Enantioselective Transesterification of Allyl Alcohols with (E)-4-Arylbut-3-en-2-ol Motif by Immobilized Lecitase™ Ultra
Abstract
:1. Introduction
2. Results and Discussion
2.1. Comparison of Immobilized Preparations of Lecitase™ Ultra as the Catalysts of Transesterification
2.2. Effect of Enzyme Dosage
2.3. Effect of Solvent
2.4. Effect of Acyl Donor
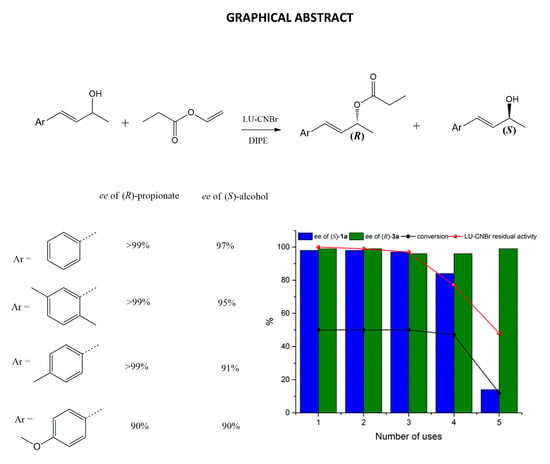
2.5. Enzyme Reuse
2.6. Resolution of (E)-4-Phenylbut-3-en-2-ol Analogues (1b-d)
3. Materials and Methods
3.1. Chemicals and Enzyme
3.2. Analysis
3.3. Enzymatic Activity Assays
3.4. Immobilization of LU in Calcium Alginate Beads
3.5. Adsorption of LU on Polyacrylic Resin (Supelite™ DAX-8)
3.6. Immobilization of LU on Cyanogen Bromide—Activated Crosslinked 4% Agarose
3.7. Immobilization of LU on Modified Bacterial Cellulose
3.8. Enzymatic Transesterification of Racemic Alcohols 1a-d Catalyzed by Immobilized LU Preparations
3.8.1. General Procedure
3.8.2. Effect of Enzyme Dosage
3.8.3. Enzyme Reusability
3.8.4. Isolation of Products Obtained by Transesterification of Racemic Alcohols (1a-d)—General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Meyer, H.P.; Eichhorn, E.; Hanlon, S.; Lütz, S.; Schürmann, M.; Wohlgemuth, R.; Coppolecchia, R. The use of enzymes in organic synthesis and the life sciences: Perspectives from the Swiss Industrial Biocatalysis Consortium (SIBC). Catal. Sci. Technol. 2013, 3, 29–40. [Google Scholar] [CrossRef]
- Sen, S.; Puskas, J.E. Green polymer chemistry: Enzyme catalysis for polymer functionalization. Molecules 2015, 20, 9358–9379. [Google Scholar] [CrossRef] [Green Version]
- Winkler, M.; Geier, M.; Hanlon, S.P.; Nidetzky, B.; Glieder, A. Human enzymes for organic synthesis. Angew. Chem. Int. Ed. 2018, 57, 13406–13423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ran, N.; Zhao, L.; Chen, Z.; Tao, J. Recent applications of biocatalysis in developing green chemistry for chemical synthesis at the industrial scale. Green Chem. 2008, 10, 361–372. [Google Scholar] [CrossRef]
- Robles-Medina, A.; González-Moreno, P.A.; Esteban-Cerdán, L.; Molina-Grima, E. Biocatalysis: Towards ever greener biodiesel production. Biotechnol. Adv. 2009, 27, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Leitão, V.S.; Cammarota, M.C.; Aguieiras, E.C.G.; de Sá, L.R.V.; Fernandez-Lafuente, R.; Freire, D.M.G. The protagonism of biocatalysis in green chemistry and its environmental benefits. Catalysts 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Arrojo, L.; Guazzaroni, M.E.; López-Cortés, N.; Beloqui, A.; Ferrer, M. Metagenomic era for biocatalyst identification. Curr. Opin. Biotechnol. 2010, 21, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Reetz, M.T. Biocatalysis in organic chemistry and biotechnology: Past, present, and future. J. Am. Chem. Soc. 2013, 135, 12480–12496. [Google Scholar] [CrossRef]
- Castilla, I.A.; Woods, D.F.; Reen, F.J.; O’Gara, F. Harnessing marine biocatalytic reservoirs for green chemistry applications through metagenomic technologies. Mar. Drugs 2018, 16, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Bornscheuer, U.T.; Pohl, M. Improved biocatalysts by directed evolution and rational protein design. Curr. Opin. Chem. Biol. 2001, 5, 137–143. [Google Scholar] [CrossRef]
- Morley, K.L.; Kazlauskas, R.J. Improving enzyme properties: When are closer mutations better? Trends Biotechnol. 2005, 23, 231–237. [Google Scholar] [CrossRef]
- Strohmeier, G.A.; Pichler, H.; May, O.; Gruber-Khadjawi, M. Application of designed enzymes in organic synthesis. Chem. Rev. 2011, 111, 4141–4164. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortíz, J.J.; dos Santos, J.C.S.; Ortiz, C.; Berenguer-Murcia, Á.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Lecitase Ultra: A phospholipase with great potential in biocatalysis. Mol. Catal. 2019, 473, 110405. [Google Scholar] [CrossRef] [Green Version]
- Clausen, K. Enzymatic oil-degumming by a novel microbial phospholipase. Eur. J. Lipid Sci. Technol. 2001, 103, 333–340. [Google Scholar] [CrossRef]
- Manjula, S.; Jose, A.; Divakar, S.; Subramanian, R. Degumming rice bran oil using phospholipase-A1. Eur. J. Lipid Sci. Technol. 2011, 113, 658–664. [Google Scholar] [CrossRef]
- Lamas, D.L.; Crapiste, G.H.; Constenla, D.T. Changes in quality and composition of sunflower oil during enzymatic degumming process. LWT-Food Sci. Technol. 2014, 58, 71–76. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Zyaykina, N.; Wozniak, B.; Tsukamoto, J.; Greyt, W.D.; Stevens, C.V. Enzymatic degumming: Degumming efficiency versus yield increase. Eur. J. Lipid Sci. Technol. 2015, 117, 81–86. [Google Scholar] [CrossRef]
- Mardani, M.; Farmani, J.; Kenari, R.E. Efficacy of some commercial lipases in hydrolysis of palm olein for the production of free fatty acids and diacylglycerol oil. J. Oil Palm Res. 2015, 27, 250–260. [Google Scholar]
- Wang, Y.; Zhao, M.; Song, K.; Wang, L.; Tang, S.; Riley, W.W. Partial hydrolysis of soybean oil by phospholipase A1 (Lecitase Ultra). Food Chem. 2010, 121, 1066–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, M.; Song, K.; Wang, L.; Han, X.; Tang, S.; Wang, Y. Separation of diacylglycerols from enzymatically hydrolyzed soybean oil by molecular distillation. Sep. Purif. Technol. 2010, 75, 114–120. [Google Scholar] [CrossRef]
- Goñi, M.L.; Pacheco, C.; Constenla, D.T.; Carelli, A.A. Solvent-free enzymatic hydrolysis of non-polar lipids in crude sunflower lecithin using phospholipase A1 (Lecitase® Ultra). Biocatal. Biotransform. 2018, 36, 341–351. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Zhao, M. Production of palm oil-based diacylglycerol using Lecitase Ultra-catalyzed glycerolysis and molecular distillation. Food Sci. Biotechnol. 2014, 23, 365–371. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Hu, C.; Cao, Q.; Yang, X.; Zhao, M. Preparation of diacylglycerol-enriched oil from free fatty acids using Lecitase Ultra-catalyzed esterification. J. Am. Oil Chem. Soc. 2011, 88, 1557–1565. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Zhang, Q.; Zhao, M. Fast synthesis of 1,3-DAG by Lecitase® Ultra-catalyzed esterification in solvent-free system. Eur. J. Lipid Sci. Technol. 2011, 113, 973–979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Han, K.L.; Park, J. (S)-selective dynamic kinetic resolution of allylic alcohols by enzyme-metal bicatalysis. Bull. Korean Chem. Soc. 2007, 28, 2096–2098. [Google Scholar]
- Baeza-Jiménez, R.; González-Rodríguez, J.; Kim, I.H.; García, H.S.; Otero, C. Use of immobilized phospholipase A1-catalyzed acidolysis for the production of structured phosphatidylcholine with an elevated conjugated linoleic acid content. Grasas y Aceites 2012, 63, 44–52. [Google Scholar]
- Zhao, T.; No, D.S.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef]
- Xi, X.; Feng, X.; Shi, N.; Ma, X.; Lin, H.; Han, Y. Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. J. Mol. Catal. B Enzym. 2016, 126, 46–55. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Effect of the immobilization protocol in the activity, stability, and enantioselectivity of Lecitase® Ultra. J. Mol. Catal. B Enzym. 2007, 47, 99–104. [Google Scholar] [CrossRef]
- Mishra, M.K.; Harini, M.; Kumaraguru, T.; Lakshmi Prasanna, T.; Fadnavis, N.W. A porous vessel bioreactor for gel entrapped biocatalysts: Kinetic resolution of trans-methyl (4-methoxyphenyl)glycidate by Lecitase® Ultra in gelatin organogel (Gelozyme). J. Mol. Catal. B Enzym. 2011, 71, 56–62. [Google Scholar] [CrossRef]
- Kumaraguru, T.; Harini, T.; Basetty, S. Immobilization of Lecitase® Ultra on recyclable polymer support: Application in resolution of trans-methyl (4-methoxyphenyl) glycidate in organic solvents. Tetrahedron Asymmetry 2017, 28, 1612–1617. [Google Scholar] [CrossRef]
- Mishra, M.K.; Kumaraguru, T.; Sheelu, G.; Fadnavis, N.W. Lipase activity of Lecitase® Ultra: Characterization and applications in enantioselective reactions. Tetrahedron Asymmetry 2009, 20, 2854–2860. [Google Scholar] [CrossRef]
- Leśniarek, A.; Chojnacka, A.; Gładkowski, W. Application of Lecitase® Ultra-catalyzed hydrolysis to the kinetic resolution of (E)-4-phenylbut-3-en-2-yl esters. Catalysts 2018, 8, 423. [Google Scholar] [CrossRef] [Green Version]
- Leśniarek, A.; Chojnacka, A.; Drozd, R.; Szymańska, M.; Gładkowski, W. Free and immobilized LecitaseTM Ultra as the biocatalyst in the kinetic resolution of (E)-4-arylbut-3-en-2-yl esters. Molecules 2020, 25, 1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of different enzyme immobilization strategies to improve enzyme performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Adlercreutz, P. Immobilisation and application of lipases in organic media. Chem. Soc. Rev. 2013, 42, 6406–6436. [Google Scholar] [CrossRef] [Green Version]
- Di Cosimo, R.; Mc Auliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Noreen, S.; Shah, S.Z.H.; Bharagava, R.N.; Iqbal, H.M.N. Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal. Biotransform. 2019, 37, 159–182. [Google Scholar] [CrossRef]
- Alves, J.S.; Garcia-Galan, C.; Danelli, D.; Paludo, N.; Barbosa, O.; Rodrigues, R.C.; Fernandez-Lafuente, R. Use of Lecitase-Ultra immobilized on styrene-divinylbenzene beads as catalyst of esterification reactions: Effects of ultrasounds. Catal. Today 2015, 255, 27–32. [Google Scholar] [CrossRef]
- Rueda, N.; Dos Santos, J.C.S.; Torres, R.; Ortiz, C.; Barbosa, O.; Fernandez-Lafuente, R. Improved performance of lipases immobilized on heterofunctional octyl-glyoxyl agarose beads. RSC Adv. 2015, 5, 11212–11222. [Google Scholar] [CrossRef] [Green Version]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzyme Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Arana-Peña, S.; Mendez-Sanchez, C.; Rios, N.S.; Ortiz, C.; Gonçalves, L.R.B.; Fernandez-Lafuente, R. New applications of glyoxyl-octyl agarose in lipases co-immobilization: Strategies to reuse the most stable lipase. Int. J. Biol. Macromol. 2019, 131, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.B.d.; Morais Júnior, W.G.d.; Silva, C.V.d.; Vieira, A.T.; Batista, A.C.F.; Faria, A.M.d.; Assunção, R.M.N. Preparation and characterization of cellulose triacetate as support for Lecitase Ultra immobilization. Molecules 2017, 22, 1930. [Google Scholar] [CrossRef] [Green Version]
- Baeza-Jiménez, R.; Noriega-Rodríguez, J.A.; García, H.S.; Otero, C. Structured phosphatidylcholine with elevated content of conjugated linoleic acid: Optimization by response surface methodology. Eur. J. Lipid Sci. Technol. 2012, 114, 1261–1267. [Google Scholar] [CrossRef]
- Gan, L.J.; Wang, X.Y.; Yang, D.; Zhang, H.; Shin, J.A.; Hong, S.T.; Park, S.H.; Lee, K.T. Emulsifying properties of lecithin containing different fatty acids obtained by immobilized Lecitase Ultra-catalyzed reaction. J. Am. Oil Chem. Soc. 2014, 91, 579–590. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; Zhao, Q.; Cui, C.; Fu, M.; Zhao, M. Immobilisation of Lecitase® Ultra for production of diacylglycerols by glycerolysis of soybean oil. Food Chem. 2012, 134, 301–307. [Google Scholar] [CrossRef]
- Liu, N.; Fu, M.; Wang, Y.; Zhao, Q.; Sun, W.; Zhao, M. Immobilization of Lecitase® Ultra onto a novel polystyrene DA-201 resin: Characterization and biochemical properties. Appl. Biochem. Biotechnol. 2012, 168, 1108–1120. [Google Scholar] [CrossRef]
- Baeza-Jiménez, R.; López-Martínez, L.X.; Otero, C.; Kim, I.H.; García, H.S. Enzyme-catalysed hydrolysis of phosphatidylcholine for the production of lysophosphatidylcholine. J. Chem. Technol. Biotechnol. 2013, 88, 1859–1863. [Google Scholar] [CrossRef]
- Gonçalves, K.M.; Sutili, F.K.; Júnior, I.I.; Flores, M.C.; Soter De Mariz, E.; Miranda, L.; Leal, I.C.R.; Cordeiro, Y.; Luque, R.; De Souza, R.O.M.A. A comprehensive study on the activity and deactivation of immobilized Lecitase Ultra in esterifications of food waste streams to monoacylglycerols. ChemSusChem 2013, 6, 872–879. [Google Scholar] [CrossRef]
- Yu, D.; Jiang, L.; Li, Z.; Shi, J.; Xue, J.; Kakuda, Y. Immobilization of phospholipase A1 and its application in soybean oil degumming. J. Am. Oil Chem. Soc. 2012, 89, 649–656. [Google Scholar] [CrossRef]
- Sheelu, G.; Kavitha, G.; Fadnavis, N.W. Efficient immobilization of Lecitase in gelatin hydrogel and degumming of rice bran oil using a spinning basket reactor. J. Am. Oil Chem. Soc. 2008, 85, 739–748. [Google Scholar] [CrossRef]
- Gonçalves, K.M.; Junior, I.I.; Papadimitriou, V.; Zoumpanioti, M.; Leal, I.C.R.; De Souza, R.O.M.A.; Cordeiro, Y.; Xenakis, A. Nanoencapsulated Lecitase Ultra and Thermomyces lanuginosus lipase, a comparative structural study. Langmuir 2016, 32, 6746–6756. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Z.; Fernandez-Lorente, G.; Palomo, J.M.; Guisan, J.M.; Fernandez-Lafuente, R. Asymmetric hydrolysis of dimethyl 3-phenylglutarate catalyzed by Lecitase® Ultra. Effect of the immobilization protocol on its catalytic properties. Enzyme Microb. Technol. 2008, 43, 531–536. [Google Scholar] [CrossRef]
- Fernandez-Lorente, G.; Filice, M.; Terreni, M.; Guisan, J.M.; Fernandez-Lafuente, R.; Palomo, J.M. Lecitase® Ultra as regioselective biocatalyst in the hydrolysis of fully protected carbohydrates. Strong modulation by using different immobilization protocols. J. Mol. Catal. B Enzym. 2008, 51, 110–117. [Google Scholar] [CrossRef]
- Pinheiro, M.P.; Monteiro, R.R.C.; Silva, F.F.M.; Lemos, T.L.G.; Fernandez-Lafuente, R.; Gonçalves, L.R.B.; dos Santos, J.C.S. Modulation of Lecitase properties via immobilization on differently activated Immobead-350: Stabilization and inversion of enantiospecificity. Process. Biochem. 2019, 87, 128–137. [Google Scholar] [CrossRef]
- Drozd, R.; Szymańska, M.; Rakoczy, R.; Junka, A.; Szymczyk, P.; Fijałkowski, K. Functionalized magnetic bacterial cellulose beads as carrier for Lecitase® Ultra immobilization. Appl. Biochem. Biotechnol. 2019, 187, 176–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenna, E.; Caraccia, N.; Fuganti, C.; Fuganti, D.; Grasselli, P. Enantioselective synthesis of β-substituted butyric acid derivatives via orthoester Claisen rearrangement of enzymatically resolved allylic alcohols: Application to the synthesis of (R)-(−)-baclofen. Tetrahedron Asymmetry 1997, 8, 3801–3805. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Grasselli, P.; Serra, S. Enzyme-mediated synthesis of (S)- and (R)-Verapamil. Eur. J. Org. Chem. 2001, 1349–1357. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Gliszczyńska, A.; Czarnecka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Maciejewska, G.; Białońska, A. Chiral δ-iodo-γ-lactones derived from cuminaldehyde, 2,5-dimethylbenzaldehyde and piperonal: Chemoenzymatic synthesis and antiproliferative activity. Tetrahedron Asymmetry 2016, 27, 227–237. [Google Scholar] [CrossRef]
- Gładkowski, W.; Włoch, A.; Pawlak, A.; Sysak, A.; Białońska, A.; Mazur, M.; Mituła, P.; Maciejewska, G.; Obmińska-Mrukowicz, B.; Kleszczyńska, H. Preparation of enantiomeric β-(2′,5′-dimethylphenyl)bromolactones, their antiproliferative activity and effect on biological membranes. Molecules 2018, 23, 3035. [Google Scholar] [CrossRef] [Green Version]
- Gumel, A.M.; Annuar, M.S.M. Thermomyces lanuginosus lipase-catalyzed synthesis of natural flavor esters in a continuous flow microreactor. 3 Biotech. 2016, 6, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Pawlak, A.; Obmińska-Mrukowicz, B.; Białońska, A.; Poradowski, D.; Drynda, A.; Urbaniak, M. Synthesis and anticancer activity of novel halolactones with β-aryl substituents from simple aromatic aldehydes. Tetrahedron 2013, 69, 10414–10423. [Google Scholar] [CrossRef]
- Yamane, T. Importance of moisture content control for enzymatic reactions in organic solvents: A novel concept of “microaqueous”. Biocatal. Biotransform. 1988, 2, 1–9. [Google Scholar] [CrossRef]
- Colombo, G.; Ottolina, G.; Carrea, G. Modelling of enzyme properties in organic solvents. Mon. Chem. 2000, 131, 527–547. [Google Scholar] [CrossRef]
- Kumar, A.; Dhar, K.; Kanwar, S.S.; Arora, P.K. Lipase catalysis in organic solvents: Advantages and applications. Biol. Proced. Online 2016, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Kamal, Z.; Yedavalli, P.; Deshmukh, M.V.; Rao, N.M. Lipase in aqueous-polar organic solvents: Activity, structure, and stability. Protein Sci. 2013, 22, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Kazlauskas, R. Hydrolysis and formation of carboxylic acid and alcohol derivatives. In Organic Synthesis Using Biocatalysis; Goswami, A., Stewart, J.D., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 127–148. [Google Scholar]
- Laane, C.; Boeren, S.; Vos, K.; Veeger, C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. [Google Scholar] [CrossRef]
- Paravidino, M.; Hanefeld, U. Enzymatic acylation: Assessing the greenness of different acyl donors. Green Chem. 2011, 13, 2651–2657. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Aboul-Enein, H.Y. Application of lipases in kinetic resolution of racemates. Chirality 2005, 17, 1–15. [Google Scholar] [CrossRef]
- Kawasaki, M.; Goto, M.; Kawabata, S.; Kometani, T. The effect of vinyl esters on the enantioselectivity of the lipase-catalysed transesterification of alcohols. Tetrahedron Asymmetry 2001, 12, 585–596. [Google Scholar] [CrossRef]
- Chojnacka, A.; Obara, R.; Wawrzeńczyk, C. Kinetic resolution of racemic secondary aliphatic allylic alcohols in lipase-catalyzed transesterification. Tetrahedron Asymmetry 2007, 18, 101–107. [Google Scholar] [CrossRef]
- Chênevert, R.; Pelchat, N.; Morin, P. Lipase-mediated enantioselective acylation of alcohols with functionalized vinyl esters: Acyl donor tolerance and applications. Tetrahedron Asymmetry 2009, 20, 1191–1196. [Google Scholar] [CrossRef]
- Netto, C.G.C.M.; Andrade, L.H.; Toma, H.E. Enantioselective transesterification catalysis by Candida antarctica lipase immobilized on superparamagnetic nanoparticles. Tetrahedron Asymmetry 2009, 20, 2299–2304. [Google Scholar] [CrossRef]
- Dong, F.; Li, L.; Lin, L.; He, D.; Chen, J.; Wei, W.; Wei, D. Transesterification synthesis of chloramphenicol esters with the lipase from Bacillus amyloliquefaciens. Molecules 2017, 22, 1523. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Schurig, V. Lipase-catalyzed irreversible transesterification of secondary alcohols using isopropenyl acetate. Monatshefte für Chemie 2003, 134, 1151–1157. [Google Scholar] [CrossRef] [Green Version]
- Ghanem, A.; Schurig, V. Lipase-catalyzed irreversible transesterification of 1-(2-furyl)ethanol using isopropenyl acetate. Chirality 2001, 13, 118–123. [Google Scholar] [CrossRef]
- Ghanem, A. The utility of cyclodextrins in lipase-catalyzed transesterification in organic solvents: Enhanced reaction rate and enantioselectivity. Org. Biomol. Chem. 2003, 1, 1282–1291. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Passoni, M.; Serra, S. Enantioselective synthesis of benzylic stereocentres via Claisen rearrangement of enantiomerically pure allylic alcohols: Preparation of (R)- and (S)-3-methyl-2-phenylbutylamine. Tetrahedron Asymmetry 2003, 14, 2401–2406. [Google Scholar] [CrossRef]
- Brenna, E.; Dei Negri, C.; Fuganti, C.; Gatti, F.G.; Serra, S. Enantioselective synthesis of cis-7-methoxy-calamenene via Claisen rearrangement of an enzymatically resolved allyl alcohol. Tetrahedron Asymmetry 2004, 15, 335–340. [Google Scholar] [CrossRef]
- Gładkowski, W.; Gliszczyńska, A.; Siepka, M.; Czarnecka, M.; Maciejewska, G. Kinetic resolution of (E)-4-(2′,5′-dimethylphenyl)-but-3-en-2-ol and (E)-4-(benzo[d][1′,3′]dioxol-5′-yl)-but-3-en-2-ol through lipase-catalyzed transesterification. Tetrahedron Asymmetry 2015, 26, 702–709. [Google Scholar] [CrossRef]
- Gładkowski, W.; Skrobiszewski, A.; Mazur, M.; Siepka, M.; Białońska, A. Convenient chemoenzymatic route to optically active β-aryl-δ-iodo-γ-lactones and β-aryl-γ-iodo-δ-lactones with the defined configurations of stereogenic centers. Eur. J. Org. Chem. 2015, 2015, 605–615. [Google Scholar] [CrossRef]
- Mazur, M. Synthesis of halolactones with methoxyphenyl ring. Przem. Chem. 2011, 5, 918–922. [Google Scholar]
- Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Gniłka, R. Improving of hydrolases biosythesis by solid-state fermentation of Penicillium camemberti on rapeseed cake. Catalysts 2018, 8, 28. [Google Scholar] [CrossRef] [Green Version]
- Skrobiszewski, A.; Gładkowski, W.; Maciejewska, G.; Wawrzeńczyk, C. Chemoenzymatic synthesis of trans-β-aryl-δ-hydroxy-γ-lactones and enzymatic kinetic resolution of their racemic mixtures. Molecules 2016, 21, 1552. [Google Scholar] [CrossRef] [PubMed] [Green Version]




 | ||||||
|---|---|---|---|---|---|---|
| Entry | Biocatalyst | t [h] | c [%] 1 | ees [%] | eep [%] | E2 |
| 1 | LU-ALG | 48 | 48 | 90 | 98 | >200 |
| 72 | 49 | 91 | 93 | 83 | ||
| 2 | LU-MBC | 48 | 27 | 36 | >99 | >200 |
| 72 | 49 | 92 | 98 | >200 | ||
| 3 | LU-CNBr | 4 | 47 | 89 | >99 | >200 |
| 8 | 49 | 94 | >99 | >200 | ||
| 24 | 50 | 97 | >99 | >200 | ||
| 4 | LU-DAX | 2 | 48 | 87 | 96 | 140 |
| 4 | 52 | 94 | 88 | 55 | ||
 | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Substrate | Ar | t [h] | c [%]1 | ees [%] | eep [%] | E2 |
| 1 | 1b |  | 6 | 48 | 91 | >99 | >200 |
| 2 | 1c |  | 8 | 49 | 95 | >99 | >200 |
| 3 | 1d |  | 24 | 50 | 90 | 90 | 58 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leśniarek, A.; Chojnacka, A.; Drozd, R.; Szymańska, M.; Gładkowski, W. Enantioselective Transesterification of Allyl Alcohols with (E)-4-Arylbut-3-en-2-ol Motif by Immobilized Lecitase™ Ultra. Catalysts 2020, 10, 798. https://doi.org/10.3390/catal10070798
Leśniarek A, Chojnacka A, Drozd R, Szymańska M, Gładkowski W. Enantioselective Transesterification of Allyl Alcohols with (E)-4-Arylbut-3-en-2-ol Motif by Immobilized Lecitase™ Ultra. Catalysts. 2020; 10(7):798. https://doi.org/10.3390/catal10070798
Chicago/Turabian StyleLeśniarek, Aleksandra, Anna Chojnacka, Radosław Drozd, Magdalena Szymańska, and Witold Gładkowski. 2020. "Enantioselective Transesterification of Allyl Alcohols with (E)-4-Arylbut-3-en-2-ol Motif by Immobilized Lecitase™ Ultra" Catalysts 10, no. 7: 798. https://doi.org/10.3390/catal10070798
APA StyleLeśniarek, A., Chojnacka, A., Drozd, R., Szymańska, M., & Gładkowski, W. (2020). Enantioselective Transesterification of Allyl Alcohols with (E)-4-Arylbut-3-en-2-ol Motif by Immobilized Lecitase™ Ultra. Catalysts, 10(7), 798. https://doi.org/10.3390/catal10070798