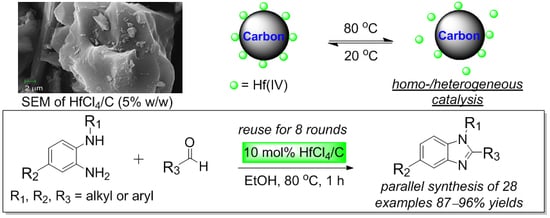
Activated Carbon Supported Hafnium(IV) Chloride as an Efficient, Recyclable, and Facile Removable Catalyst for Expeditious Parallel Synthesis of Benzimidazoles
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for Preparation of HfCl4/C Catalyst
3.3. General Procedure for Preparation of Other Supported HfCl4 Catalysts
3.4. General Synthetic Procedure and Characterization of Benzimidazoles
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sreerama, R.; Barnali, M.; Balamurali, M.M.; Chanda, K. Synthesis and medicinal applications of benzimidazoles: An overview. Curr. Org. Synth. 2017, 14, 40–60. [Google Scholar]
- Carvalho, L.C.R.; Fernandes, E.; Marques, M.M.B. Developments towards regioselective synthesis of 1,2-disubstituted benzimidazoles. Chem. Eur. J. 2011, 17, 12544–12555. [Google Scholar] [CrossRef] [PubMed]
- Preston, P.N. Synthesis, reactions, and spectroscopic properties of benzimidazoles. Chem. Rev. 1974, 74, 279–314. [Google Scholar] [CrossRef]
- Yadav, G.; Ganguly, S. Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: A mini-review. Eur. J. Med. Chem. 2015, 97, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Chhabra, S.; Shrivastava, S.K.; Mishra, P. Benzimidazole: A promising pharmacophore. Med. Chem. Res. 2013, 22, 5077–5104. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Alamgir, M.; Black, D.S.C.; Kumar, N. Synthesis, reactivity and biological activity of benzimidazoles. Top. Heterocycl. Chem. 2007, 21, 87–118. [Google Scholar]
- Wallace, M.B.; Feng, J.; Zhang, Z.; Skene, R.J.; Shi, L.; Caster, C.L.; Kassel, D.B.; Xu, R.; Gwaltney, S.L., II. Structure-based design and synthesis of benzimidazole derivatives as dipeptidyl peptidase IV inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 2362–2367. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Shinde, D.B. BF3OEt2 Promoted solvent-free synthesis of benzimidazole derivative. Chin. Chem. Lett. 2006, 17, 453–456. [Google Scholar]
- Mayer, J.P.; Lewis, G.S.; McGee, C.; Bankaitis-Davis, D. Solid-phase synthesis of benzimidazoles. Tetrahedron Lett. 1998, 39, 6655–6658. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, K.H.; Chung, Y.K. Manganese (IV) dioxide-catalyzed synthesis of quinoxalines under microwave irradiation. Chem. Commun. 2005, 1321–1323. [Google Scholar] [CrossRef] [PubMed]
- Gogoi, P.; Konwar, D. An efficient and one-pot synthesis of imidazolines and benzimidazoles via anaerobic oxidation of carbon–nitrogen bonds in water. Tetrahedron Lett. 2006, 47, 79–82. [Google Scholar] [CrossRef]
- Beaulieu, P.L.; Hache, B.; von Moos, E. A practical oxone®-mediated, high-throughput, solution-phase synthesis of benzimidazoles from 1,2-phenylenediamines and aldehydes and its application to preparative scale synthesis. Synthesis 2003, 11, 1683–1692. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wu, R.; Cai, S.; Lin, Y.; Sellers, L.; Sakamoto, K.; He, B.; Peterson, B.R. Synthesis and biological evaluation of analogues of AKT (protein kinase B) inhibitor-IV. J. Med. Chem. 2011, 54, 1126–1139. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wang, C.-J.; Gong, S.-S.; Ai, Y.-J.; Sun, H.-B. Cp2ZrCl2-catalyzed synthesis of 2-aminovinyl benzimidazoles under microwave conditions. Chin. Chem. Lett. 2015, 26, 297–300. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Luo, Y.; Wang, J.; Jian, Y.; Sun, H.; Zhang, G.; Zhang, W.; Gao, Z. Solvent strategy for unleashing the Lewis acidity of titanocene dichloride for rapid Mannich reactions. RSC Adv. 2016, 6, 15298–15303. [Google Scholar] [CrossRef]
- Qiu, R.; Xu, X.; Peng, L.; Zhao, Y.; Li, N.; Yin, S. Strong Lewis acids of air-stable metallocene bis(perfluorooctanesulfonate)s as high-efficiency catalysts for carbonyl-group transformation reactions. Chem. Eur. J. 2012, 18, 6172–6182. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium (IV) salts. Science 2000, 290, 1140–1142. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakayama, M.; Ohara, S.; Yamamoto, H. Direct ester condensation from a 1:1 mixture of carboxylic acids and alcohols catalyzed by hafnium (IV) or zirconium (IV) salts. Tetrahedron 2002, 58, 8179–8188. [Google Scholar] [CrossRef]
- Lundberg, H.; Adolfsson, H. Hafnium-catalyzed direct amide formation at room temperature. ACS Catal. 2015, 5, 3271–3277. [Google Scholar] [CrossRef]
- Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Highly efficient synthesis of α-aminophosphonates catalyzed by hafnium(IV) chloride. Tetrahedron Lett. 2016, 57, 1782–1785. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.-Z.; Zheng, X.-A.; Kong, R.; Gong, S.-S.; Sun, Q. Hafnium (IV) triflate as a potent catalyst for selective 1-O-deacetylation of peracetylated saccharides. Carbohydr. Res. 2018, 455, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly efficient synthesis of substituted 3,4-dihydropyrimidin-2-(1H)-ones (DHPMs) catalyzed by Hf(OTf)4: Mechanistic insights into reaction pathways under metal Lewis acid catalysis and solvent-free conditions. Molecules 2019, 24, 364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Liu, R.; Gong, S.-S.; Sun, Q. Hf(OTf)4 as a highly potent catalyst for the synthesis of Mannich bases under solvent-free conditions. Molecules 2020, 25, 388. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.-H.; Yin, L.; Wang, Y.-M. An expeditious synthesis of benzimidazole derivatives catalyzed by Lewis acids. Catal. Commun. 2007, 8, 1126–1131. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Shinde, D.B. Zirconyl (IV) chloride-promoted synthesis of benzimidazole derivatives. Russ. J. Org. Chem. 2006, 42, 453–454. [Google Scholar] [CrossRef]
- Wei, J.-Y.; Han, S.-B.; Peng, X.-C.; Wang, C.-J.; Zeng, D.-Y.; Gong, S.-S.; Sun, Q. Efficient synthesis of fluorinated benzimidazolines, benzoxazolines and benzothiazolines catalyzed by Hf(OTf)4. Heterocycles 2020, 100, 371–382. [Google Scholar]
- Trivedi, R.; De, S.K.; Gibbs, R.A. A convenient one-pot synthesis of 2-substituted benzimidazoles. J. Mol. Catal. A Chem. 2006, 245, 8–11. [Google Scholar] [CrossRef]
- Sharghi, H.; Aberi, M.; Doroodmanda, M.M. Reusable cobalt(III)-salen complex supported on activated carbon as an efficient heterogeneous catalyst for synthesis of 2-arylbenzimidazole derivatives. Adv. Synth. Catal. 2008, 350, 2380–2390. [Google Scholar] [CrossRef]
- Chakrabarty, M.; Karmakar, S.; Mukherjee, R.; Arima, S.; Harigaya, Y. A mild and expedient one-pot synthesis of substituted benzimidazoles in water using a phase-transfer catalyst. Monatsh. Chem. 2009, 140, 375–380. [Google Scholar] [CrossRef]




| Entry | Catalyst | Reaction Time (h) | Isolated Yield of 3{1,1} (%) |
|---|---|---|---|
| 1 | No | 120 | 82 |
| 2 | TiCl4 | 20 | 67 |
| 3 | ZrOCl2·8H2O | 20 | 89 |
| 4 | ZrCp2Cl2 | 20 | 92 |
| 5 | ZrCl4 | 16 | 90 |
| 6 | HfCl4 | 12 | 96 |
| Entry | Temperature (°C) | Solvent | Reaction Time (h) | Isolated Yield of 3{1,1} (%) |
|---|---|---|---|---|
| 1 | 20 | EtOH | 16 | 96 |
| 2 | 40 | EtOH | 12 | 97 |
| 3 | 60 | EtOH | 4 | 97 |
| 4 | 80 | EtOH | 1 | 97 |
| 5 | 80 | DMF | 1 | 88 |
| 6 | 80 | CH3CN | 1.5 | 92 |
| 7 | 80 | DCE | 24 | 90 |
| 8 | 70 | THF | 6 | 64 |
| Entry | Solid Support | Reaction Time 1st/2nd (h) | Isolated Yield of 3{1,1} 1st/2nd (%) |
|---|---|---|---|
| 1 | SiO2 | 2/4 | 92/78 |
| 2 | activated carbon | 1/1 | 96/96 |
| 3 | Al2O3 | 1/4 | 93/75 |
| 4 | K-10 montmorillonite | 1/4 | 94/79 |
| Method | Weight Loss (mg) | |||||
|---|---|---|---|---|---|---|
| 1st | 2nd | 3rd | 4th | 5th | 6th | |
| A a | 8 | 7 | 5 | 3 | 1 | 0 |
| B b | 0 | 0 | 0 | 0 | 0 | 1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.-C.; Gong, S.-S.; Zeng, D.-Y.; Duo, S.-W.; Sun, Q. Activated Carbon Supported Hafnium(IV) Chloride as an Efficient, Recyclable, and Facile Removable Catalyst for Expeditious Parallel Synthesis of Benzimidazoles. Catalysts 2020, 10, 436. https://doi.org/10.3390/catal10040436
Peng X-C, Gong S-S, Zeng D-Y, Duo S-W, Sun Q. Activated Carbon Supported Hafnium(IV) Chloride as an Efficient, Recyclable, and Facile Removable Catalyst for Expeditious Parallel Synthesis of Benzimidazoles. Catalysts. 2020; 10(4):436. https://doi.org/10.3390/catal10040436
Chicago/Turabian StylePeng, Xiao-Chong, Shan-Shan Gong, De-Yun Zeng, Shu-Wang Duo, and Qi Sun. 2020. "Activated Carbon Supported Hafnium(IV) Chloride as an Efficient, Recyclable, and Facile Removable Catalyst for Expeditious Parallel Synthesis of Benzimidazoles" Catalysts 10, no. 4: 436. https://doi.org/10.3390/catal10040436
APA StylePeng, X. -C., Gong, S. -S., Zeng, D. -Y., Duo, S. -W., & Sun, Q. (2020). Activated Carbon Supported Hafnium(IV) Chloride as an Efficient, Recyclable, and Facile Removable Catalyst for Expeditious Parallel Synthesis of Benzimidazoles. Catalysts, 10(4), 436. https://doi.org/10.3390/catal10040436