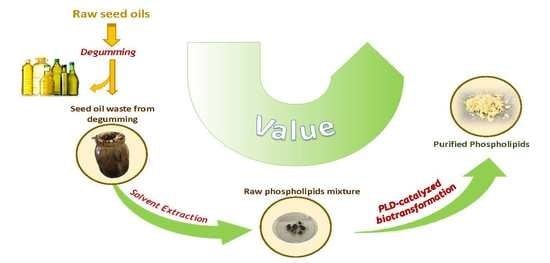
Valorization of Corn Seed Oil Acid Degumming Waste for Phospholipids Preparation by Phospholipase D-Mediated Processes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Biomass Treatment
2.2. Characterization of Fraction BF2-S
2.3. Enrichment of PLs Mixture
2.3.1. PS-Enriched BF2-S Preparation
2.3.2. PE-Enriched BF2-S Preparation
2.3.3. PG-Enriched BF2-S Preparation
3. Materials and Methods
3.1. 31P NMR Sample Preparation
3.2. Thin Layer Chromatography (TLC)
3.3. High Performance Liquid Chromatography (HPLC)
3.4. Column Chromatography
3.5. Gas Chromatography
3.6. Electrospray Ionization Mass Spectrometry
3.7. PLD Preparation
3.7.1. Microorganism Fermentation
3.7.2. Precipitation and Dialysis
3.8. PLD Activity Determination
3.9. Synthesis of Phosphatidyl-p-Nitrophenol
3.9.1. Preparation of PA
3.9.2. Transformation of PA in PpNP
3.10. Biomass Recovery
3.11. Corn Waste Gums Treatment
3.12. Determination of Phosphorous Content
3.13. Preparation of PX-enriched BF2-S
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dowhan, W.; Bogdanov, M.; Mileykovskaja, E. Functional Roles of Lipids in Membranes, Biochemistry of Lipids, Lipoproteins and Membranes, 5th ed.; Elsevier Science: San Diego, CA, USA, 2008; pp. 1–37. [Google Scholar]
- D’Arrigo, P.; Servi, S. Synthesis of Lysophospholipids. Molecules 2010, 15, 1354–1377. [Google Scholar] [CrossRef] [Green Version]
- David, F.; Medvedovici, A.; Sandra., P. Oils Fat and Grasses: Supercritical Fluid Chromatography; Encyclopedia of Separation Science: Kortrijk, Belgium, 2000; pp. 3567–3575. [Google Scholar]
- Robert, C.; Couedelo, L.; Vayesse, C.; Michalski, M.C. Vegetable lecithins: A review of their compositional diversity, impact on lipid metabolism and potential in cardiometabolic disease prevention. Biochimie 2020, 169, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Trziszka, T.; Wawrzeńczyk, C. Isolation of Pure Phospholipid Fraction from Egg Yolk. J. Am. Oil Chem. Soc. 2012, 89, 179–182. [Google Scholar] [CrossRef]
- Weber, E.J. Compositions of commercial corn and soybean lecithins. J. Am. Oil Chem. Soc. 1981, 58, 898–901. [Google Scholar] [CrossRef]
- Hoogevest, P.; Wendel, A. The use of natural and synthetic phospholipids as pharmaceutical excipients. Eur. J. Lipid Sci. Technol. 2014, 116, 1088–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, Y.C.; Yadav, M.; Upadhyay, S.N. Latest advances in degumming feedstock oils for large-scale biodiesel production. Biofuels Bioprod. Biorefin. 2019, 13, 174–191. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M. Practical Guide to Vegetable Oil Processing, 2nd ed.; Elsevier Science: Linwood, New Zealand, 2017; pp. 41–78. [Google Scholar]
- Sein, A.; Hitchman, T.; Dayton, C.L. Industrial Enzyme Applications. In Enzymes in Vegetable Oil Degumming Processes; Vogel, A., May, O., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 323–350. [Google Scholar] [CrossRef]
- Kullenberg, D.; Taylor, L.A.; Schneider, M.; Massing, U. Health effects of dietary phospholipids. Lipid. Health Dis. 2012, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- Gładkowski, W.; Kiełbowicz, G.; Chojnacka, A.; Gil, M.; Trziszka, T.; Dobrzański, Z.; Wawrzeńczyk, C. Fatty acid composition of egg yolk phospholipid fractions following feed supplementation of Lohmann Brown hens with humic-fat preparations. Food Chem. 2011, 126, 1013–1018. [Google Scholar] [CrossRef]
- Van Nieuwenhuyzen, W.; Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- Bandu, R.; Mok, H.J.; Kim, K.P. Phospholipids as cancer biomarkers: Mass spectrometry-based analysis. Mass Spectrom. Rev. 2018, 37, 107–138. [Google Scholar] [CrossRef]
- Gliszczyńska, A.; Niezgoda, N.; Gładkowski, W.; Świtalska, M.; Wietrzyk, J. Isoprenoid-phospholipid conjugates as potential therapeutic agents: Synthesis, characterization and antiproliferative studies. PLoS ONE 2017, 12, e0172238. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Ciccone, S.; D’Arrigo, P.; Viani, F.; Sorge, R.; Novelli, G.; Patrizi, P.; Desideri, A.; Biocca, S. Design of a novel LOX-1 receptor antagonist mimicking the natural substrate. Biochem. Biophys. Res. Commun. 2013, 438, 340–345. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, F.; Allegretti, C.; Tessaro, D.; Carata, E.; Citti, C.; Vergaro, V.; Nobile, C.; Cannazza, G.; D’Arrigo, P.; Mele, A.; et al. Biocatalytic Synthesis of Phospholipids and Their Application as Coating Agents for CaCO3 Nano-crystals: Characterization and Intracellular Localization Analysis. Chem. Sel. 2016, 1, 6507–6514. [Google Scholar] [CrossRef]
- Alavi, M.; Karimi, N.; Safaei, M. Application of Various Types of Liposomes in Drug Delivery Systems. Adv. Pharm. Bull. 2017, 7, 3–9. [Google Scholar] [CrossRef]
- Vertzoni, M.; Markopoulos, C.; Symillides, M.; Goumas, C. Luminal lipid phases after administration of a triglyceride solution of danazol in the fed state and their contribution to the flux of danazol across Caco-2 cell monolayers. Mol. Pharm. 2012, 9, 1189–1198. [Google Scholar] [CrossRef]
- Joensuu, M.; Wallis, T.P.; Saber, H.S.; Meunier, F.A. Phospholipases in neuronal function: A role in learning and memory. J. Neurochem. 2020, 153, 1–32. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Cerioli, L.; Chiappe, C.; Panzeri, W.; Tessaro, D.; Mele, A. Improvements in the enzymatic synthesis of phosphatidylserine employing ionic liquids. J. Mol. Catal. B Enzym. 2012, 84, 132–135. [Google Scholar] [CrossRef]
- Kim, H.Y.; Huang, B.X.; Spector, A.A. Phosphatidylserine in the brain: Metabolism and function. Prog. Lipid Res. 2014, 56, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mozzi, R.; Buratta, S.; Goracci, G. Metabolism and function of phosphatidylserine in mammalian brain. Neurochem. Res. 2003, 28, 195–214. [Google Scholar] [CrossRef]
- Juneja, L.; Kazuoka, T.; Yamane, T.; Shimizu, S. Kinetic evaluation of conversion of phosphatidylcholine to phosphatidylethanolamine by phospholipase D from different sources. Biochim. Biophys. Acta 1988, 960, 334–341. [Google Scholar] [CrossRef]
- Van Der Veen, J.N.; Lingrell, S.; McCloskey, N.; LeBlond, N.D.; Galleguillos, D.; Zhao, Y.; Curtis, J.M.; Sipione, S.; Fullerton, M.D.; Vance, D.E.; et al. A role for phosphatidylcholine and phosphatidylethanolamine in hepatic insulin signaling. FASEB J. 2019, 33, 5045–5057. [Google Scholar] [CrossRef] [PubMed]
- Mazela, J.; Merritt, T.A.; Gadzinowski, J.; Sinha, S. Evolutiom of pulmonary surfactants for the treatment of neonatal respiratory distress syndrome and paediatric lung diseases. Acta Paediatr. 2006, 95, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Fasoli, E.; Arnone, A.; Caligiuri, A.; D’Arrigo, P.; de Ferra, L.; Servi, S. Tin-mediated synthesis of lyso-phospholipids. Organ. Biomol. Chem. 2006, 4, 2974–2978. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Fasoli, E.; Pedrocchi-Fantoni, G.; Rossi, C.; Saraceno, C.; Servi, S.; Tessaro, D. A practical selective synthesis of mixed short/long chains glycerophosphocholines. Chem. Phys. Lipid. 2007, 147, 113–118. [Google Scholar] [CrossRef]
- Gagnon, M.C.; Dautrey, S.; Bertrand, X.; Auger, M.; Paquin, J.F. A flexible synthetic approach to phosphatidylglycerols. Eur. J. Org. Chem. 2017, 43, 6401–6407. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Servi, S. Using phospholipases for phospholipid modification. Trends Biotechnol. 1997, 15, 90–96. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, M.; Xu, W.; Zhang, W.; Zhang, T.; Guang, C.; Mu, W. Microbial phospholipase D: Identification, modification and application. Trends Food Sci. Technol. 2020, 96, 145–156. [Google Scholar] [CrossRef]
- Anthonsen, T.; D’Arrigo, P.; Pedrocchi-Fantoni, G.; Secundo, F.; Servi, S.; Sundby, E. Phospholipids hydrolysis in organic solvents catalysed by immobilised phospholipase C. J. Mol. Catal. B Enzym. 1999, 6, 125–132. [Google Scholar] [CrossRef]
- Chen, W.; Guo, W.; Gao, F. Phospholipase A1-Catalysed Synthesis of Docosahexaenoic Acid-Enriched Phosphatidylcholine in Reverse Micelles System. Appl. Biochem. Biotechnol. 2017, 182, 1037–1052. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; White, P.J. Lipids of the Kernel. In Corn: Chemistry and Technology; Serna-Saldivar, S.O., Ed.; AACC International Press: St. Paul, MN, USA, 2018; pp. 337–368. [Google Scholar] [CrossRef]
- Sampaio, K.A.; Zyaykina, N.; Uitterhaegen, E.; De Greyt, W.; Verhé, R.; de Almeida Meirelles, A.J.; Stevens, C.V. Enzymatic degumming of corn oil using phospholipase C from a selected strain of Pichia pastoris. LWT 2019, 107, 145–150. [Google Scholar] [CrossRef]
- Sotirhos, N.; Herslof, B.; Kenne, L. Quantitative analysis of phospholipids by 31P-NMR. J. Lipid Res. 1986, 27, 386–392. [Google Scholar] [PubMed]
- Estrada, R.; Stolowich, N.; Yappert, M.C. Influence of temperature on 31P NMR chemical shifts of phospholipids and their metabolites I. In chloroform–methanol–water. Anal. Biochem. 2008, 380, 41–50. [Google Scholar] [CrossRef]
- Servi, S. Phospholipases as Synthetic Catalysts. In Biocatalysis—From Discovery to Application. Topics in Current Chemistry; Fessner, W.D., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 127–158. [Google Scholar] [CrossRef]
- Bossi, L.; D’Arrigo, P.; Pedrocchi-Fantoni, G.; Mele, A.; Servi, S.; Leiros, I. The substrate requirements of phospholipase D. J. Mol. Catal. B Enzym. 2001, 11, 433–438. [Google Scholar] [CrossRef]
- Cardillo, R.; D’Arrigo, P.; De Ferra, L.; Piergianni, V.; Scarcelli, D.; Servi, S. A simple assay for the quantitative evaluation of Phospholipase D activity. Biotechnol. Tech. 1993, 7, 795–798. [Google Scholar] [CrossRef]
- Comfurius, P.; Zwaal, R.F.A. The enzymatic synthesis of phosphatidylserine and purification by CM-cellulose column chromatography. BBA Lipid. Lipid Metab. 1977, 488, 36–42. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Pedrocchi-Fantoni, G.; Servi, S.; Strini, A. Enzyme-mediated synthesis of two diastereoisomeric forms of phosphatidylglycerol and of diphosphatidylglycerol (cardiolipin). J. Chem. Soc. Perkin Trans. 1996, 1, 2657–2660. [Google Scholar] [CrossRef]
- Suzuri, K.; Yamamoto, Y.; Hosokawa, M.; Miyashita, K. Effect of α-tocopherol on the synthesis of phosphatidylglycerol catalyzed by phospholipase D in an aqueous system. Biotechnol. Lett. 2009, 31, 719–723. [Google Scholar] [CrossRef]
- Piazza, G.J.; Marmer, W.N. Conversion of phosphatidylcholine to phosphatidylglycerol with phospholipase D and glycerol. J. Am. Oil Chem. Soc. 2007, 84, 645–651. [Google Scholar] [CrossRef]
- Christie, W.W. Gas chromatography-mass spectrometry methods for structural analysis of fatty acids. Lipids 1998, 33, 343–353. [Google Scholar] [CrossRef] [PubMed]
- D’Arrigo, P.; Piergianni, V.; Scarcelli, D.; Servi, S. A spectrophotometric assay for phospholipase D. Anal. Chim. Acta 1995, 304, 249–254. [Google Scholar] [CrossRef]







| Sample | Phosphorus Quantification (mg/kg) |
|---|---|
| Waste corn gums | 1093 |
| BF2-S fraction | 1619 |
| Identified Phospholipids | Chains sn-1/sn-2 | Positive m/z | Negative m/z |
|---|---|---|---|
| PC-A | 16:0/18:2 | 757.8 + 23 | - |
| PC-B | 18:2/18:1 | 783.8 + 23 | - |
| 2-LPC-A | 16:0 | 495.4 + 23 | - |
| 2-LPC-B | 18:2 | 519.5 + 23 | - |
| PG-A | 16:0/18:2 | - | 745.6 |
| PG-B | 18:2/18:0 | - | 771.6 |
| 2-LPG-B | 18:2 | - | 507.2 |
| PA-A | 16:0/18:2 | - | 669.8 |
| PA-B | 18:2/18:1 | - | 695.8 |
| 2-LPA-A | 16:0 | - | 409.0 |
| 2-LPA-B | 18:2 | - | 433.0 |
| PI-A | 16:0/18:2 | - | 833.6 |
| PI-B | 18:2/18:0 | - | 771.6 |
| 2-LPI-A | 16:0 | - | 571.3 |
| 2-LPI-B | 18:2 | - | 595.3 |
| PE-A | 16:0/18:2 | - | 714.6 |
| PE-B | 18:2/18:0 | - | 740.6 |
| Fatty Acid | % in Raw Corn Oil | % in BF2-S Fraction | |
|---|---|---|---|
| palmitic | C16:0 | 11.5 | 26.3 |
| palmitoleic | C16:1 | 0.17 | - |
| stearic | C18:0 | 1.72 | 2.0 |
| oleic | C18:1 | 29.7 | 29.2 |
| linoleic | C18:2 | 54.8 | 39.5 |
| linolenic | C18:3 | 1.03 | - |
| arachidic | C20:0 | 0.39 | 0.30 |
| eicosenoic | C20:1 | 0.33 | - |
| eicosadienoic | C20:2 | 0.001 | - |
| behenic acid | C22:0 | 0.11 | 0.38 |
| Phospholipid | BF2-S Fraction | PS-Enriched BF2-S | PE-Enriched BF2-S | PG-Enriched BF2-S |
|---|---|---|---|---|
| PA | 12% | 23% | 16% | 15% |
| PG | 24% | - | 22% | 36% |
| PI | 15% | 22% | 19% | 25% |
| PE | 5.4% | - | 15% | - |
| PC | 17% | - | - | - |
| LPC | 13% | 0.2% | 2.0% | 5.5% |
| PS | - | 9.4% | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegretti, C.; Bono, A.; D’Arrigo, P.; Denuccio, F.; De Simeis, D.; Di Lecce, G.; Serra, S.; Tessaro, D.; Viola, M. Valorization of Corn Seed Oil Acid Degumming Waste for Phospholipids Preparation by Phospholipase D-Mediated Processes. Catalysts 2020, 10, 809. https://doi.org/10.3390/catal10070809
Allegretti C, Bono A, D’Arrigo P, Denuccio F, De Simeis D, Di Lecce G, Serra S, Tessaro D, Viola M. Valorization of Corn Seed Oil Acid Degumming Waste for Phospholipids Preparation by Phospholipase D-Mediated Processes. Catalysts. 2020; 10(7):809. https://doi.org/10.3390/catal10070809
Chicago/Turabian StyleAllegretti, Chiara, Andrea Bono, Paola D’Arrigo, Francesca Denuccio, Davide De Simeis, Giuseppe Di Lecce, Stefano Serra, Davide Tessaro, and Mariacristina Viola. 2020. "Valorization of Corn Seed Oil Acid Degumming Waste for Phospholipids Preparation by Phospholipase D-Mediated Processes" Catalysts 10, no. 7: 809. https://doi.org/10.3390/catal10070809
APA StyleAllegretti, C., Bono, A., D’Arrigo, P., Denuccio, F., De Simeis, D., Di Lecce, G., Serra, S., Tessaro, D., & Viola, M. (2020). Valorization of Corn Seed Oil Acid Degumming Waste for Phospholipids Preparation by Phospholipase D-Mediated Processes. Catalysts, 10(7), 809. https://doi.org/10.3390/catal10070809