain edema volume, (mean ± SD), cm3 | 15.5 ± 12.6 |
| Sphericity, (mean ± SD) | 0.75 ± 0.17 |
| Simpson grade | |
| I | 27 (36.5%) |
| II | 23 (31.1%) |
| III | 7 (9.5%) |
| IV | 17 (23.0%) |
| Tumor consistency | |
| Soft | 37 (50.0%) |
| Firm | 37 (50.0%) |
| MIB-1 index, (mean ± SD), | 3.9 ± 3.1 |
| WHO grade | |
| 1 | 68 (91.9%) |
| 2 | 6 (8.1%) |
| Characteristics | Regularly Shaped (n = 43) | Irregularly Shaped (n = 31) | p-Value |
|---|---|---|---|
| Median age (years ± SD) | 60.5 ± 11.4 | 58.6 ± 17.3 | 0.60 |
| Female sex | 30 (69.8%) | 18 (58.1%) | 0.33 |
| Median preoperative KPS (±SD) | 76.7 ± 11.5 | 73.9 ± 12.3 | 0.31 |
| Preoperative visual deterioration (CN II) | 14 (32.6%) | 12 (38.7%) | 0.63 |
| Preoperative CN III palsy | 2 (4.7%) | 7 (9.5%) | 0.12 |
| Preoperative CN IV dysfunction | 2 (4.7%) | 8 (25.8%) | 0.014 |
| Preoperative CN VI dysfunction | 5 (11.6%) | 3 (9.7%) | 0.99 |
| Cavernous sinus infiltration | 7 (16.3%) | 9 (29.0%) | 0.25 |
| Vascular encasement | 17 (39.5%) | 16 (51.6%) | 0.35 |
| Calcification | 2 (4.7%) | 4 (12.9%) | 0.23 |
| Cystic appearance | 2 (4.7%) | 4 (12.9%) | 0.23 |
| Pial blood supply | 12 (27.9%) | 12 38.7%) | 0.45 |
| Arachnoid layer disruption | 11 (25.6%) | 15 (48.4%) | 0.052 |
| Brain edema | 19 (44.2%) | 8 (25.8%) | 0.14 |
| Surface area, (mean ± SD), mm2 | 3112.9 ± 2736.6 | 5355.6 ± 4262.8 | 0.02 |
| Tumor volume, (mean ± SD), cm3 | 18.9 ± 22.9 | 29.7 ± 34.9 | 0.14 |
| Brain edema volume, (mean ± SD), cm3 (present in 27 cases) | 13.9 ± 10.9 | 19.2 ± 16.3 | 0.33 |
| Sphericity, (mean ± SD) | 0.81 ± 0.14 | 0.69 ± 0.18 | 0.006 |
| Simpson grade | 0.10 | ||
| I | 18 (41.9%) | 9 (29.0%) | |
| II | 16 (37.2%) | 7 (22.6%) | |
| III | 2 (4.7%) | 5 (16.1%) | |
| IV | 7 (16.3%) | 10 (32.3%) | |
| Tumor consistency | 0.99 | ||
| Soft | 22 (51.2%) | 15 (48.4%) | |
| Firm | 21 (48.8%) | 16 (51.6%) | |
| WHO grade | 0.23 | ||
| 1 | 41 (95.3%) | 27 (87.1%) | |
| 2 | 2 (4.7%) | 4 (12.9%) | |
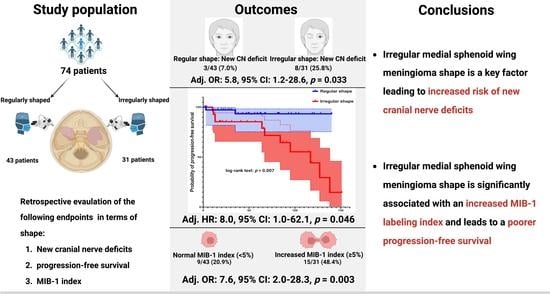
| MIB-1 index, (mean ± SD) | 3.2 ± 2.2 | 5.0 ± 3.8 | 0.009 |
| Adjuvant radiation therapy | 2 (4.7%) | 4 (12.9%) | 0.23 |
| New cranial nerve deficit | 3 (7.0%) | 8 (25.8%) | 0.04 |
| Mean (95% CI) PFS time (months) | 132.1 (121.5–142.7) | 97.0 (76.3–117.8) | 0.007a |
| Name/Year of Study | Study Design/Level of Evidence | Country | Definition/Measurement of Shape | No. Patients of Entire Cohort | No. Patients with Irregularly Shaped Meningioma | No. Patients with Regularly Shaped Meningiomas | Endpoints | MIB-1/Ki-67 Index | WHO Grade | Age | Sex |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Hashiba et al. 2006 [35] | Retrospective/Level III | Japan | Shape of the tumor was classified as either smooth or irregular. The so-called mush-rooming tumors, i.e., tumors with fringes and a lobulated appearance, were considered irregular. | Total: 90 | 38 | 52 | MIB-1 | Regular: 1.82% ± 1.75% Irregular 4.58% ± 4.84% | 79 WHO grade 1, 8 WHO grade 2 (atypical), 3 WHO grade 3 (anaplastic) | Mean age: 57.6 (range: 20–93) | Female:male ratio = 3.5:1 |
| Nakasu et al. 1995 [36] | Retrospective study/Level III | Japan | Tumor shape was described as round or lobular. Round tumors had smoothly curved surfaces without notches, whereas lobular tumors showed globoid appearances with at least one notch. | 120 | 26 | 94 | MIB-1 | Regular: 1.06 ± 0.67% Irregular: 2.85 ± 3.68% | 107 WHO grade 1, 10 WHO grade 2 (atypical), 3 WHO grade 3 (anaplastic) | Mean age: 57.5 ± 13.2 | Female:male ratio = 3:1 |
| Present series | Level III | Germany | Tumor shape was considered irregular if the edges were irregular, mushroom-shaped, lobulated, and the boundary with adjacent cortex was unclear [25]. The shape was judged independently by two reviewers (JW & FA). The shape of MSWM was further quantified using sphericity, which measures how closely an object’s shape resembles a perfect sphere. | 74 | 31 | 43 | MIB-1, Cranial nerve deficits, PFS | Regular: 3.16 ± 2.25 Irregular: 5.03 ± 3.75 | 68 WHO grade 1, 6 WHO grade 2 | Median age: 59.0 ± 14.1 | Female:male ratio: 1.85:1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wach, J.; Naegeli, J.; Vychopen, M.; Seidel, C.; Barrantes-Freer, A.; Grunert, R.; Güresir, E.; Arlt, F. Impact of Shape Irregularity in Medial Sphenoid Wing Meningiomas on Postoperative Cranial Nerve Functioning, Proliferation, and Progression-Free Survival. Cancers 2023, 15, 3096. https://doi.org/10.3390/cancers15123096
Wach J, Naegeli J, Vychopen M, Seidel C, Barrantes-Freer A, Grunert R, Güresir E, Arlt F. Impact of Shape Irregularity in Medial Sphenoid Wing Meningiomas on Postoperative Cranial Nerve Functioning, Proliferation, and Progression-Free Survival. Cancers. 2023; 15(12):3096. https://doi.org/10.3390/cancers15123096
Chicago/Turabian StyleWach, Johannes, Johannes Naegeli, Martin Vychopen, Clemens Seidel, Alonso Barrantes-Freer, Ronny Grunert, Erdem Güresir, and Felix Arlt. 2023. "Impact of Shape Irregularity in Medial Sphenoid Wing Meningiomas on Postoperative Cranial Nerve Functioning, Proliferation, and Progression-Free Survival" Cancers 15, no. 12: 3096. https://doi.org/10.3390/cancers15123096
APA StyleWach, J., Naegeli, J., Vychopen, M., Seidel, C., Barrantes-Freer, A., Grunert, R., Güresir, E., & Arlt, F. (2023). Impact of Shape Irregularity in Medial Sphenoid Wing Meningiomas on Postoperative Cranial Nerve Functioning, Proliferation, and Progression-Free Survival. Cancers, 15(12), 3096. https://doi.org/10.3390/cancers15123096