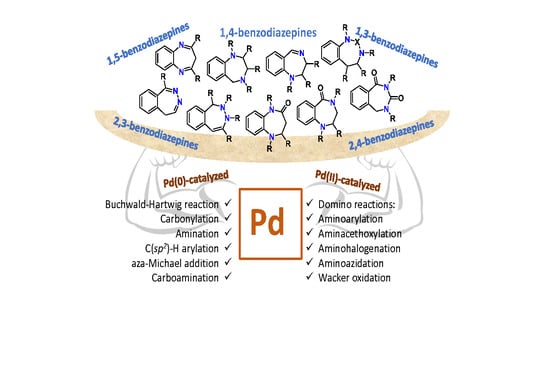
Palladium-Catalyzed Benzodiazepines Synthesis
Abstract
:1. Introduction
2. Synthesis of Benzodiazepines
2.1. 1,5-Benzodiazepines
2.2. 1,4-Benzodiazepines
2.3. 1,3-Benzodiazepines
2.4. 2,3-Benzodiazepines
2.5. 2,4-Benzodiazepines
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ashcroft, G.W. Benzodiazepines in Clinical Practice. By David J. Greenblatt and Richard L. Shader. Amsterdam: North-Holland Publishing Co. for Raven Press. 1974. Pp. v + 305. Price $16.90. Br. J. Psychiatry 1975, 126, 295–296. [Google Scholar]
- Trimble, M.R. Benzodiazepines Divided: A Multidisciplinary Review; John Wiley: Chicester, UK, 1983; p. 329. [Google Scholar]
- Evans, B.E.; Rittle, K.E.; Bock, M.G.; DiPardo, R.M.; Freidinger, R.M.; Whitter, W.L.; Lundell, G.F.; Veber, D.F.; Anderson, P.S.; Chang, R.S.L.; et al. Methods for drug discovery: Development of potent, selective, orally effective cholecystokinin antagonists. J. Med. Chem. 1988, 31, 2235–2246. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.G.; DiPardo, R.M.; Evans, B.E.; Rittle, K.E.; Whitter, W.L.; Veber, D.F.; Anderson, P.S.; Freidinger, R.M. Benzodiazepine gastrin and brain cholecystokinin receptor ligands; L-365,260. J. Med. Chem. 1989, 32, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Walser, A.; Fryer, R.I. Dihydro-1,4-Benzodiazepinones and Thiones. Chem. Heterocycl. Compd. 1991, 50, 631–848. [Google Scholar]
- Thurston, D.E.; Bose, D.S. Synthesis of DNA-Interactive Pyrrolo[2,1-c][1,4]benzodiazepines. Chem. Rev. 1994, 94, 433–465. [Google Scholar] [CrossRef]
- Riemann, D.; Perlis, M.L. The treatments of chronic insomnia: A review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med. Rev. 2009, 13, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Hester, J.B.; Rudzik, A.D.; Kamdar, B.V. 6-Phenyl-4H-s-triazolo[4,3-a][1,4]benzodiazepines which have central nervous system depressant activity. J. Med. Chem. 1971, 14, 1078–1081. [Google Scholar] [CrossRef]
- Kaneko, T.; Wong, H.; Doyle, T.W.; Rose, W.C.; Bradner, W.T. Bicyclic and tricyclic analogs of anthramycin. J. Med. Chem. 1985, 28, 388–392. [Google Scholar] [CrossRef]
- Keenan, R.M.; Callahan, J.F.; Samanen, J.M.; Bondinell, W.E.; Calvo, R.R.; Chen, L.; DeBrosse, C.; Eggleston, D.S.; Haltiwanger, R.C.; Hwang, S.M.; et al. Conformational Preferences in a Benzodiazepine Series of Potent Nonpeptide Fibrinogen Receptor Antagonists. J. Med. Chem. 1999, 42, 545–559. [Google Scholar] [CrossRef]
- Grossi, G.; Di Braccio, M.; Roma, G.; Ballabeni, V.; Tognolini, M.; Calcina, F.; Barocelli, E. 1,5-Benzodiazepines: Part XIII. Substituted 4H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepin-5-amines and 4H-imidazo[1,2-a][1,5]benzodiazepin-5-amines as analgesic, anti-inflammatory and/or antipyretic agents with low acute toxicity. Eur. J. Med. Chem. 2002, 37, 933–944. [Google Scholar] [CrossRef]
- Kusanur, R.A.; Ghate, M.; Kulkarni, M.V. Synthesis of spiro[indolo-1,5-benzodiazepines] from 3-acetyl coumarins for use as possible antianxiety agents. J. Chem. Sci. 2004, 116, 265–270. [Google Scholar] [CrossRef]
- Rajarao, S.J.R.; Platt, B.; Sukoff, S.J.; Lin, Q.; Bender, C.N.; Nieuwenhuijsen, B.W.; Ring, R.H.; Schechter, L.E.; Rosenzweig-Lipson, S.; Beyer, C.E. Anxiolytic-like activity of the non-selective galanin receptor agonist, galnon. Neuropeptides 2007, 41, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.K.; Shobha, D.; Moon, E.; Chari, M.A.; Mukkanti, K.; Kim, S.-H.; Ahn, K.-H.; Kim, S.Y. Anti-neuroinflammatory activity of 1,5-benzodiazepine derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 3969–3971. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Joshi, Y.C. Synthesis and antimicrobial, antifungal and anthelmintic activities of 3h-1,5-benzodiazepine derivatives. J. Serbian Chem. Soc. 2008, 73, 937–943. [Google Scholar] [CrossRef]
- Shaikh, S.; Baseer, M. A Synthesis and antimicrobial activities of some new 2,3-dihydro-1,5-benzodiazepine derivatives. Int. J. Pharm. Sci. Res. 2013, 4, 2717–2720. [Google Scholar]
- Bjørklund, L.; Horsdal, H.T.; Mors, O.; Østergaard, S.D.; Gasse, C. Trends in the psychopharmacological treatment of bipolar disorder: A nationwide register-based study. Acta Neuropsychiatr. 2016, 28, 75–84. [Google Scholar] [CrossRef]
- Wingård, L.; Taipale, H.; Reutfors, J.; Westerlund, A.; Bodén, R.; Tiihonen, J.; Tanskanen, A.; Andersen, M. Initiation and long-term use of benzodiazepines and Z-drugs in bipolar disorder. Bipolar Disord. 2018, 20, 634–646. [Google Scholar] [CrossRef]
- Nardi, A.E.; Cosci, F.; Balon, R.; Weintraub, S.J.; Freire, R.C.; Krystal, J.H.; Roth, T.; Silberman, E.K.; Sonino, N.; Fava, G.A.; et al. The Prescription of Benzodiazepines for Panic Disorder: Time for an Evidence-Based Educational Approach. J. Clin. Psychopharmacol. 2018, 38, 283–285. [Google Scholar] [CrossRef]
- Annor-Gyamfi, J.K.; Jarrett, J.M.; Osazee, J.O.; Bialonska, D.; Whitted, C.; Palau, V.E.; Shilabin, A.G. Synthesis and biological activity of fused tetracyclic Pyrrolo[2,1-c][1,4]benzodiazepines. Heliyon 2018, 4, e00539. [Google Scholar] [CrossRef]
- Malayeri, S.O.; Tayarani-Najaran, Z.; Behbahani, F.S.; Rashidi, R.; Delpazir, S.; Ghodsi, R. Synthesis and biological evaluation of benzo[b]furo[3,4-e][1,4]diazepin-1-one derivatives as anti-cancer agents. Bioorg. Chem. 2018, 80, 631–638. [Google Scholar] [CrossRef]
- Praveen Kumar, C.; Reddy, T.S.; Mainkar, P.S.; Bansal, V.; Shukla, R.; Chandrasekhar, S.; Hügel, H.M. Synthesis and biological evaluation of 5,10-dihydro-11H-dibenzo[b,e][1,4]diazepin-11-one structural derivatives as anti-cancer and apoptosis inducing agents. Eur. J. Med. Chem. 2016, 108, 674–686. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, P.-H.; Lin, Y.-Y.; Yu, W.-T.; Hu, W.-P.; Hsu, C.-C.; Lin, Y.-T.; Chang, L.-S.; Hsiao, C.-T.; Wang, J.-J.; et al. Synthesis, DNA-binding abilities and anticancer activities of triazole-pyrrolo[2,1-c][1,4]benzodiazepines hybrid scaffolds. Bioorg. Med. Chem. Lett. 2013, 23, 6854–6859. [Google Scholar] [CrossRef] [PubMed]
- Colussi, G.; Catena, C.; Darsiè, D.; Sechi, L.A. Benzodiazepines: An Old Class of New Antihypertensive Drugs? Am. J. Hypertens. 2017, 31, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.; Kukla, M.J.; Breslin, H.J.; Ludovici, D.W.; Grous, P.P.; Diamond, C.J.; Miranda, M.; Rodgers, J.D.; Ho, C.Y. Synthesis and Anti-HIV-1 Activity of 4,5,6,7-Tetrahydro-5-methylimidazo[4,5,1-jk][1,4]benzodiazepin-2(1H)-one (TlBO) Derivatives. 4. J. Med. Chem. 1995, 38, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Merluzzi, V.J.; Hargrave, K.D.; Labadia, M.; Grozinger, K.; Skoog, M.; Wu, J.C.; Shih, C.K.; Eckner, K.; Hattox, S.; Adams, J.; et al. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science 1990, 250, 1411–1413. [Google Scholar] [CrossRef]
- Witt, A.; Bergman, J. Total Syntheses of the Benzodiazepine Alkaloids Circumdatin F and Circumdatin C. J. Org. Chem. 2001, 66, 2784–2788. [Google Scholar] [CrossRef]
- Ookura, R.; Kito, K.; Ooi, T.; Namikoshi, M.; Kusumi, T. Structure Revision of Circumdatins A and B, Benzodiazepine Alkaloids Produced by Marine Fungus Aspergillus ostianus, by X-ray Crystallography. J. Org. Chem. 2008, 73, 4245–4247. [Google Scholar] [CrossRef]
- Cui, C.-M.; Li, X.-M.; Li, C.-S.; Sun, H.-F.; Gao, S.-S.; Wang, B.-G. Benzodiazepine Alkaloids from Marine-Derived Endophytic Fungus Aspergillus ochraceus. Helv. Chim. Acta 2009, 92, 1366–1370. [Google Scholar] [CrossRef]
- Cairns, J.; Clarkson, T.R.; Hamersma, J.A.M.; Rae, D.R. 11-(Tetrahydro-3 and 4-pyridinyl)dibenzo[b,e][1,4]diazepines undergo novel rearrangements on treatment with concentrated HBr. Tetrahedron Lett. 2002, 43, 1583–1585. [Google Scholar] [CrossRef]
- Xu, X.; Guo, S.; Dang, Q.; Chen, J.; Bai, X. A New Strategy toward Fused-Pyridine Heterocyclic Scaffolds: Bischler–Napieralski-type Cyclization, Followed by Sulfoxide Extrusion Reaction. J. Comb. Chem. 2007, 9, 773–782. [Google Scholar] [CrossRef]
- Shi, F.; Xu, X.; Zheng, L.; Dang, Q.; Bai, X. Method Development for a Pyridobenzodiazepine Library with Multiple Diversification Points. J. Comb. Chem. 2008, 10, 158–161. [Google Scholar] [CrossRef]
- Umemiya, H.; Fukasawa, H.; Ebisawa, M.; Eyrolles, L.; Kawachi, E.; Eisenmann, G.; Gronemeyer, H.; Hashimoto, Y.; Shudo, K.; Kagechika, H. Regulation of Retinoidal Actions by Diazepinylbenzoic Acids. Retinoid Synergists Which Activate the RXR–RAR Heterodimers. J. Med. Chem. 1997, 40, 4222–4234. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Che, X.; Dang, Q.; Wei, Z.; Gao, S.; Bai, X. Synthesis of Tricyclic 4-Chloro-pyrimido[4,5-b][1,4]benzodiazepines. Org. Lett. 2005, 7, 1541–1543. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lee, G.T.; Prasad, K.; Repič, O. A Practical Synthesis of a Diazepinylbenzoic Acid, a Retinoid X Receptor Antagonist. Org. Process Res. Dev. 2008, 12, 1137–1141. [Google Scholar] [CrossRef]
- Smits, R.A.; Lim, H.D.; Stegink, B.; Bakker, R.A.; de Esch, I.J.P.; Leurs, R. Characterization of the Histamine H4 Receptor Binding Site. Part 1. Synthesis and Pharmacological Evaluation of Dibenzodiazepine Derivatives. J. Med. Chem. 2006, 49, 4512–4516. [Google Scholar] [CrossRef]
- Su, J.; Tang, H.; McKittrick, B.A.; Burnett, D.A.; Zhang, H.; Smith-Torhan, A.; Fawzi, A.; Lachowicz, J. Modification of the clozapine structure by parallel synthesis. Bioorg. Med. Chem. Lett. 2006, 16, 4548–4553. [Google Scholar] [CrossRef]
- Joshua, A.V.; Sharma, S.K.; Strelkov, A.; Scott, J.R.; Martin-Iverson, M.T.; Abrams, D.N.; Silverstone, P.H.; McEwan, A.J.B. Synthesis and biodistribution of 8-iodo-11-(4-methylpiperazino)-5H-dibenzo[b,e][1,4]-diazepine: Iozapine. Bioorg. Med. Chem. Lett. 2007, 17, 4066–4069. [Google Scholar] [CrossRef]
- Liao, Y.; Venhuis, B.J.; Rodenhuis, N.; Timmerman, W.; Wikström, H.; Meier, E.; Bartoszyk, G.D.; Böttcher, H.; Seyfried, C.A.; Sundell, S. New (Sulfonyloxy)piperazinyldibenzazepines as Potential Atypical Antipsychotics: Chemistry and Pharmacological Evaluation. J. Med. Chem. 1999, 42, 2235–2244. [Google Scholar] [CrossRef]
- Sharma, U.K.; Sharma, N.; Vachhani, D.D.; Van der Eycken, E.V. Metal-mediated post-Ugi transformations for the construction of diverse heterocyclic scaffolds. Chem. Soc. Rev. 2015, 44, 1836–1860. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, D.K.; Maity, P.K.; Shabab, M.; Khan, M.I. Click chemistry based rapid one-pot synthesis and evaluation for protease inhibition of new tetracyclic triazole fused benzodiazepine derivatives. Bioorg. Med. Chem. Lett. 2009, 19, 5241–5245. [Google Scholar] [CrossRef]
- Donald, J.R.; Martin, S.F. Synthesis and Diversification of 1,2,3-Triazole-Fused 1,4-Benzodiazepine Scaffolds. Org. Lett. 2011, 13, 852–855. [Google Scholar] [CrossRef] [Green Version]
- Loudni, L.; Roche, J.; Potiron, V.; Clarhaut, J.; Bachmann, C.; Gesson, J.-P.; Tranoy-Opalinski, I. Design, synthesis and biological evaluation of 1,4-benzodiazepine-2,5-dione-based HDAC inhibitors. Bioorg. Med. Chem. Lett. 2007, 17, 4819–4823. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.C.; Nicotra, F.; Airoldi, C.; Costa, B.; Giagnoni, G.; Fumagalli, P.; Cipolla, L. Synthesis and Biological Evaluation of Novel Rigid 1,4-Benzodiazepine-2,5-dione Chimeric Scaffolds. Eur. J. Org. Chem. 2008, 2008, 635–639. [Google Scholar] [CrossRef]
- De Silva, R.A.; Santra, S.; Andreana, P.R. A Tandem One-Pot, Microwave-Assisted Synthesis of Regiochemically Differentiated 1,2,4,5-Tetrahydro-1,4-benzodiazepin-3-ones. Org. Lett. 2008, 10, 4541–4544. [Google Scholar] [CrossRef] [PubMed]
- Ried, W.; Torinus, E. Über heterocyclische Siebenringsysteme, X. Synthesen kondensierter 5-, 7- und 8-gliedriger Heterocyclen mit 2 Stickstoffatomen. Chem. Ber. 1959, 92, 2902–2916. [Google Scholar] [CrossRef]
- Lewis, J.C.; Bergman, R.G.; Ellman, J.A. Direct Functionalization of Nitrogen Heterocycles via Rh-Catalyzed C–H Bond Activation. Acc. Chem. Res. 2008, 41, 1013–1025. [Google Scholar] [CrossRef] [Green Version]
- Gribble, M.W.; Ellman, J.A.; Bergman, R.G. Synthesis of a Benzodiazepine-Derived Rhodium NHC Complex by C–H Bond Activation. Organometallics 2008, 27, 2152–2155. [Google Scholar] [CrossRef] [Green Version]
- Chemistry, O.; Mahavidyalaya, Y. Copper-Bronze Catalyst: An Efficient Green Approach for the Synthesis of Dibenzo[b,e][1,4]diazepine Derivatives. Chem. Sci. Trans. 2015, 4, 194–198. [Google Scholar]
- Majumdar, K.C.; Ganai, S. CuI/l-Proline-Catalyzed Intramolecular Aryl Amination: An Efficient Route for the Synthesis of 1,4-Benzodiazepinones. Synlett 2011, 2011, 1881–1887. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Guo, S.; Zha, S.; Zhu, J. Synthesis of 2,3-Benzodiazepines via Rh(III)-Catalyzed C–H Functionalization of N-Boc Hydrazones with Diazoketoesters. Org. Lett. 2017, 19, 3640–3643. [Google Scholar] [CrossRef]
- Miri, N.S.; Safaei-Ghomi, J. Synthesis of benzodiazepines catalyzed by CoFe2O4@SiO2-PrNH2 nanoparticles as a reusable catalyst. Z. Nat. B. 2017, 72, 497–503. [Google Scholar] [CrossRef]
- Gawande, S.D.; Kavala, V.; Zanwar, M.R.; Kuo, C.-W.; Huang, W.-C.; Kuo, T.-S.; Huang, H.-N.; He, C.-H.; Yao, C.-F. Synthesis of Dibenzodiazepinones via Tandem Copper(I)- Catalyzed C–N Bond Formation. Adv. Synth. Catal. 2014, 356, 2599–2608. [Google Scholar] [CrossRef]
- Arora, N.; Dhiman, P.; Kumar, S.; Singh, G.; Monga, V. Recent advances in synthesis and medicinal chemistry of benzodiazepines. Bioorg. Chem. 2020, 97, 103668. [Google Scholar] [CrossRef]
- Singh, R.K.; Sharma, S.; Sandhar, A.; Saini, M.; Kumar, S. Current development in multicomponent catalytic synthesis of 1,5-benzodiazepines: A systematic review. Iran. J. Catal. 2016, 6, 1–21. [Google Scholar]
- Kaur, N.; Kishore, D. Synthetic strategies applicable in the synthesis of privileged scaffold: 1,4-benzodiazepine. Synth. Commun. 2014, 44, 1375–1413. [Google Scholar] [CrossRef]
- Kumar, S.S.; Kavitha, H.P.; Arulmurugan, S.; Venkatraman, B.R. Review on Synthesis of Biologically Active Diazepam Derivatives. Mini. Rev. Org. Chem. 2012, 9, 285–302. [Google Scholar] [CrossRef]
- Horton, D.A.; Bourne, G.T.; Smythe, M.L. The Combinatorial Synthesis of Bicyclic Privileged Structures or Privileged Substructures. Chem. Rev. 2003, 103, 893–930. [Google Scholar] [CrossRef] [PubMed]
- Weers, M.; Lühning, L.H.; Lührs, V.; Brahms, C.; Doye, S. One-Pot Procedure for the Synthesis of 1,5-Benzodiazepines from N-Allyl-2-bromoanilines. Chem. A Eur. J. 2017, 23, 1237–1240. [Google Scholar] [CrossRef]
- Cropper, E.L.; Yuen, A.P.; Ford, A.; White, A.J.P.; (Mimi) Hii, K.K. Delineating ligand effects in intramolecular aryl amidation reactions: Formation of a novel spiro-heterocycle by a tandem cyclisation process. Tetrahedron 2009, 65, 525–530. [Google Scholar] [CrossRef]
- Willy, B.; Dallos, T.; Rominger, F.; Schönhaber, J.; Müller, T.J.J. Three-component synthesis of cryofluorescent 2,4-disubstituted 3H-1,5-benzodiazepines—Conformational control of emission properties. Eur. J. Org. Chem. 2008, 4796–4805. [Google Scholar] [CrossRef]
- Shahi, C.K.; Bhattacharyya, A.; Nanaji, Y.; Ghorai, M.K. A Stereoselective Route to Tetrahydrobenzoxazepines and Tetrahydrobenzodiazepines via Ring-Opening and Aza-Michael Addition of Activated Aziridines with 2-Hydroxyphenyl and 2-Aminophenyl Acrylates. J. Org. Chem. 2017, 82, 37–47. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Paladino, G.; Penoni, A.; Zoni, C. Regioselective formation of six- and seven-membered ring by intramolecular Pd-catalyzed amination of N-allyl-anthranilamides. J. Org. Chem. 2004, 69, 5627–5630. [Google Scholar] [CrossRef]
- Thireau, J.; Schneider, C.; Baudequin, C.; Gaurrand, S.; Angibaud, P.; Meerpoel, L.; Levacher, V.; Querolle, O.; Hoarau, C. Chemoselective Palladium-Catalyzed Direct C–H Arylation of 5-Carboxyimidazoles: Unparalleled Access to Fused Imidazole-Based Tricycles Containing Six-, Seven- or Eight-Membered Rings. Eur. J. Org. Chem. 2017, 2017, 2491–2494. [Google Scholar] [CrossRef]
- Virelli, M.; Moroni, E.; Colombo, G.; Fiengo, L.; Porta, A.; Ackermann, L.; Zanoni, G. Expedient Access to 2-Benzazepines by Palladium-Catalyzed C–H Activation: Identification of a Unique Hsp90 Inhibitor Scaffold. Chem. A Eur. J. 2018, 24, 16516–16520. [Google Scholar] [CrossRef] [PubMed]
- Kalinski, C.; Umkehrer, M.; Ross, G.; Kolb, J.; Burdack, C.; Hiller, W. Highly substituted indol-2-ones, quinoxalin-2-ones and benzodiazepin-2,5-diones via a new Ugi(4CR)-Pd assisted N-aryl amidation strategy. Tetrahedron Lett. 2006, 47, 3423–3426. [Google Scholar] [CrossRef]
- Catellani, M.; Catucci, C.; Celentano, G.; Ferraccioli, R. Palladium-catalysed synthesis of enantiopure 1,2,4,5-tetrahydro-1,4-benzodiazepin-3-(3H)-one derivatives. Synlett 2001, 6, 803–805. [Google Scholar] [CrossRef]
- Beccalli, E.M.; Broggini, G.; Paladino, G.; Zoni, C. Palladium-mediated approach to dibenzo[b,e][1,4]diazepines and benzopyrido-analogues. An efficient synthesis of tarpane. Tetrahedron 2005, 61, 61–68. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hirokawa, T.; Watanabe, M.; Fujita, S.; Ogura, Y.; Enomoto, M.; Kuwahara, S. First synthesis of BU-4664L. Tetrahedron Lett. 2015, 56, 5670–5672. [Google Scholar] [CrossRef]
- Mitra, S.; Darira, H.; Chattopadhyay, P. Efficient synthesis of imidazole-fused benzodiazepines using palladium-catalyzed intramolecular C-N bond formation reaction. Synthesis 2013, 45, 85–92. [Google Scholar] [CrossRef]
- Tsvelikhovsky, D.; Buchwald, S.L. Concise palladium-catalyzed synthesis of dibenzodiazepines and structural analogues. J. Am. Chem. Soc. 2011, 133, 14228–14231. [Google Scholar] [CrossRef] [Green Version]
- Maddess, M.L.; Li, C. Metal Catalyzed Synthesis of Dihydropyridobenzodiazepines. Organometallics 2019, 38, 81–84. [Google Scholar] [CrossRef]
- Surman, M.D.; Mulvihill, M.J.; Miller, M.J. Novel 1,4-benzodiazepines from acylnitroso-derived hetero-Diels-Alder cycloadducts. Org. Lett. 2002, 4, 139–141. [Google Scholar] [CrossRef]
- Tardibono, L.P.; Miller, M.J. Synthesis and anticancer activity of new hydroxamic acid containing 1,4-benzodiazepines. Org. Lett. 2009, 11, 1575–1578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikura, M.; Mori, M.; Ikeda, T.; Terashima, M.; Ban, Y. New Synthesis of Diazepam and the Related 1,4-Benzodiazepines by means of Palladium-Catalyzed Carbonylation. J. Org. Chem. 1982, 47, 2456–2461. [Google Scholar] [CrossRef]
- Mori, M.; Purvaneckas, G.E.; Ishikura, M.; Ban, Y. New synthesis of pyrrolo-1,4-benzodiazepines by utilizing palladium-catalyzed carbonylation. Chem. Pharm. Bull. (Tokyo) 1984, 32, 3840–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, M.; Uozumi, Y.; Ban, Y. Total synthesis of neothramycin. J. Chem. Soc. Chem. Commun. 1986, 841–842. [Google Scholar] [CrossRef]
- Mori, M.; Kimura, M.; Uozumi, Y.; Ban, Y. A one step synthesis of 1,4-benzodiazepines: Synthetic studies on neothramycin. Tetrahedron Lett. 1985, 26, 5947–5950. [Google Scholar] [CrossRef]
- Mori, M.; Uozumi, Y.; Kimura, M.; Ban, Y. Total Syntheses of Prothracarcin and Tomaymycin by Use of Palladium Catalyzed Carbonylation. Tetrahedron 1986, 42, 3793–3806. [Google Scholar] [CrossRef]
- Lu, S.M.; Alper, H. Intramolecular carbonylation reactions with recyclable palladium-complexed dendrimers on silica: Synthesis of oxygen, nitrogen, or sulfur-containing medium ring fused heterocycles. J. Am. Chem. Soc. 2005, 127, 14776–14784. [Google Scholar] [CrossRef]
- Cuny, G.; Bois-Choussy, M.; Zhu, J. One-Pot Synthesis of Polyheterocycles by a Palladium-Catalyzed Intramolecular N-Arylation/C-H Activation/Aryl-Aryl Bond-Forming Domino Process. Angew. Chem. Int. Ed. 2003, 42, 4774–4777. [Google Scholar] [CrossRef]
- Cuny, G.; Bois-Choussy, M.; Zhu, J. Palladium- and copper-catalyzed synthesis of medium- and large-sized ring-fused dihydroazaphenanthrenes and 1,4-benzodiazepine-2,5-diones. Control of reaction pathway by metal-switching. J. Am. Chem. Soc. 2004, 126, 14475–14484. [Google Scholar] [CrossRef]
- Salcedo, A.; Neuville, L.; Rondot, C.; Retailleau, P.; Zhu, J. Palladium-catalyzed domino intramolecular N-arylation/Lntermolecular C-C bond formation for the synthesis of functionalized benzodiazepinediones. Org. Lett. 2008, 10, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, D.; Locati, A.; Marques, C.S.; Goth, A.; Ramalho, J.P.P.; Burke, A.J. A catalytic route to dibenzodiazepines involving Buchwald-Hartwig coupling: Reaction scope and mechanistic consideration. RSC Adv. 2015, 5, 99990–99999. [Google Scholar] [CrossRef]
- Neukom, J.D.; Aquino, A.S.; Wolfe, J.P. Synthesis of saturated 1,4-benzodiazepines via Pd-catalyzed carboamination reactions. Org. Lett. 2011, 13, 2196–2199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundu, P.; Mondal, A.; Das, B.; Chowdhury, C. A Straightforward Approach for the Stereoselective Synthesis of (E)-2-Aryl/vinylmethylidene-1,4-benzodiazepines and -1,4-benzodiazepin-5-ones through Palladium/Charcoal-Catalyzed Reactions. Adv. Synth. Catal. 2015, 357, 3737–3752. [Google Scholar] [CrossRef]
- Rigamonti, M.; Prestat, G.; Broggini, G.; Poli, G. Synthesis of 1,4-benzodiazepinones via palladium-catalysed allene carbopalladation/amination domino sequence Dedicated to Professor Maria José Calhorda in occasion of her 65th birthday. J. Organomet. Chem. 2014, 760, 149–155. [Google Scholar] [CrossRef]
- Hu, W.; Teng, F.; Hu, H.; Luo, S.; Zhu, Q. Pd-Catalyzed C(sp2)-H Imidoylative Annulation: A General Approach to Construct Dibenzoox(di)azepines. J. Org. Chem. 2019, 84, 6524–6535. [Google Scholar] [CrossRef]
- Rosewall, C.F.; Sibbald, P.A.; Liskin, D.V.; Michael, F.E. Palladium-catalyzed carboamination of alkenes promoted by N-fluorobenzenesulfonimide via C-H activation of arenes. J. Am. Chem. Soc. 2009, 131, 9488–9489. [Google Scholar] [CrossRef]
- Gao, Y.; Li, C.; Xu, B.; Liu, H. Rapid access to difluoroalkylated pyrrolobenzodiazepines: Via a Pd-catalyzed C-H difluoroalkylation/cyclization cascade reaction. Org. Chem. Front. 2019, 6, 410–414. [Google Scholar] [CrossRef]
- Manick, A.D.; Duret, G.; Tran, D.N.; Berhal, F.; Prestat, G. Synthesis of 1,4-benzodiazepinones and 1,4-benzoxazepinones via palladium-catalyzed amino and oxyacetoxylation. Org. Chem. Front. 2014, 1, 1058–1061. [Google Scholar] [CrossRef]
- Manick, A.D.; Berhal, F.; Prestat, G. Synthesis of Six- and Seven-Membered Chloromethyl-Substituted Heterocycles via Palladium-Catalyzed Amino- and Oxychlorination. Synthesis 2016, 48, 3719–3729. [Google Scholar]
- Foschi, F.; Loro, C.; Sala, R.; Oble, J.; Lo Presti, L.; Beccalli, E.M.; Poli, G.; Broggini, G. Intramolecular Aminoazidation of Unactivated Terminal Alkenes by Palladium-Catalyzed Reactions with Hydrogen Peroxide as the Oxidant. Org. Lett. 2020, 22, 1402–1406. [Google Scholar] [CrossRef] [PubMed]
- Thikekar, T.U.; Sun, C.M. Palladium-Catalyzed Regioselective Synthesis of 1,2-Fused Indole-Diazepines via [5+2] Annulation of o-Indoloanilines with Alkynes. Adv. Synth. Catal. 2017, 359, 3388–3396. [Google Scholar] [CrossRef]
- Ferrini, S.; Ponticelli, F.; Taddei, M. Rapid approach to 3,5-disubstituted 1,4-benzodiazepines via the photo-fries rearrangement of anilides. J. Org. Chem. 2006, 71, 9217–9220. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Hao, J.H.; Zhang, C.P.; Zhang, J.; Feng, Y.; Qin, H.L. Diversified facile synthesis of benzimidazoles, quinazolin-4(3H)-ones and 1,4-benzodiazepine-2,5-diones via palladium-catalyzed transfer hydrogenation/condensation cascade of nitro arenes under microwave irradiation. RSC Adv. 2015, 5, 11132–11135. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Grady, M.J.W.; Guzei, I.A.; Gandhi, B.A.; Bunel, E.E.; Stahl, S.S. PdII complexes possessing a seven-membered N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 2005, 44, 5269–5272. [Google Scholar] [CrossRef]
- Scarborough, C.C.; Bergant, A.; Sazama, G.T.; Guzei, I.A.; Spencer, L.C.; Stahl, S.S. Synthesis of PdII complexes bearing an enantiomerically resolved seven-membered N-heterocyclic carbene ligand and initial studies of their use in asymmetric Wacker-type oxidative cyclization reactions. Tetrahedron 2009, 65, 5084–5092. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.J.; Wang, W.; Zhou, R.; Zhang, L.; Fu, H.Y.; Zheng, X.L.; Chen, H.; Li, R.X. 6H-Dibenzo[d,f-[1,3]diazepin-6-ylidene,5,7-dihydro-5,7-diphenylphosphanyl]: A new ligand for palladium-catalyzed Mizoroki-Heck coupling. Catal. Commun. 2014, 57, 14–18. [Google Scholar] [CrossRef]
- Wezeman, T.; Hu, Y.; McMurtrie, J.; Bräse, S.; Masters, K.S. Synthesis of Non-Symmetrical and Atropisomeric Dibenzo[1,3]diazepines: Pd/CPhos-Catalysed Direct Arylation of Bis-Aryl Aminals. Aust. J. Chem. 2015, 68, 1859–1865. [Google Scholar] [CrossRef]
- Wang, G.; Liu, C.; Li, B.; Wang, Y.; Van Hecke, K.; Van der Eycken, E.V.; Pereshivko, O.P.; Peshkov, V.A. Diversity-oriented synthesis of 1,3-benzodiazepines. Tetrahedron 2017, 73, 6372–6380. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, D.; Hu, W. Regio- and Diastereoselective Three-Component Reactions via Trapping of Ammonium Ylides with N-Alkylquinolinium Salts: Synthesis of Multisubstituted Tetra- and Dihydroquinoline Derivatives. Org. Lett. 2017, 19, 3783–3786. [Google Scholar] [CrossRef]
- Chan, C.K.; Tsai, Y.L.; Chan, Y.L.; Chang, M.Y. Synthesis of Substituted 2,3-Benzodiazepines. J. Org. Chem. 2016, 81, 9836–9847. [Google Scholar] [CrossRef] [PubMed]
- Asamdi, M.; Shaikh, M.M.; Chauhan, P.M.; Chikhalia, K.H. Palladium-catalyzed [5+2] oxidative annulation of N-Arylhydrazones with alkynes through C–H activation to synthesize Benzo[d][1,2]diazepines. Tetrahedron 2018, 74, 3719–3727. [Google Scholar] [CrossRef]
- Ding, Y.L.; Zhao, Y.L.; Niu, S.S.; Wu, P.; Cheng, Y. Asymmetric Synthesis of Multifunctionalized 2,3-Benzodiazepines by a One-Pot N-heterocyclic Carbene/Chiral Palladium Sequential Catalysis. J. Org. Chem. 2020, 85, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Bocelli, G.; Catellani, M.; Cugini, F.; Ferraccioli, R. New and Efficient Palladium-catalyzed Synthesis of a 2,3,4,5-Tetrahydro-lH-2,4-benzodiazepine- 1,3-dione Derivative. Tetrahedron 1999, 40, 2623–2624. [Google Scholar] [CrossRef]

















































© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christodoulou, M.S.; Beccalli, E.M.; Giofrè, S. Palladium-Catalyzed Benzodiazepines Synthesis. Catalysts 2020, 10, 634. https://doi.org/10.3390/catal10060634
Christodoulou MS, Beccalli EM, Giofrè S. Palladium-Catalyzed Benzodiazepines Synthesis. Catalysts. 2020; 10(6):634. https://doi.org/10.3390/catal10060634
Chicago/Turabian StyleChristodoulou, Michael S., Egle M. Beccalli, and Sabrina Giofrè. 2020. "Palladium-Catalyzed Benzodiazepines Synthesis" Catalysts 10, no. 6: 634. https://doi.org/10.3390/catal10060634
APA StyleChristodoulou, M. S., Beccalli, E. M., & Giofrè, S. (2020). Palladium-Catalyzed Benzodiazepines Synthesis. Catalysts, 10(6), 634. https://doi.org/10.3390/catal10060634