Can Asiatic Acid from Centella asiatica Be a Potential Remedy in Cancer Therapy?—A Review
Abstract
:Simple Summary
Abstract
1. Introduction
2. Triterpenes: Biological Effects and Mechanism of Action
3. In Vitro Studies
4. In Vivo Studies
5. Challenges and Limitations
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5FU | 5-fluorouracil |
| AA | asiatic acid |
| AChE | acetylcholinesterase |
| ACOX | acyl-coenzyme A oxidase 1 |
| ACS | acetyl-CoA synthetase |
| AIF | apoptosis-inducing factor |
| Akt | protein kinase B |
| AMPK/CREB | AMP-activated protein kinase/cAMP response element-binding |
| AP-1 | activator protein 1 |
| Apaf-1 | apoptotic protease activating factor 1 |
| Arf6 | ADP-ribosylation factor 6 |
| ATM | ataxia telangiectasia mutated protein |
| Bak1 | BCL2 antagonist/killer 1 |
| Bax | Bcl-2-associated X protein |
| BChE | butyrylcholinesterase |
| Bcl-2 | B-cell lymphoma 2 |
| BDNF | brain-derived neurotrophic factor |
| CA | Centella asiatica |
| Cdc42 | cell division control protein 42 homolog |
| CINC-1/CXCL1 | cytokine-induced neutrophil chemoattractant 1/chemokine (C-X-C motif) ligand 1 |
| Col1a1 | collagen, type I, alpha 1 |
| COX-2 | cyclooxygenase 2 |
| CPT1 | carnitine palmitoyltransferase 1 |
| CTGF | connective tissue growth factor |
| CXCL1/KC | chemokine (C-X-C motif) ligand 1/keratinocyte-derived chemokine |
| CYPs | cytochromes P450 |
| DMSO | dimethyl sulfoxide |
| DR | death receptor |
| EMT | epithelial-mesenchymal transition |
| eNOS | endothelial nitric oxide synthase |
| ER | endoplasmic reticulum |
| ERK | extracellular-regulated kinase |
| Fas | Fas Cell Surface Death Receptor |
| FATP4 | fatty acid transport protein 4; |
| FGF | fibroblast growth factor |
| GB | glioblastoma |
| GSK-3β | glycogen synthase kinase 3β |
| HO-1 | heme oxygenase-1 |
| ICAM-1 | intercellular adhesion molecule-1 |
| IFN-γ | interferon gamma |
| IL-1 | interleukin 1 |
| IL-4 | interleukin 4 |
| IL-5 | interleukin 5 |
| IL-6 | interleukin 6 |
| IL-10 | interleukin 10 |
| iNOS | inducible nitric oxide synthase |
| JNK1/2 | c-Jun N-terminal kinase ½ |
| LC3 | Microtuble-associated protein light chain 3 |
| LLC | lung carcinoma |
| LOX | lipoxygenase |
| MAPK | mitogen-activated protein kinase |
| MCP-1 | monocyte chemoattractant protein-1 |
| mdm2 | mouse double minute 2 homolog |
| MIP-2 | macrophage inflammatory protein 2 |
| MMP9 | matrix metallopeptidase 9 |
| MMP15 | matrix metallopeptidase 15 |
| MPO | myeloperoxidase |
| MRI | magnetic resonance imaging |
| mTOR | mammalian target of rapamycin |
| NAG | N-acetylglutamate |
| NF-κB | nuclear factor kappa B |
| NG | naringenin |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| NOX | NADPH oxidase |
| NPC | nasopharyngeal cancer |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| Pdcd4 | Programmed Cell Death 4 |
| PGC-1α | peroxisome proliferator-activated receptor γ coactivator 1α |
| P-gP | glycoprotein P |
| PI3K/Akt | phosphoinositide 3-kinase/protein kinase B |
| PPAR-γ | peroxisome proliferator-activated receptor gamma |
| p-p38MAPK | Phosphorylated-p38 mitogen-activated protein kinase |
| Rac1 | Rac family small GTPase 1 |
| RCC | Renal Carcinoma Cells |
| ROS | reactive oxidative species |
| SCD1 | stearoyl-CoA desaturase-1 |
| SIRT1 | sirtuin 1 |
| Smad3 | Mothers against decapentaplegic homolog 3 |
| Smad7 | Mothers against decapentaplegic homolog 7 |
| SREBP-1c | sterol regulatory element binding protein-1 |
| STATs | signal transducer and activator of transcription proteins |
| TGF | transforming growth factor |
| Tgfb1 | transforming growth factor beta 1 |
| Timp1 | tissue inhibitor of metalloproteinases 1 |
| TLRs | toll-like receptors |
| TNF-α | tumor necrosis factor alpha |
| VCAM-1 | vascular cell adhesion molecule 1 |
| VEGF | vascular endothelial growth factor |
| WHO | World Health Organization |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.E. Centella asiatica (L.) Urban: From Traditional Medicine to Modern Medicine with Neuroprotective Potential. Evid. Based Complement. Alternat Med. 2012, 2012, 946259. [Google Scholar] [CrossRef]
- Brinkhaus, B.; Lindner, M.; Schuppan, D.; Hahn, E.G. Chemical, pharmacological and clinical profile of the East Asian medical plant Centella asiatica. Phytomedicine 2000, 7, 427–448. [Google Scholar] [CrossRef] [PubMed]
- Idris, F.N.; Mohd Nadzir, M. Comparative Studies on Different Extraction Methods of Centella asiatica and Extracts Bioactive Compounds Effects on Antimicrobial Activities. Antibiotics 2021, 10, 457. [Google Scholar] [CrossRef] [PubMed]
- Gohil, K.J.; Patel, J.A.; Gajjar, A.K. Pharmacological Review on Centella asiatica: A Potential Herbal Cure-all. Indian. J. Pharm. Sci. 2010, 72, 546–556. [Google Scholar] [CrossRef]
- Razali, N.N.M.; Ng, C.T.; Fong, L.Y. Cardiovascular Protective Effects of Centella Asiatica and Its Triterpenes: A Review. Planta Med. 2019, 85, 1203–1215. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.R.L.; Calado, L.L.; Duarte, A.B.S.; de Sousa, D.P. Centella asiatica and Its Metabolite Asiatic Acid: Wound Healing Effects and Therapeutic Potential. Metabolites 2023, 13, 276. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Bylka, W.; Znajdek-Awiżeń, P.; Studzińska-Sroka, E.; Dańczak-Pazdrowska, A.; Brzezińska, M. Centella asiatica in dermatology: An overview. Phytother. Res. 2014, 28, 1117–1124. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. A Systematic Review of the Effect of Centella asiatica on Wound Healing. Int. J. Environ. Res. Public. Health 2022, 19, 3266. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.H.; Barron, A.M.; Abdullah, J.M. Mitoprotective effects of Centella asiatica (L.) Urb.: Anti-inflammatory and neuroprotective opportunities in neurodegenerative disease. Front. Pharmacol. 2021, 12, 687935. [Google Scholar] [CrossRef]
- Puttarak, P.; Dilokthornsakul, P.; Saokaew, S.; Dhippayom, T.; Kongkaew, C.; Sruamsiri, R.; Chuthaputti, A.; Chaiyakunapruk, N. Effects of Centella asiatica (L.) Urb. on cognitive function and mood related outcomes: A Systematic Review and Meta-analysis. Sci. Rep. 2017, 7, 10646. [Google Scholar] [CrossRef] [PubMed]
- Wannasarit, S.; Mahattanadul, S.; Issarachot, O.; Puttarak, P.; Wiwattanapatapee, R. Raft-forming gastro-retentive formulations based on Centella asiatica extract-solid dispersions for gastric ulcer treatment. Eur. J. Pharm. Sci. 2020, 143, 105204. [Google Scholar] [CrossRef] [PubMed]
- Emran, T.B.; Dutta, M.; Nasir Uddin, M.M.; Nath, A.; Zia Uddin, M. Anti-diabetic Potential of the Leaf Extract of Centella asiatica in Alloxan-Induced Rat with diabetics. J. Biosci. 2015, 4, 5. [Google Scholar]
- Chen, Y.H.; Wu, J.X.; Yang, S.F.; Hsiao, Y.H. Synergistic Combination of Luteolin and Asiatic Acid on Cervical Cancer In Vitro and In Vivo. Cancers 2023, 15, 548. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.H.; Yeh, J.Y.; Chen, Y.S.; Li, M.H.; Huang, C.H. Wound-healing effect of electrospun gelatin nanofibres containing Centella asiatica extract in a rat model. J. Tissue Eng. Regen. Med. 2017, 11, 905–915. [Google Scholar] [CrossRef]
- Pantia, S.; Kangsamaksin, T.; Janvilisri, T.; Komyod, W. Asiatic Acid Inhibits Nasopharyngeal Carcinoma Cell Viability and Migration via Suppressing STAT3 and Claudin-1. Pharmaceuticals 2023, 16, 902. [Google Scholar] [CrossRef]
- Ferah Okkay, I.; Okkay, U.; Aydin, I.C.; Bayram, C.; Ertugrul, M.S.; Mendil, A.S.; Hacimuftuoglu, A. Centella asiatica extract protects against cisplatin-induced hepatotoxicity via targeting oxidative stress, inflammation, and apoptosis. Environ. Sci. Pollut. Res. Int. 2022, 29, 33774–33784. [Google Scholar] [CrossRef]
- Kandasamy, A.; Aruchamy, K.; Rangasamy, P.; Varadhaiyan, D.; Gowri, C.; Oh, T.H.; Ramasundaram, S.; Athinarayanan, B. Phytochemical Analysis and Antioxidant Activity of Centella Asiatica Extracts: An Experimental and Theoretical Investigation of Flavonoids. Plants 2023, 12, 3547. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Li, L.; Song, W.; Li Min, H.; Wang, Y.; Yuan, J.; Xue, Z. Natural products of pentacyclic triterpenoids: From discovery to heterologous biosynthesis. Nat. Prod. Rep. 2023, 40, 1303–1353. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef]
- Zulkipli, N.N.; Zakaria, R.; Long, I.; Abdullah, S.F.; Muhammad, E.F.; Wahab, H.A.; Sasongko, T.H. In Silico Analyses and Cytotoxicity Study of Asiaticoside and Asiatic Acid from Malaysian Plant as Potential mTOR Inhibitors. Molecules 2020, 25, 3991. [Google Scholar] [CrossRef]
- Welbat, J.U.; Chaisawang, P.; Pannangrong, W.; Wigmore, P. Neuroprotective Properties of Asiatic Acid against 5-Fluorouracil Chemotherapy in the Hippocampus in an Adult Rat Model. Nutrients 2018, 10, 1053. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Chuang, Y.-C.; Lo, Y.-S.; Lin, C.-C.; Hsi, Y.-T.; Hsieh, M.-J.; Chen, M.-K. Asiatic Acid, Extracted from Centella asiatica and Induces Apoptosis Pathway through the Phosphorylation p38 Mitogen-Activated Protein Kinase in Cisplatin-Resistant Nasopharyngeal Carcinoma Cells. Biomolecules 2020, 10, 184. [Google Scholar] [CrossRef] [PubMed]
- Nagoor Meeran, M.F.; Goyal, S.N.; Suchal, K.; Sharma, C.; Patil, C.R.; Ojha, S.K. Pharmacological Properties, Molecular Mechanisms, and Pharmaceutical Development of Asiatic Acid: A Pentacyclic Triterpenoid of Therapeutic Promise. Front. Pharmacol. 2018, 9, 892. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Liu, T.; Ma, J. Neuroprotective mechanisms of Asiatic acid. Heliyon 2023, 9, e15853. [Google Scholar] [CrossRef]
- Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer (accessed on 12 March 2024).
- Hao, Y.; Huang, J.; Ma, Y.; Chen, W.; Fan, Q.; Sun, X.; Shao, M.; Cai, H. Asiatic acid inhibits proliferation, migration and induces apoptosis by regulating Pdcd4 via the PI3K/Akt/mTOR/p70S6K signaling pathway in human colon carcinoma cells. Oncol. Lett. 2018, 15, 8223. [Google Scholar] [CrossRef] [PubMed]
- Heise, N.; Becker, S.; Mueller, T.; Bache, M.; Csuk, R.; Güttler, A. Mitochondria-Targeting 1,5-Diazacyclooctane-Spacered Triterpene Rhodamine Conjugates Exhibit Cytotoxicity at Sub-Nanomolar Concentration against Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 10695. [Google Scholar] [CrossRef]
- Sommerwerk, S.; Heller, L.; Kerzig, C.; Kramell, A.E.; Csuk, R. Rhodamine B conjugates of triterpenoic acids are cytotoxic mitocans even at nanomolar concentrations. Eur. J. Med. Chem. 2017, 127, 1–9. [Google Scholar] [CrossRef]
- Reungpatthanaphong, P.; Dechsupa, S.; Meesungnoen, J.; Loetchutinat, C.; Mankhetkorn, S. Rhodamine B as a mitochondrial probe for measurement and monitoring of mitochondrial membrane potential in drug-sensitive and -resistant cells. J. Biochem. Biophys. Methods 2003, 57, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kraft, O.; Hartmann, A.-K.; Brandt, S.; Hoenke, S.; Heise, N.V.; Csuk, R.; Mueller, T. Asiatic acid as a leading structure for derivatives combining sub-nanomolar cytotoxicity, high selectivity, and the ability to overcome drug resistance in human preclinical tumor models. Eur. J. Med. Chem. 2023, 250, 115189. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.; Wu, H.; Zhan, Z.; Wu, T.; Xiang, M.; Wang, Z.; Song, L.; Wei, B. Exploration of anti-osteosarcoma activity of asiatic acid based on network pharmacology and in vitro experiments. Oncol. Rep. 2024, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Hung, T.W.; Yang, S.F.; Tsai, Y.L.; Yang, J.T.; Lin, C.L.; Hsieh, Y.H. Asiatic acid from Centella asiatica exert anti-invasive ability in human renal cancer cells by modulation of ERK/p38MAPK-mediated MMP15 expression. Phytomedicine 2022, 100, 154036. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Lee, L.M.; Lee, W.J.; Chu, C.Y.; Tan, P.; Yang, Y.C.; Chen, W.Y.; Yang, S.F.; Hsiao, M.; Chien, M.H. Melatonin inhibits MMP-9 transactivation and renal cell carcinoma metastasis by suppressing Akt-MAPKs pathway and NF-κB DNA-binding activity. J. Pineal Res. 2016, 60, 277–290. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, T.; Rameshkumar, A.; Krishnan, R.; Bose, D.; Vasanthi, V.; Nandhini, G. Evaluation of the Anti-Carcinogenic Effect of Centella asiatica on Oral Cancer Cell Line: In vitro Study. Asian Pac. J. Cancer Prev. 2023, 24, 1695–1700. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, C.V.; Jain, A.K.; Agarwal, C.; Pierce, A.; Keating, A.; Huber, K.M.; Serkova, N.J.; Wempe, M.F.; Agarwal, R.; Deep, G. Asiatic acid induces endoplasmic reticulum stress and apoptotic death in glioblastoma multiforme cells both in vitro and in vivo. Mol. Carcinog. 2015, 54, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, K.; Huang, J.; Chu, D.; Tian, M.; Huang, K.; Ma, C. Asiatic Acid Induces Endoplasmic Reticulum Stress and Activates the Grp78/IRE1α/JNK and Calpain Pathways to Inhibit Tongue Cancer Growth. Front. Pharmacol. 2021, 12, 690612. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Chen, K.; Huang, J.; Chu, D.; Li, J.; Huang, K.; Ma, C. Asiatic acid inhibits angiogenesis and vascular permeability through the VEGF/VEGFR2 signaling pathway to inhibit the growth and metastasis of breast cancer in mice. Phytother. Res. 2021, 35, 6389–6400. [Google Scholar] [CrossRef]
- Wu, T.; Geng, J.; Guo, W.; Gao, J.; Zhu, X. Asiatic acid inhibits lung cancer cell growth in vitro and in vivo by destroying mitochondria. Acta Pharm. Sin. B 2017, 7, 65–72. [Google Scholar] [CrossRef]
- Lian, G.Y.; Wang, Q.M.; Tang, P.M.; Zhou, S.; Huang, X.R.; Lan, H.Y. Combination of Asiatic Acid and Naringenin Modulates NK Cell Anti-cancer Immunity by Rebalancing Smad3/Smad7 Signaling. Mol. Ther. 2018, 26, 2255–2266. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Wang, G.; Xiao, Q.; Zhou, Y.; Wei, Y.; Gong, Z. Antiangiogenic effects of AA-PMe on HUVECs in vitro and zebrafish in vivo. Onco Targets Ther. 2018, 11, 1871–1884. [Google Scholar] [CrossRef]
- Dutta, S.; Chakraborty, P.; Basak, S.; Ghosh, S.; Ghosh, N.; Chatterjee, S.; Dewanjee, S.; Sil, P.C. Synthesis, characterization, and evaluation of in vitro cytotoxicity and in vivo antitumor activity of asiatic acid-loaded poly lactic-co-glycolic acid nanoparticles: A strategy of treating breast cancer. Life Sci. 2022, 307, 120876. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Bravo, M.; Loureiro, J.A.; Lima, J.; Pereira, M.C. Transferrin-modified nanoparticles for targeted delivery of Asiatic acid to glioblastoma cells. Life Sci. 2022, 296, 120435. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhang, H.; Sun, F.; Sun, S.; Zhu, Z.; Chai, Y. Biopharmaceutical and pharmacokinetic characterization of asiatic acid in Centella asiatica as determined by a sensitive and robust HPLC–MS method. J. Ethnopharmacol. 2015, 163, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Imran, M.; Hussain, M.; Saeed, F.; Imran, A.; Umar, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ahmed, A.; Alsagaby, S.A.; et al. Asiatic acid: A review on its polypharmacological properties and therapeutic potential against various Maladies. Int. J. Food Properties. 2023, 26, 12441263. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zhao, P.; Chen, Y.; Zhou, Y.; Wang, S.; Yin, L. Preparation and evaluation of PEGylated asiatic acid nanostructured lipid carriers on anti-fibrosis effects. Drug Dev. Ind. Pharm. 2020, 46, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Fard, S.E.; Tafvizi, F.; Torbati, M.B. Silver nanoparticles biosynthesised using Centella asiatica leaf extract: Apoptosis induction in MCF-7 breast cancer cell line. IET Nanobiotechnol. 2018, 12, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Franczyk, B.; Rysz, J.; Gluba-Brzózka, A. Pharmacogenetics of Drugs Used in the Treatment of Cancers. Genes 2022, 13, 311. [Google Scholar] [CrossRef]
- Cayún, J.P.; Cerpa, L.C.; Colombo, A.; Cáceres, D.D.; Leal, J.L.; Reyes, F.; Gutiérrez-Cáceres, C.; Calfunao, S.; Varela, N.M.; Quiñones, L.A. Genetic Polymorphisms and Tumoral Mutational Profiles over Survival in Advanced Colorectal Cancer Patients: An Exploratory Study. Curr. Oncol. 2024, 31, 274–295. [Google Scholar] [CrossRef]
- Songvut, P.; Chariyavilaskul, P.; Tantisira, M.H.; Khemawoot, P. Safety and Pharmacokinetics of Standardized Extract of Centella asiatica (ECa 233) Capsules in Healthy Thai Volunteers: A Phase 1 Clinical Study. Planta Med. 2019, 85, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Bollen, M.; David, J.; Speers, A.B.; Brandes, M.S.; Gray, N.E.; Alcázar Magaña, A.; McClure, C.; Stevens, J.F.; Maier, C.S.; et al. Pharmacokinetics and Pharmacodynamics of Key Components of a Standardized Centella asiatica Product in Cognitively Impaired Older Adults: A Phase 1, Double-Blind, Randomized Clinical Trial. Antioxidants 2022, 11, 215. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Bollen, M.; David, J.; Mepham, B.; Alcázar Magaña, A.; McClure, C.; Maier, C.S.; Quinn, J.F.; Soumyanath, A. Bioanalytical method validation and application to a phase 1, double-blind, randomized pharmacokinetic trial of a standardized Centella asiatica (L.) Urban water extract product in healthy older adults. Front. Pharmacol. 2023, 14, 1228030. [Google Scholar] [CrossRef] [PubMed]
- Yunianto, I.; Das, S.; Mat, N.M. Antispermatogenic and antifertility effect of Pegaga (Centella asiatica L) on the testis of male Sprague-Dawley rats. Clin. Ter. 2010, 161, 235–239. [Google Scholar] [PubMed]
- Jorge, O.A.; Jorge, A.D. Hepatotoxicity associated with the ingestion of Centella asiatica. Rev. Esp Enferm. Dig. 2005, 97, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Arora, R.; Sarangi, S.C.; Ganeshan, N.S.; Agarwal, A.; Kaleekal, T.; Gupta, Y.K. Pharmacodynamic and pharmacokinetic interactions of hydroalcoholic leaf extract of Centella asiatica with valproate and phenytoin in experimental models of epilepsy in rats. J. Ethnopharmacol. 2021, 270, 113784. [Google Scholar] [CrossRef]
- Wright, K.M.; Magana, A.A.; Laethem, R.M.; Moseley, C.L.; Banks, T.T.; Maier, C.S.; Stevens, J.F.; Quinn, J.F.; Soumyanath, A. Centella asiatica Water Extract Shows Low Potential for Cytochrome P450-Mediated Drug Interactions. Drug Metab. Dispos. 2020, 48, 1053–1063. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ (accessed on 12 March 2024).


| Molecular Targets | Examples |
|---|---|
| Cytokines | TNF-α, IFN-γ, IL-1, IL-4, IL-5, IL-6, IL-10 |
| Chemokines | CINC-1/CXCL1, CXCL1/KC, MIP-2, MCP-1 |
| Growth factors | VEGF, TGF, CTGF, FGF, BDNF |
| Enzymes | AChE, BChE, NOX, eNOS, iNOS, FATP4, ACS, CPT1, ACOX, CYPs, COX-2, LOX, MMP9, MPO, NAG, MAPK |
| Signaling molecules | ERK, JNK1/2, PGC-1α, PI3K/Akt, AMPK/CREB, mTOR, Akt, Akt/GSK-3β |
| Adhesion molecules | ICAM-1, VCAM-1 |
| Apoptosis-related proteins | Bcl-2, mdm2, cmyb, Bax, Bak1, Apaf-1, caspases, p53, p38, ATM, DR, Fas, AIF |
| Cell cycle proteins | cyclin D1 |
| G-proteins | Arf6, Rac1, Cdc42, heat shock proteins 60 and P-gP |
| Genes | Col1a1, Tgfb1, Timp1, SREBP-1c, SCD1, HO-1, NLRP3 |
| Receptors | μ-opioid, PPAR-γ, TLRs |
| Transcription factors | NF-κB, AP-1, SIRT1, STATs, Nrf2 |
| Authors | Asiatic Acid Form | Observed Effects | References |
|---|---|---|---|
| Heise et al. | 1,5–diazacyclooctane-spacered AA-rhodamine conjugate | 250 nM of AA conjugate in MDA-MB-231 cells (basal, triple-negative breast cancer cells) significantly inhibits proliferation (under 20% compared to the control cells). 250 nM of AA conjugate in HS578T cells (basal, triple-negative breast cancer cells) → decreases proliferation by about 50%. 500 nM of AA conjugate in HS578T cells reduces cell number by up to 20% compared to control cells. | [30] |
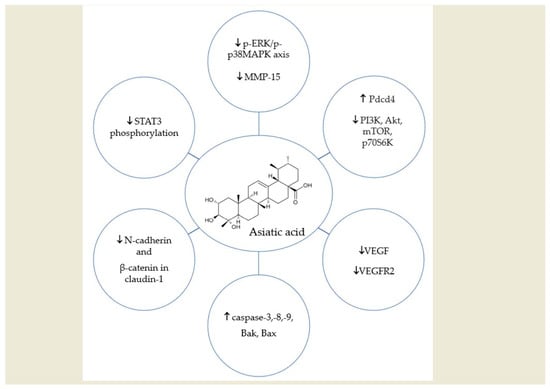
| Pantia et al. | asiatic acid (97%) prepared as a stock solution of 100 mM by dissolving 4.887 mg of AA in 100 μL of DMSO | Inhibition of STAT3 phosphorylation → reduction of NPC cell viability. Reduction in the expression of mesenchymal markers N-cadherin and β-catenin in claudin-1 → ↓ cell migration and metastasis. Induction of caspase-3 expression → induction of cell death. | [18] |
| Liu Y. et al. | AA isolated from C. asiatica (>98% purity) dissolved in DMSO | Upregulating caspase-3,-8,-9, Bak, Bax expression, phosphorylation of p38, ERK ½ pathway → induction of cell death. | [25] |
| Hao et al. | AA isolated from C. asiatica dissolved in DMSO | Changes in the morphology of colon cancer cells → induction of apoptosis. ↓Expression of E-cadherin; ↑expression of vimentin and N-cadherin → inhibition of migration of colon cancer cells. ↑ Expression of Pdcd4 protein; ↓ Expression of PI3K, Akt, mTOR, p70S6K → induction of apoptosis, anticancer effect. | [29] |
| He Pang et al. | No data | ↓ Expression of BCl2, ↑ expression of Bax → mitochondrial dysfunction → induction of apoptosis. Inhibition of the PI3K/AKT signaling pathway and activation of the ROS/MAPK signaling pathway → ↑ intracellular ROS content. ↑ LC3II/I ratio and ↓ levels of p62 → autophagy promotion. | [34] |
| Huang et al. | asiatic acid (purity > 98%). The origin stock solution of AA is 100 mM in DMSO solvent. | Inhibition of p-ERK/p-p38MAPK axis and ↓ MMP-15 expression → suppression of migration and invasion of RCC. | [35] |
| Authors | Asiatic Acid Dose | Observed Effects | References |
|---|---|---|---|
| Kavitha CV. et al. | 30 mg/kg/d in ectopic xenograft group or 30 mg/kg/twice a day in orthotopic xenograft group | ↑apoptosis ↑activation of caspases ↓ tumor volume ↓ tumor weight ↑ ER stress ↑ intracellular calcium level | [38] |
| Li J. et al. | 15 mg/kg/d | ↑apoptosis ↓ tumor volume ↓ tumor weight ↓ downregulation of Bcl2 family proteins ↑upregulation of Bax and cleaved caspase-3 levels ↑ ER stress | [39] |
| Tian M. et al. | 50 mg/kg/d | ↑apoptosis ↓ tumor volume ↓ tumor weight ↓VEGF expression ↓VEGFR2 expression ↓ lung metastasis | [40] |
| Wu T. et al. | 50 mg/kg/d or 100 mg/kg/d | ↑apoptosis ↓ tumor volume ↓ tumor weight ↑ROS Collapse of mitochondrial membrane potential | [41] |
| Lian et al. | 10 mg/kg/d or 10 mg/kg/d (AA) + 50 mg/kg/d (NG) | ↓ tumor volume ↓ tumor weight ↑ NK-cells maturation ↑ NK-cells differentiation ↑ NK-cells anti-tumor cytotoxicity | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiciński, M.; Fajkiel-Madajczyk, A.; Kurant, Z.; Gajewska, S.; Kurant, D.; Kurant, M.; Sousak, M. Can Asiatic Acid from Centella asiatica Be a Potential Remedy in Cancer Therapy?—A Review. Cancers 2024, 16, 1317. https://doi.org/10.3390/cancers16071317
Wiciński M, Fajkiel-Madajczyk A, Kurant Z, Gajewska S, Kurant D, Kurant M, Sousak M. Can Asiatic Acid from Centella asiatica Be a Potential Remedy in Cancer Therapy?—A Review. Cancers. 2024; 16(7):1317. https://doi.org/10.3390/cancers16071317
Chicago/Turabian StyleWiciński, Michał, Anna Fajkiel-Madajczyk, Zuzanna Kurant, Sandra Gajewska, Dominik Kurant, Marcin Kurant, and Masaoud Sousak. 2024. "Can Asiatic Acid from Centella asiatica Be a Potential Remedy in Cancer Therapy?—A Review" Cancers 16, no. 7: 1317. https://doi.org/10.3390/cancers16071317
APA StyleWiciński, M., Fajkiel-Madajczyk, A., Kurant, Z., Gajewska, S., Kurant, D., Kurant, M., & Sousak, M. (2024). Can Asiatic Acid from Centella asiatica Be a Potential Remedy in Cancer Therapy?—A Review. Cancers, 16(7), 1317. https://doi.org/10.3390/cancers16071317