Facile Formation of Anatase Nanoparticles on H-Titanate Nanotubes at Low Temperature for Efficient Visible Light-Driven Degradation of Organic Pollutants
Abstract
:1. Introduction
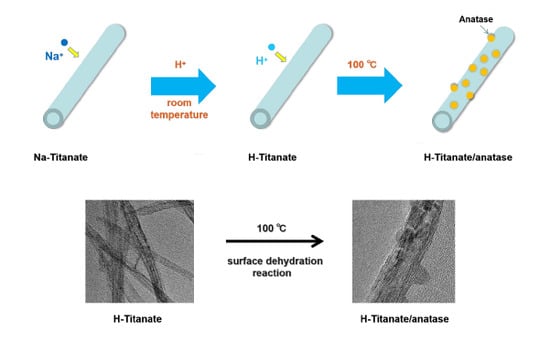
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Photocatalytic Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cao, S.W.; Low, J.X.; Yu, J.G.; Jaroniec, M. Polymeric Photocatalysts Based on Graphitic Carbon Nitride. Adv. Mater. 2015, 27, 2150–2176. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, Y.; Xiao, Y.; Zhou, W.; Tian, G.; Fu, H. Enhanced charge transfer and separation of hierarchical hydrogenated TiO2 nanothorns/carbon nanofibers composites decorated by NiS quantum dots for remarkable photocatalytic H2 production activity. Nanoscale 2018, 10, 4041–4050. [Google Scholar] [CrossRef]
- Dai, G.; Qin, H.; Zhou, H.; Wang, W.; Luo, T. Template-free fabrication of hierarchical macro/mesoporpous SnS2/TiO2 composite with enhanced photocatalytic degradation of Methyl Orange (MO). Appl. Surf. Sci. 2018, 430, 488–495. [Google Scholar] [CrossRef]
- Lyu, J.; Gao, J.; Zhang, M.; Fu, Q.; Sun, L.; Hu, S.; Zhong, J.; Wang, S.; Li, J. Construction of homojunction-adsorption layer on anatase TiO2 to improve photocatalytic mineralization of volatile organic compounds. Appl. Catal. B 2017, 202, 664–670. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. TiO2−MnOx−Pt Hybrid Multiheterojunction Film Photocatalyst with Enhanced Photocatalytic CO2-Reduction Activity. ACS Appl. Mater. Interfaces 2018, 11, 5581–5589. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Li, H.; Wang, L.; Shen, W.; Chen, H. Novel α-Fe2O3/CdS Cornlike Nanorods with Enhanced Photocatalytic Performance. ACS Appl. Mater. Interfaces 2012, 4, 4800–4806. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Jaroniec, M.; Qiao, S.Z. Cocatalysts in Semiconductor-based Photocatalytic CO2 Reduction: Achievements, Challenges, and Opportunities. Adv. Mater. 2018, 30, 1704649(1)–1704649(31). [Google Scholar] [CrossRef]
- Fu, W.W.; Li, G.D.; Wang, Y.; Zeng, S.J.; Yan, Z.J.; Wang, J.W.; Xin, S.G.; Zhang, L.; Wu, S.W.; Zhang, Z.T. Facile formation of mesoporous structured mixed-phase (anatase/rutile) TiO2 with enhanced visible light photocatalytic activity. Chem. Commun. 2018, 54, 58–61. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660(1)–1807660(31). [Google Scholar]
- Niu, S.; Zhang, R.; Zhang, Z.; Zheng, J.; Jiao, Y.; Guo, C. In situ construction of the BiOCl/Bi2Ti2O7 heterojunction with enhanced visible-light photocatalytic activity. Inorg. Chem. Front. 2019, 6, 791–798. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Friedrich, J.M.; Walsh, F.C. Protonated Titanates and TiO2 Nanostructured Materials: Synthesis, Properties, and Applications. Adv. Mater. 2006, 18, 2807–2824. [Google Scholar] [CrossRef]
- Mao, Y.; Wong, S.S. Size- and Shape-Dependent Transformation of Nanosized Titanate into Analogous Anatase Titania Nanostructures. J. Am. Chem. Soc. 2006, 128, 8217–8226. [Google Scholar] [CrossRef] [PubMed]
- Buchholcz, B.; Haspel, H.; Kukovecz, Á.; Kónya, Z. Low-temperature conversion of titanate nanotubes into nitrogen-doped TiO2 nanoparticles. CrystEngComm 2014, 16, 7486–7492. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhao, X. Nitrogen-doped titanate-anatase core-shell nanobelts with exposed {101} anatase facets and enhanced visible light photocatalytic activity. J. Am. Chem. Soc. 2012, 134, 5754–5757. [Google Scholar] [CrossRef]
- Yan, Y.; Qiu, X.; Wang, H.; Li, L.; Fu, X.; Wu, L.; Li, G. Synthesis of titanate/anatase composites with highly photocatalytic decolorization of dye under visible light irradiation. J. Alloys Compd. 2008, 460, 491–495. [Google Scholar] [CrossRef]
- Harsha, N.; Ranya, K.R.; Babitha, K.B.; Shukla, S.; Biju, S.; Reddy, M.L.P.; Warrier, K.G.K. Hydrothermal Processing of Hydrogen Titanate/Anatase-Titania Nanotubes and Their Application as Strong Dye-Adsorbents. J. Nanosci. Nanotechnol. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Wang, P.; Yi, X.; Lu, Y.; Yu, H.; Yu, J. In-situ synthesis of amorphous H2TiO3-modified TiO2 and its improved photocatalytic H2-evolution performance. J. Colloid Interface Sci. 2018, 532, 272–279. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Huang, Y.Z.; Kanhere, P.D.; Subramaniam, V.P.; Gong, D.G.; Zhang, S.; Highfield, J.; Schreyer, M.K.; Chen, Z. Dual-Phase Titanate/Anatase with Nitrogen Doping for Enhanced Degradation of Organic Dye under Visible Light. Chem. Eur. J. 2011, 17, 2575–2578. [Google Scholar] [CrossRef]
- Cai, J.; Zhu, Y.; Liu, D.; Meng, M.; Hu, Z.; Jiang, Z. Synergistic Effect of Titanate-Anatase Heterostructure and Hydrogenation-Induced Surface Disorder on Photocatalytic Water Splitting. ACS Catal. 2015, 5, 1708–1716. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, H.; Wang, N.; Lin, C.; Li, J. The evolution of morphology and crystal form of titanate nanotubes under calcination and its mechanism. J. Alloys Compd. 2007, 431, 230–235. [Google Scholar] [CrossRef]
- Xiong, Z.; Dou, H.; Pan, J.; Ma, J.; Xu, C.; Zhao, X.S. Synthesis of mesoporous anatase TiO2 with a combined template method and photocatalysis. CrystEngComm 2010, 12, 3455–3457. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. TiO2 NPs Assembled into a Carbon Nanofifiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107(1)–046107(3). [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Zhang, M.S.; Yin, Z.; Chen, Q. Photoluminescence in anatase titanium dioxide nanocrystals. Appl. Phys. B Laser Opt. 2000, 70, 261–265. [Google Scholar] [CrossRef]
- Ng, J.; Xu, S.; Zhang, X.; Yang, H.Y.; Sun, D.D. Hybridized Nanowires and Cubes: A Novel Architecture of a Heterojunctioned TiO2/SrTiO3 Thin Film for Efficient Water Splitting. Adv. Funct. Mater. 2010, 20, 4287–4294. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, B.; Lu, X.; Zhang, X.; Zhu, H.; Li, B. Multifunctional 3D K2Ti6O13 nanobelt-built architectures towards wastewater remediation: Selective adsorption, photodegradation, mechanism insight and photoelectrochemical investigation. Catal. Sci. Technol. 2018, 8, 6180–6195. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofifibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef]
- Fu, W.; Ding, S.; Wang, Y.; Wu, L.; Zhang, D.; Pan, Z.; Wang, R.; Zhang, Z.; Qiu, S. F, Ca co-doped TiO2 nanocrystals with enhanced photocatalytic activity. Dalton Trans. 2014, 43, 16160–16163. [Google Scholar] [CrossRef]
- Nguyen-Le, M.-T.; Lee, B.-K. Novel fabrication of a nitrogen-doped mesoporous TiO2-nanorod titanate heterojunction to enhance the photocatalytic degradation of dyes under visible light. RSC Adv. 2016, 6, 31347–31350. [Google Scholar]
- Niu, F.; Chen, D.; Qin, L.; Zhang, N.; Wang, J.; Chen, Z.; Huang, Y. Facile Synthesis of Highly Efficient p–n Heterojunction CuO/BiFeO3 Composite Photocatalysts with Enhanced Visible-Light Photocatalytic Activity. ChemCatChem 2015, 7, 3279–3289. [Google Scholar] [CrossRef]
- Liu, C.; Liang, J.-Y.; Han, R.-R.; Wang, Y.-Z.; Zhao, J.; Huang, Q.-J.; Chen, J.; Hou, W.-H. S-doped Na2Ti6O13@TiO2 core-shell nanorods with enhanced visible light photocatalytic performance. Phys. Chem. Chem. Phys. 2015, 17, 15165–15172. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, J.; Zheng, F.; Li, Y.; Huang, F. Synthesis of the double-shell anatase-rutile TiO2 hollow spheres with enhanced photocatalytic activity. Nanoscale 2013, 5, 12150–12155. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Jiao, J.; Li, Z.; Guo, Y.; Feng, C.; Liu, Y.; Wang, L.; Wu, M. Efficient Separation of Electron–Hole Pairs in Graphene Quantum Dots by TiO2 Heterojunctions for Dye Degradation. ACS Sustain. Chem. Eng. 2015, 3, 2405–2413. [Google Scholar] [CrossRef]
- Tang, Y.; Lai, Y.; Gong, D.; Goh, K.-H.; Lim, T.-T.; Dong, Z.; Chen, Z. Ultrafast Synthesis of Layered Titanate Microspherulite Particles by Electrochemical Spark Discharge Spallation. Chem. Eur. J. 2010, 16, 7704–7708. [Google Scholar] [CrossRef]
- Lim, Y.W.L.; Tang, Y.X.; Cheng, Y.H.; Chen, Z. Morphology, crystal structure and adsorption performance of hydrothermally synthesized titania and titanate nanostructures. Nanoscale 2010, 2, 2751–2757. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Poyraz, A.S.; Kuo, C.-H.; Miao, R.; Meng, Y.; Chen, S.-Y.; Jiang, T.; Wenos, C.; Suib, S.L. Crystalline Mixed Phase (Anatase/Rutile) Mesoporous Titanium Dioxides for Visible Light Photocatalytic Activity. Chem. Mater. 2015, 27, 6–17. [Google Scholar] [CrossRef]
- Gurulakshmi, M.; Selvaraj, M.; Selvamani, A.; Vijayan, P.; Sasi Rekha, N.R.; Shanthi, K. Enhanced visible-light photocatalytic activity of V2O5/S-TiO2 nanocomposites. Appl. Catal. A 2012, 449, 31–46. [Google Scholar] [CrossRef]
- Kho, Y.K.; Iwase, A.; Teoh, W.Y.; Ma¨dler, L.; Kudo, A.; Amal, R. Photocatalytic H2 Evolution over TiO2 Nanoparticles. The Synergistic Effect of Anatase and Rutile. J. Phys. Chem. C 2010, 114, 2821–2829. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual cocatalysts on {101} and {001} facets. Appl. Catal. B 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, Q.; Wang, L.; Zhang, L. Magnetically separable CdFe2O4/graphene catalyst and its enhanced photocatalytic properties. J. Mater. Chem. A 2015, 3, 3576–3585. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Y.; Sun, D.D.; Guo, P.; Leckie, J.O. One-Step Fabrication and High Photocatalytic Activity of Porous TiO2 Hollow Aggregates by Using a Low-Temperature Hydrothermal Method Without Templates. Chem. Eur. J. 2007, 13, 1851–1855. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, W.; Shi, Z.; Bai, H.; Dai, J.; Lu, Z.; Lei, F.; Zhang, D.; Zhao, L.; Zhang, Z. Facile Formation of Anatase Nanoparticles on H-Titanate Nanotubes at Low Temperature for Efficient Visible Light-Driven Degradation of Organic Pollutants. Catalysts 2020, 10, 695. https://doi.org/10.3390/catal10060695
Fu W, Shi Z, Bai H, Dai J, Lu Z, Lei F, Zhang D, Zhao L, Zhang Z. Facile Formation of Anatase Nanoparticles on H-Titanate Nanotubes at Low Temperature for Efficient Visible Light-Driven Degradation of Organic Pollutants. Catalysts. 2020; 10(6):695. https://doi.org/10.3390/catal10060695
Chicago/Turabian StyleFu, Weiwei, Zhiqiang Shi, Helong Bai, Jinyu Dai, Zhiming Lu, Feifei Lei, Deguang Zhang, Lun Zhao, and Zongtao Zhang. 2020. "Facile Formation of Anatase Nanoparticles on H-Titanate Nanotubes at Low Temperature for Efficient Visible Light-Driven Degradation of Organic Pollutants" Catalysts 10, no. 6: 695. https://doi.org/10.3390/catal10060695
APA StyleFu, W., Shi, Z., Bai, H., Dai, J., Lu, Z., Lei, F., Zhang, D., Zhao, L., & Zhang, Z. (2020). Facile Formation of Anatase Nanoparticles on H-Titanate Nanotubes at Low Temperature for Efficient Visible Light-Driven Degradation of Organic Pollutants. Catalysts, 10(6), 695. https://doi.org/10.3390/catal10060695