Parametric Sensitivity Analysis for the Industrial Case of O-Xylene Oxidation to Phthalic Anhydride in a Packed Bed Catalytic Reactor
Abstract
:1. Introduction
- van Welsenaere and Froment, (1970) [7] introduced, for an ideal, one-dimensional PBR model, with constant wall temperature, two criteria for runaway based on an intrinsic property of the reactive system. They transposed the peak temperature and the conditions of the inflexion points for the prediction of the critical values for the operating variables. Their work also treats the related problem of a hotspot which has to be limited for reasons other than runaway.
- Soria-López et al. (1981) [1], as an extension of van Welsenaere and Froment’s [7] work, analyzed an ideal, one-dimensional PBR model, with co-current external cooling, under two types of operation modes: (i) the temperature along the reactor axis increases monotonically; that is, without a hotspot can occur under certain conditions (referred to as pseudo-adiabatic operations, PAO), and (ii) the temperature shows a maximum at a finite axial reactor position (MFARP). Limiting operation conditions were defined between MFARP temperature curves and PAO curves.
- Akella and Lee (1983) [22] studied, for an ideal, one-dimensional PBR model, a counter-current operation. The runaway conditions were derived from a phase plane of feed and coolant inlet temperatures.
- Later, Arandes and de Lasa (1995) [3] extended the PAO analysis to the following operation regimens in PBRs: isothermal, decreasing temperature profile, cold spot, hotspot–cold spot, and cold spot–hotspot.
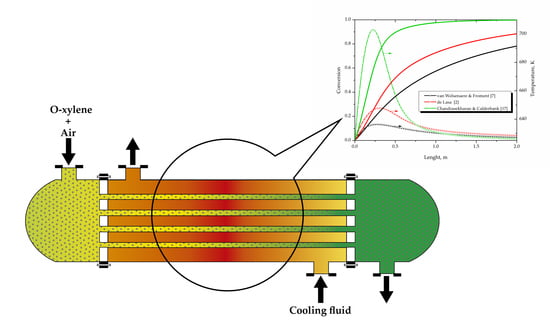
2. Reactor Model
- The PBR is operating at steady state conditions.
- Molar flows, pressure and temperature gradients only occur in the axial direction. The only transport mechanism operating in this direction is the overall flow itself, and this is considered to be of the plug flow type.
- The parameters M, u, ρg, ρc, wc, U, G, Rep are assumed to be independent of temperature.
- There are neither temperature nor concentration differences between the fluid and the catalyst particle.
3. Kinetic Models
4. Computation of Sensitivity Indices
5. Numerical Simulation
5.1. For Constant Temperature or Co-Current Flow in the Shell Side
5.2. For Countercurrent Flow in the Shell Side
6. Results
6.1. Constant Cooling Temperature (TC = T0 = 625 K)
6.2. Variable Cooling Temperature According to Co-Current or Countercurrent Pattern (TCo = To = 625 K)
6.3. Variable Cooling Temperature According to Co-Current or Countercurrent Pattern (PAo = 0.9322 kPa)
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Notation
| a | Kinetic parameter (K−1) | u | Superficial velocity (m/h) | ||
| Heat capacity of the cooling medium | wc | Cooling medium flow rate (kg/h) | |||
| Heat capacity of the gaseous mixture | y | Dependent’s variables vector | |||
| Dp | Particle diameter (m) | z | Axial coordinate (m) | ||
| Dt | Tube diameter (m) | ΔHrxn | Heat of reaction (kcal/kmol) | ||
| f | Continuous function | Greek letters | |||
| F | Molar flow (kmol/h) | α | Kinetic constant | ||
| Ft | Total molar flow (kmol/h) | ε | Bed porosity | ||
| G | Mass flow | θv | Kinetic parameter | ||
| k | Kinetic constant | ρb | Bulk density of the fixed bed (kg/m3) | ||
| L | Total reactor length (m) | ρg | Gas mixture density (kg/m3) | ||
| M | Molecular weight of gaseous mixture (kg/m3) | ρL | Linear density (kgcat/m3) | ||
| N | Total number of reactions | φ | Input parameters | ||
| P | Total pressure (kPa) | Subscripts | |||
| Oxygen’s partial pressure (kPa) | i | Counter for reactions | |||
| q | Total number of compounds | j | Counter for compounds | ||
| r | Reaction rate | o | Inlet conditions | ||
| Rep | Particle Reynolds number | 1 | O-xylene to phthalic anhydride | ||
| S | Sensitivity coefficient | 21 | O-xylene to phthalic anhydride step | ||
| S(F,To) | Molar flow sensitivity coefficient with respect to the inlet temperature | 22 | Phthalic anhydride to CO and CO2 step | ||
| S(T,To) | Temperature sensitivity coefficient with respect to the inlet temperature | 23 | O-xylene to CO and CO2 step | ||
| S(Tc,To) | Cooling medium sensitivity coefficient with respect to the inlet temperature | 31 | O-xylene to O-tolualdehyde step | ||
| T | Temperature inside the reactor (K) | 32 | O-xylene to phthalic anhydride step | ||
| Tc | Temperature of the cooling medium (K) | 33 | O-xylene to COx step | ||
| tn | Number of tubes | 34 | O-tolualdehyde to Phthalide step | ||
| U | Overall heat transfer coefficient | 35 | Phthalide to phthalic anhydride step | ||
References
- Lopez, A.; De Lasa, H.; Porras, J. Parametric Sensitivity of a Fixed Bed Catalytic Reactor. Chem. Eng. Sci. 1981, 36, 285–291. [Google Scholar] [CrossRef]
- De Lasa, H. Application of the Pseudoadiabatic Operation to Catalytic Fixed Bed Reactors Case of the Orthoxylene Oxidation. Can. J. Chem. Eng. 1983, 61, 710–718. [Google Scholar] [CrossRef]
- Arandes, J.; De Lasa, H. Pseudoadiabatic Operation for Fixed-Bed Catalytic Reactors: Methods for Finding the Limits of the Regime. Chem. Eng. J. Biochem. Eng. J. 1995, 58, 33–44. [Google Scholar] [CrossRef]
- Richter, O.; Mestl, G. Deactivation of Commercial, High-Load o-Xylene Feed VOx/TiO2 Phthalic Anhydride Catalyst by Unusual Over-Reduction. Catalisis 2019, 9, 435. [Google Scholar] [CrossRef] [Green Version]
- Bilous, O.; Amundson, N.R. Chemical Reactor Stability and Sensitivity: II. Effect of Parameters on Sensitivity of Empty Tubular Reactors. AIChE J. 1956, 2, 117–126. [Google Scholar] [CrossRef]
- Balakotaiah, V.; Luss, D. Explicit Runaway Criterion for Catalytic Reactors with Transport Limitations. AIChE J. 1991, 37, 1780–1788. [Google Scholar] [CrossRef]
- Van Welsenaere, R.; Froment, G. Parametric Sensitivity and Runaway in Fixed Bed Catalytic Reactors. Chem. Eng. Sci. 1970, 25, 1503–1516. [Google Scholar] [CrossRef]
- Varma, A.; Morbidelli, M.; Wu, H. Parametric Sensitivity in Chemical Systems; Cambridge University Press (CUP): Cambridge, UK, 1999. [Google Scholar]
- Strozzi, F.; Zaldívar, J. A General Method for Assessing the Thermal Stability of Batch Chemical Reactors by Sensitivity Calculation Based on Lyapunov Exponents. Chem. Eng. Sci. 1994, 49, 2681–2688. [Google Scholar] [CrossRef]
- Bosch, J.; Strozzi, F.; Snee, T.; Hare, J.; Zaldívar, J. A Comparative Analysis between Temperature and Pressure Measurements for Early Detection of Runaway Initiation. J. Loss Prev. Process. Ind. 2004, 17, 389–395. [Google Scholar] [CrossRef]
- Rase, H.F. Fixed-Bed Reactor Design and Diagnostics; Butterworth-Heinemann: Oxford, UK, 1990. [Google Scholar]
- Papageorgiou, J.; Froment, G. Phthalic Anhydride Synthesis. Reactor Optimization Aspects. Chem. Eng. Sci. 1996, 51, 2091–2098. [Google Scholar] [CrossRef]
- Gimeno, M.P.; Gascón, J.; Téllez, C.; Herguido, J.; Menéndez, M. Selective Oxidation of O-Xylene to Phthalic Anhydride over V2O5/TiO2: Kinetic Study in a Fluidized Bed Reactor. Chem. Eng. Process 2008, 47, 1844–1852. [Google Scholar] [CrossRef]
- Anastov, A.I. Deactivation of an Industrial V2O5–TiO2 Catalyst for Oxidation of O-Xylene into Phthalic Anhydride. Chem. Eng. Process 2003, 42, 449–460. [Google Scholar] [CrossRef]
- Anastov, A.I.; Nikolov, V.A. Optimal Policies of Operation of a Fixed-Bed Reactor for Oxidation of O-Xylene into Phthalic Anhydride. Ind. Eng. Chem. Res. 1998, 37, 3424–3433. [Google Scholar] [CrossRef]
- Henning, G.P.; Perez, G.A. Parametric Sensivity in Fixed-Bed Catalytic Reactors. Chem. Eng. Sci. 1986, 41, 83–88. [Google Scholar] [CrossRef]
- Chandrasekharan, K.; Calderbank, P. Prediction of packed-bed catalytic reactor performance for a complex reaction (oxidation of o-xylene to phthalic anhydride). Chem. Eng. Sci. 1979, 34, 1323–1331. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Foster, N.R. Catalysts, Kinetics and Reactor Design in Phthalic Anhydride Synthesis. Catal. Rev. 1979, 19, 211–292. [Google Scholar] [CrossRef]
- Skrzypek, J.; Grzesik, M.; Galantowicz, M.; Solinski, J. Kinetics of the Catalytic Air Oxidation of O-Xylene over a Commercial V2O5/TiO2 Catalyst. Chem. Eng. Sci. 1985, 40, 611–620. [Google Scholar] [CrossRef]
- Dias, C.R.; Farinha Portela, M.; Bond, G.C. Oxidation of O-Xylene to Phthalic Anhydride over V2O5/TiO2 Catalysts. IV Mathematical Modelling Study and Analysis of the Reaction Network. J. Catal. 1996, 164, 276–287. [Google Scholar] [CrossRef]
- Yabrov, A.A.; Ivanov, A.A. Response studies of the mechanism of o-xylene oxidation over a vanadium-titanium oxide catalyst. React. Kinet. Catal. Lett. 1980, 14, 347–351. [Google Scholar] [CrossRef]
- Akella, L.M.; Lee, H.H. A Design Approach Based on Pase Plane Analysis: Countercurrent Reactor/Heat Exchanger with Parametric Sensitivity. AIChE J. 1983, 29, 87–94. [Google Scholar] [CrossRef]
- Hosten, L.; Froment, G. Parametric Sensitivity in co-Currently Cooled Tubular Reactors. Chem. Eng. Sci. 1986, 41, 1073–1080. [Google Scholar] [CrossRef]
- Rajadhyaksha, R.; Vasudeva, K.; Doraiswamy, L. Parametric Sensitivity in Fixed-Bed Reactors. Chem. Eng. Sci. 1975, 30, 1399–1408. [Google Scholar] [CrossRef]
- McGreavy, C.; Adderley, C.I. Parametric Sensitivity and Temperature Runaway in Heterogeneous Fixed Bed Reactors. Adv. Chem. 1974, 133, 519–531. [Google Scholar] [CrossRef]
- Fogler, H.S. Elements of Chemical Reaction Engineering. Chem. Eng. Sci. 1987, 42, 2493. [Google Scholar] [CrossRef]
- Rosendall, B.; Finlayson, B.A. Transport Effects in Packed–Bed Oxidation Reactors. Comput. Chem. Eng. 1995, 19, 1207–1218. [Google Scholar] [CrossRef]
- Hlavacek, V. Aspects in Design of Packed Catalytic Reactors. Ind. Eng. Chem. 1970, 62, 8–26. [Google Scholar] [CrossRef]
- Elnashaie, S.S.E.H.; Elshishini, S.S. Modelling, Simulation and Optimization of Industrial Fixed Bed Reactors; Gordon & Breach Science Publishers: Yverdon, Switzerland, 1993; pp. 383–384. [Google Scholar]





| Mechanism | Reference | Reaction Step | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | ||
| 1 | [7] | X | |||||||||||
| 2 | [2,16] | X | X | X | |||||||||
| 3 | [17] | X | X | X | X | X | |||||||
| 4 | [12] | X | X | X | X | X | |||||||
| 5 | [14] | X | X | X | X | X | X | ||||||
| 6 | [18] | X | X | X | X | X | X | ||||||
| 7 | [19] | X | X | X | X | X | X | X | |||||
| 8 | [20] | X | X | X | X | X | X | X | X | X | X | ||
| 9 | [21] | X | X | X | X | X | X | X | X | X | X | X | |
| Molecular weight of gaseous mixture | ||
| Superficial velocity | ||
| Total pressure | ||
| Heat capacity of the gaseous mixture | ||
| Tube diameter | ||
| Number of tubes | ||
| Gas mixture density | ||
| Oxygen’s partial pressure | ||
| Bulk density of the fixed bed | ||
| Heat capacity of the cooling medium | ||
| Cooling medium flow rate | ||
| Overall heat transfer coefficient | ||
| Total reactor length | ||
| Particle diameter | ||
| Bed porosity | ||
| Particle Reynolds number |
| van Welsenaere and Froment [7] |  |
| de Lasa [2] |  |
| Chandrasekharan and Calderbank [17] |  |
| Kinetic Model | Constant Tc = To = 625 K | Co-Current Tco = To = 625 K | Countercurrent Tco = To = 625 K | ||
|---|---|---|---|---|---|
| Author Criterion kPa | This Work kPa | Author Criterion kPa | This Work kPa | This Work kPa | |
| One reaction | 1.677 [7] 1.778 [16] 1.815 [28] | 1.863 | 1.422 [1] | 1.733 | 1.417 |
| Three reactions | 0.867 [16] a | 1.187 | 0.867 [16] a 1.014 [2] | 1.106 a 1.097 | 0.847 |
| Five reactions | - | 0.692 | 0.800 [2] | 0.692 | 0.689 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuluaga-Botero, S.; Dobrosz-Gómez, I.; Gómez-García, M.-Á. Parametric Sensitivity Analysis for the Industrial Case of O-Xylene Oxidation to Phthalic Anhydride in a Packed Bed Catalytic Reactor. Catalysts 2020, 10, 626. https://doi.org/10.3390/catal10060626
Zuluaga-Botero S, Dobrosz-Gómez I, Gómez-García M-Á. Parametric Sensitivity Analysis for the Industrial Case of O-Xylene Oxidation to Phthalic Anhydride in a Packed Bed Catalytic Reactor. Catalysts. 2020; 10(6):626. https://doi.org/10.3390/catal10060626
Chicago/Turabian StyleZuluaga-Botero, Santiago, Izabela Dobrosz-Gómez, and Miguel-Ángel Gómez-García. 2020. "Parametric Sensitivity Analysis for the Industrial Case of O-Xylene Oxidation to Phthalic Anhydride in a Packed Bed Catalytic Reactor" Catalysts 10, no. 6: 626. https://doi.org/10.3390/catal10060626
APA StyleZuluaga-Botero, S., Dobrosz-Gómez, I., & Gómez-García, M. -Á. (2020). Parametric Sensitivity Analysis for the Industrial Case of O-Xylene Oxidation to Phthalic Anhydride in a Packed Bed Catalytic Reactor. Catalysts, 10(6), 626. https://doi.org/10.3390/catal10060626