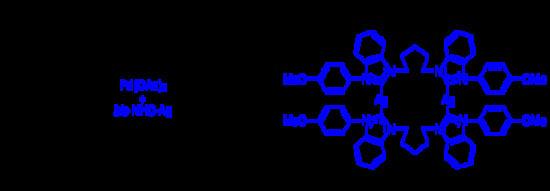
Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
3.2. Description of the Screening Experiments
3.2.1. General Procedures for Transfer Hydrogenation Reactions in Organic Solvent under N2
3.2.2. General Procedures for Transfer Hydrogenation Reactions in Aqueous Media under Air
3.3. Analytical Data of the Reduction Products
3.3.1. Transfer Hydrogenation Reactions in Organic Solvent under N2
3.3.2. Transfer Hydrogenation Reactions in Aqueous Media under Air
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herrmann, W.A. N-Heterocyclic Carbenes: A New Concept in Organometallic Catalysis. Angew. Chem. Int. Ed. 2002, 41, 1290–1309. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef] [PubMed]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Froese, R.D.J.; Lombardi, C.; Pompeo, M.; Rucker, R.P.; Organ, M.G. Designing Pd-N-Heterocyclic Carbene Complexes for High Reactivity and Selectivity for Cross-Coupling Applications. Acc. Chem. Res. 2017, 50, 2244–2253. [Google Scholar] [CrossRef] [PubMed]
- Peris, E. Smart N-Heterocyclic Carbene Ligands in Catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.; Nolan, S.P. Well-Defined N-Heterocyclic Carbenes–Palladium(II) Precatalysts for Cross-Coupling Reactions. Acc. Chem. Res. 2008, 41, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Monot, J.; Brahmi, M.M.; Ueng, S.-H.; Robert, C.; Murr, M.D.; Curran, D.P.; Malacria, M.; Fensterbank, L.; Lacôte, E. Suzuki–Miyaura Coupling of NHC-Boranes: A New Addition to the C–C Coupling Toolbox. Org. Lett. 2009, 11, 4914–4917. [Google Scholar] [CrossRef]
- Kose, O.; Saito, S. Cross-coupling reaction of alcohols for carbon–carbon bond formation using pincer-type NHC/Palladium catalysts. Org. Biomol. Chem. 2010, 8, 896–900. [Google Scholar] [CrossRef]
- Yong, B.S.; Nolan, S.P. Transition Metal-Carbene Complexes in Homogeneous Catalysis. Chemtracts: Org. Chem. 2003, 16, 205–227. [Google Scholar] [CrossRef]
- Sprengers, J.W.; Wassenaar, J.; Clement, N.D.; Cavell, K.J.; Elsevier, C.J. Palladium–(N-Heterocyclic Carbene) Hydrogenation Catalysts. Angew. Chem. Int. Ed. 2005, 44, 2026–2029. [Google Scholar] [CrossRef]
- Liu, L.-J.; Wang, F.; Shi, M. Synthesis of Chiral Bis(N-heterocyclic carbene) Palladium and Rhodium Complexes with 1,1′-Biphenyl Scaffold and Their Application in Asymmetric Catalysis. Organometallics 2009, 28, 4416–4420. [Google Scholar] [CrossRef]
- Brieger, G.; Nestrick, T.J. Catalytic Transfer Hydrogenation. Chem. Rev. 1974, 74, 567–580. [Google Scholar] [CrossRef]
- Backvall, J.-E. Transition metal hydrides as active intermediates in hydrogen transfer reactions. J. Organomet. Chem. 2002, 652, 105–111. [Google Scholar] [CrossRef]
- Gladiali, S.; Alberico, E. Asymmetric transfer hydrogenation: Chiral ligands and applications. Chem. Soc. Rev. 2006, 35, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Samec, J.S.M.; Backvall, J.-E.; Anderssonm, P.G.; Brandt, P. Mechanistic aspects of transition metal-catalyzed hydrogen transfer reactions. Chem. Soc. Rev. 2006, 35, 237–248. [Google Scholar] [CrossRef]
- Wang, D.; Astruc, D. The Golden Age of Transfer Hydrogenation. Chem. Rev. 2015, 115, 6621–6686. [Google Scholar] [CrossRef]
- Lorca, M.; Kuhn, D.; Kurosu, M. Selective reduction of aldehydes via BINOL–Zr complex. Tetrahedron Lett. 2001, 42, 6243–6246. [Google Scholar] [CrossRef]
- Selvam, P.; Sonavane, S.U.; Mohapatra, S.K.; Jayaram, R.V. Chemoselective Reduction of α,β-Unsaturated Carbonyls over Novel Mesoporous CoNMA Molecular Sieves under Hydrogen Transfer Conditions. Adv. Synth. Catal. 2004, 346, 542–544. [Google Scholar] [CrossRef]
- Miecznikowski, J.R.; Crabtree, R.H. Hydrogen Transfer Reduction of Aldehydes with Alkali-Metal Carbonates and Iridium NHC Complexes. Organometallics 2004, 23, 629–631. [Google Scholar] [CrossRef]
- Naskar, S.; Bhattacharjee, M. Ruthenium cationic species for transfer hydrogenation of aldehydes: Synthesis and catalytic properties of [(PPh3)2Ru(CH3CN)3Cl]+[A]- {A = BPh4 or ClO4} and structure of [(PPh3)2Ru(CH3CN)3Cl]+[BPh4]-. J. Organomet. Chem. 2005, 690, 5006–5010. [Google Scholar] [CrossRef]
- Baratta, W.; Siega, K.; Rigo, P. Fast and Chemoselective Transfer Hydrogenation of Aldehydes Catalyzed by a Terdentate CNN Ruthenium Complex [RuCl(CNN)(dppb)]. Adv. Synth. Catal. 2007, 349, 1633–1636. [Google Scholar] [CrossRef]
- Zweifel, T.; Naubron, J.V.; Büttner, T.; Ott, T.; Grützmacher, H. Ethanol as Hydrogen Donor: Highly Efficient Transfer Hydrogenations with Rhodium(I) Amides. Angew. Chem. Int. Ed. 2008, 47, 3245–3249. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Deraedt, C.; Ruiz, J.; Astruc, D. Sodium hydroxide-catalyzed transfer hydrogenation of carbonyl compounds and nitroarenes using ethanol or isopropanol as both solvent and hydrogen donor. J. Mol. Catal. A Chem. 2015, 400, 14–21. [Google Scholar] [CrossRef]
- Castellanos-Blanco, N.; Arévalo, A.; García, J.J. Nickel-Catalyzed Transfer Hydrogenation of Ketones Using Ethanol as Solvent and Hydrogen Donor. Dalton Trans. 2016, 45, 13604–13614. [Google Scholar] [CrossRef] [PubMed]
- Enthaler, S. Carbon Dioxide-The Hydrogen-Storage Material of the Future? ChemSusChem 2008, 1, 801–804. [Google Scholar] [CrossRef] [PubMed]
- Joó, F. Breakthroughs in hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen. ChemSusChem 2008, 1, 805–808. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.C.; Morris, D.J.; Wills, M. Hydrogen generation from formic acid and alcohols using homogeneous catalysts. Chem. Soc. Rev. 2010, 39, 81–88. [Google Scholar] [CrossRef]
- Boddien, A.; Gartner, F.; Federsel, C.; Sponholz, P.; Mellmann, D.; Jackstell, R.; Junge, H.; Beller, M. CO2-“Neutral” Hydrogen Storage Based on Bicarbonates and Formates. Angew. Chem. Int. Ed. 2011, 50, 6411–6414. [Google Scholar] [CrossRef]
- Bi, Q.-Y.; Lin, J.-D.; Liu, Y.-M.; He, H.-Y.; Huang, F.-Q.; Cao, Y. Dehydrogenation of Formic Acid at Room Temperature: Boosting Palladium Nanoparticle Efficiency by Coupling with Pyridinic Nitrogen-Doped Carbon. Angew. Chem. Int. Ed. 2016, 55, 11849–11853. [Google Scholar] [CrossRef]
- Cavell, K.J.; McGuinness, D.S. Redox processes involving hydrocarbylmetal (N-heterocyclic carbene) complexes and associated imidazolium salts: Ramifications for catalysis. Coord. Chem. Rev. 2004, 248, 671–681. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Green, M.J.; Cavell, K.J.; Skelton, B.W.; White, A.H. Synthesis and reaction chemistry of mixed ligand methylpalladium–carbene complexes. J. Organomet. Chem. 1998, 565, 165–178. [Google Scholar] [CrossRef]
- McGuinness, D.S.; Cavell, K.J.; Skelton, B.W.; White, A.H. Zerovalent Palladium and Nickel Complexes of Heterocyclic Carbenes: Oxidative Addition of Organic Halides, Carbon–Carbon Coupling Processes, and the Heck Reaction. Organometallics 1999, 18, 1596–1605. [Google Scholar] [CrossRef]
- Arnold, P.L.; Cloke, G.N.; Geldbach, T.; Hitchcock, P.B. Metal Vapor Synthesis as a Straightforward Rout to Group 10 Homoleptic Carbene Complexes. Organometallics 1999, 18, 3228–3233. [Google Scholar] [CrossRef]
- Cavell, K.J. N-Heterocyclic carbenes/imidazolium salts as substrates in catalysis: The catalytic 2-substitution and annulation of heterocyclic compounds. Dalton Trans. 2008, 6676–6685. [Google Scholar] [CrossRef]
- Clement, N.D.; Cavell, K.J.; Jones, C.; Elsevier, C.J. Oxidative Addition of Imidazolium Salts to Ni0 and Pd0: Synthesis and Structural Characterization of Unusually Stable Metal-Hydride Complexes. Angew. Chem. Int. Ed. 2004, 43, 1277–1279. [Google Scholar]
- Hauwert, P.; Maestri, G.; Sprengers, J.W.; Catellani, M.; Elsevier, C.J. Transfer Semihydrogenation of Alkynes Catalyzed by a Zero-Valent Palladium N-Heterocyclic Carbene Complex. Angew. Chem. Int. Ed. 2008, 47, 3223–3226. [Google Scholar] [CrossRef]
- Hauwert, P.; Boerleider, R.; Warsink, S.; Weigand, J.J.; Elsevier, C.J. Mechanism of Pd(NHC)-Catalyzed Transfer Hydrogenation of Alkynes. J. Am. Chem. Soc. 2010, 132, 16900–16910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warsink, S.; Bosman, S.; Weigand, J.J.; Elsevier, C.J. Rigid pyridyl substituted NHC ligands, their Pd(0) complexes and their application in selective transfer semihydrogenation of alkynes. Appl. Organometal. Chem. 2011, 25, 276–282. [Google Scholar] [CrossRef]
- Tromp, D.S.; Hauwert, P.; Elsevier, C.J. Synthesis of bis-N-alkyl imidazolium salts and their palladium(0)(NHC)(η2-MA)2 complexes. Appl. Organometal. Chem. 2012, 26, 335–341. [Google Scholar] [CrossRef]
- Hauwert, P.; Dunsford, J.J.; Tromp, D.S.; Weigand, J.J.; Lutz, M.; Cavell, K.J.; Elsevier, C.J. Zerovalent [Pd(NHC){Alkene}1,2] Complexes Bearing Expanded-Ring N-Heterocyclic Carbene Ligands in Transfer Hydrogenation of Alkynes. Organometallics 2013, 32, 131–140. [Google Scholar] [CrossRef]
- Drost, R.M.; Bouwens, T.; van Leest, N.P.; de Bruin, B.; Elsevier, C.J. Convenient Transfer Semihydrogenation Methodology for Alkynes Using a PdII-NHC Precatalyst. ACS Catal. 2014, 4, 1349–1357. [Google Scholar] [CrossRef]
- Broggi, J.; Jurčík, V.; Songis, O.; Poater, A.; Cavallo, L.; Slawin, A.M.Z.; Cazin, C.S.J. The Isolation of [Pd{OC(O)H}(H)(NHC)(PR3)] (NHC = N-Heterocyclic Carbene) and Its Role in Alene and Alynes Reductions Using Formic Acid. J. Am. Chem. Soc. 2013, 135, 4588–4591. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, S.N.; Warsink, S.; Lutz, M.; Elsevier, C.J. Synthesis of palladium(0) and –(II) complexes with chelating bis(N-heterocyclic carbene) ligands and their application in semihydrogenation. Dalton Trans. 2013, 42, 7365–7372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranganath, K.V.S.; Kloesges, J.; Schäfer, A.H.; Clorius, F. Asymmetric Nanocatalysis: N-Heterocyclic Carbenes as Chiral Modifiers of Fe3O4/Pd nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 7786–7789. [Google Scholar] [CrossRef] [PubMed]
- Planellas, M.; Pleixats, R.; Shafir, A. Palladium Nanoparticles in Suzuki Cross-Couplings: Tapping into the Potential of Tris-Imidazolium Salts for Nanoparticle Stabilization. Adv. Synth. Catal. 2012, 354, 651–662. [Google Scholar] [CrossRef]
- Khazipov, O.V.; Shevchenko, M.A.; Chemenko, A.Y.; Astakhov, A.V.; Pasyukov, D.V.; Eremin, D.B.; Zubavivhus, Y.V.; Khrustalev, V.N.; Chemyshev, V.M.; Ananikov, V.P. Fast and Slow Release of Catalytically Active Species in Metal/NHC Systems Induced by Aliphatic Amines. Organometallics 2018, 37, 1483–1492. [Google Scholar] [CrossRef]
- Chernyshev, V.M.; Denisova, E.A.; Eremin, D.B.; Ananilov, V.P. The key role of R–NHC couplings (R = C, H, heteroatom) and M–NHC bond cleavage in the evolution of M/NHC complexes and formation of catalytically active species. Chem. Sci. 2020, 11, 6957–6977. [Google Scholar] [CrossRef]
- Cerezo-Navarrete, C.; Lara, P.; Martínez-Prieto, L.M. Organometallic Nanoparticles Ligated by NHCs: Synthesis, Surface Chemistry and Ligand Effects. Catalysts 2020, 10, 1144. [Google Scholar] [CrossRef]
- Lin, Y.-R.; Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. Bis-benzimidazolium-palladium system catalyzed Suzuki-Miyaura coupling reaction of aryl bromides under mild conditions. Appl. Organometal. Chem. 2018, 32, e3896. [Google Scholar] [CrossRef]
- Chiu, C.-C.; Chiu, H.-T.; Lee, D.-S.; Lu, T.-J. An efficient class of bis-NHC salts: Applications in Pd-catalyzed reactions under mild reaction conditions. RSC Adv. 2018, 8, 26407–26415. [Google Scholar] [CrossRef] [Green Version]
- Arterburn, J.B.; Pannala, M.; Gonzalez, A.M.; Chamberlin, R.M. Palladium-catalyzed transfer hydrogenation in alkaline aqueous medium. Tetrahedron Lett. 2000, 41, 7847–7849. [Google Scholar] [CrossRef]
- Shirakawa, E.; Otuska, H.; Hayashi, T. Reduction of alkynes into 1,2-dideuterioalkenes with hexamethyldisilane and deuterium oxide in the presence of a palladium catalyst. Chem. Commun. 2005, 2005, 5885–5886. [Google Scholar] [CrossRef]
- Luo, F.; Pan, C.; Wang, W.; Ye, Z.; Cheng, J. Palladium-catalyzed reduction of alkynes employing HSiEt3: Stereoselective synthesis of trans- and cis-alkenes. Tetrahedron 2010, 66, 1399–1403. [Google Scholar] [CrossRef]
- Xuan, Q.; Song, Q. Diboron-Assisted Palladium-Catalyzed Transfer Hydrogenation of N-Heteroaromatics with Water as Hydrogen Donor and Solvent. Org. Lett. 2016, 18, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zeng, Y.; Zhang, H.; Wei, T.; Wu, X.; Li, N. Facile Pd-catalyzed chemoselective transfer hydrogenation of olefins using formic acid in water. Tetrahedron Lett. 2016, 57, 4845–4849. [Google Scholar] [CrossRef]
- Rao, S.; Prabhu, K.R. Stereodivergent Alkyne Reduction by using Water as the Hydrogen Source. Chem. Eur. J. 2018, 24, 13954–13962. [Google Scholar] [CrossRef]
- Zhao, C.-Q.; Chen, T.-G.; Giu, H.; Wei, L.; Fang, P.; Mei, T.-S. Water as a Hydrogenating Agent: Stereodivergent Pd-Catalyzed Semihydrogenation of Alkynes. Org. Lett. 2019, 21, 1412–1416. [Google Scholar] [CrossRef]
- Wang, W.; Gao, L.; Wei, H.; Qi, Z.-H.; Zeng, G.; Cheng, X.; Wang, G.; Ma, J. Selectivity control of Pd-(PMe3)4-catalyzed hydrogenation of internal alkynes to E-alkenes by reaction time and water content in formic acid. Dalton Trans. 2019, 48, 10033–10042. [Google Scholar] [CrossRef]
- De, S.; Udvardy, A.; Czégéni, C.E.; Joó, F. Poly-N-heterocyclic carbene complexes with applications in aqueous media. Coord. Chem. Rev. 2019, 400, 213038. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Gou, X.-X.; Han, Y.-F. Chelating Bis(N-Heterocyclic Carbene) Palladium-Catalyzed Reactions. Chem. Asian J. 2018, 13, 2257–2276. [Google Scholar] [CrossRef]
- Enthaler, S.; Haberberger, M.; Irran, E. Highly Selective Iron-Catalyzed Synthesis of Alkenes by the Reduction of Alkynes. Chem. Asian J. 2011, 6, 1613–1623. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, R.; Tanaka, E.; Ichihashi, T.; Idemoto, Y.; Endo, K. Semireduction of Alkynes Using Formic Acid with Reusable Pd-Catalysts. J. Org. Chem. 2018, 83, 13574–13579. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Zhou, F.; Liu, M.; Li, X.; Chan, A.S.C.; Li, C.J. Silver-Catalyzed Hydrogenation of Aldehydes in Water. Angew. Chem. Int. Ed. 2013, 52, 11871–11874. [Google Scholar] [CrossRef]
- Jurčík, V.; Nolan, S.P.; Cazin, C.S.J. Hydrogenation of C–C Multiple Bonds Mediated by [Pd(NHC)(PCy)3] (NHC = N-Heterocyclic Carbene) under Mild Reaction Conditions. Chem. Eur. J. 2009, 15, 2509–2511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, H.; Liu, S.; Pi, D.; Shen, G. Water as a hydrogen source in palladium-catalyzed reduction and reductive amination of nitroarenes mediated by diboronic acid. Tetrahedron 2017, 73, 3898–3904. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Q.; Li, J.; Liu, Z.; Zhou, B. Highly Selective Semihydrogenation of Phentlalkynes to (Z)-Styrenes Using Hantzsch Ester 1,4-Dihydropyridine Catalyzed by Pd/C. Synlett 2010, 2010, 1870–1872. [Google Scholar]
- Byrne, P.A.; Gilheany, D.G. Unequivical experimental evidence for a unified Li salt-free Wittig reaction mechanism for all phosphonium ylide types: Reactions with b-heteroatom substituted aldehydes are consistently selective for cis-oxaphosphetane derived products. J. Am. Chem. Soc. 2012, 134, 9225–9239. [Google Scholar] [CrossRef]
- Espinal-Viguri, M.; Neale, S.E.; Coles, N.T.; Macgregor, S.A.; Webster, R.L. Room Temperature Iron-Catalyzed Transfer Hydrogenation and Regioselective Deuteration of Carbon-Carbon Double Bonds. J. Am. Chem. Soc. 2019, 141, 572–582. [Google Scholar] [CrossRef]
- Chen, W.; Tao, H.; Huang, W.; Wang, G.; Li, S.; Cheng, X.; Li, G. Hantzsch Ester as a Photosensitizer for the Visible-Light-Induced Debromination of Vicinal Dibromo Compounds. Chem. Eur. J. 2016, 22, 9546–9550. [Google Scholar] [CrossRef]
- Zhou, M.; Li, T.; Xu, B. Easy-handling and Low-leaching Heterogeneous Palladium and Platinum Catalysis via Coating with a Silicone Elastomer. Tetrahedron Lett. 2019, 60, 948–952. [Google Scholar] [CrossRef]
- Buxaderas, E.; Volpe, M.A.; Radivoy, G. Selective Semi-hydrogenation of Terminal Alkynes Promoted by Bimetallic Cu-Pd Nanoparticles. Synthesis 2018, 51, 1466–1472. [Google Scholar]
- Bao, H.; Zhou, B.; Jin, H.; Liu, Y. Diboron-Assisted Copper-Catalyzed Z-Selective Semihydrogenation of Alkynes Using Ethanol as a Hydrogen Donor. J. Org. Chem. 2019, 84, 3579–3589. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Z.; Leng, X.; Zhu, H.; Liu, G.; Huang, Z. Transfer Hydrogenation of Alkenes Using Ethanol Catalyzed by a NCP Pincer Iridium Complex: Scope and Mechanism. J. Am. Chem. Soc. 2018, 140, 4417–4429. [Google Scholar] [CrossRef]
- Zhao, S.; Mankad, N.P. Cu-Catalyzed Hydroxymethylation of Unactivated Alkyl Iodides with CO to Provide One-Carbon-Extended Alcohols. Angew. Chem. Int. Ed. 2018, 57, 5867–5870. [Google Scholar] [CrossRef] [PubMed]

| Entry | bis-NHC–Ag/Pd (mol %) | TEAF (equiv) | Solvent | Conv. (%) 2 |
|---|---|---|---|---|
| 1 | 0.5 | 3.0 | DMF | 44 |
| 2 | 1.0 | 5.0 | tBuOH | 67 |
| 3 | 2.0 | 7.0 | PhMe | 42 |
| 4 | 1.0 | 3.0 | DMF | 74 |
| 5 | 1.0 | 4.0 | DMF | >99 |
| 6 3 | 1.0 | 4.0 | DMF | 8 |
| Entry | Substrate | Product | Yield (%) 2 |
|---|---|---|---|
| 1 |  |  | 98 |
| 2 |  |  | 99 |
| 3 |  |  | 99 |
| 4 |  |  | 85 |
| 5 |  |  | 99 |
| 6 |  |  | 99 |
| 7 |  |  | 92 |

| Entry | Pd Source | Base | Solvent Ratio (DMF/H2O) | Conv. (%)2 | Z:E:9a 2 |
|---|---|---|---|---|---|
| 1 | Pd(OAc)2 | NEt3 | 9/1 | 100 | 94:6:0 |
| 2 | Pd(OAc)2 | NEt3 | 5/5 | 100 | 93:7:0 |
| 3 | Pd(OAc)2 | NEt3 | 1/9 | 82 | 93:7:0 |
| 4 | Pd(OAc)2 | NEt3 | 0/1 | 32 | 93:7:0 |
| 5 | Pd(OAc)2 | K2CO3 | 5/5 | 24 | 96:4:0 |
| 6 | Pd(OAc)2 | K3PO4·H2O | 5/5 | 36 | 97:3:0 |
| 7 | PdCl2(CH3CN)2 | NEt3 | 5/5 | 100 | 93:7:0 |
| 8 | PdCl2 | NEt3 | 5/5 | 89 | 94:6:0 |
| 9 | [PdCl2(C3H5)2] | NEt3 | 5/5 | 82 | 93:7:0 |

| Entry | Substrate | Condition 2 | Time (h) | Conv. (%) 3 | Z:E:9 3 |
|---|---|---|---|---|---|
| 1 |  | A (5/5) | 24 | 100 (> 99) 4 | 93:7:0 |
| 2 | B (9/1) | 24 | 100 (> 99) 4 | 94:6:0 | |
| 3 |  | B (9/1) | 2 | 100 (97) 5 | 30:70:0 |
| 4 |  | A (5/5) | 24 | 47 | 100:0:0 |
| 5 | B (9/1) | 24 | 100 (80) 5 | 100:0:0 | |
| 6 |  | B (9/1) | 2.5 | 100 (90) 5 | 95:5:0 |
| 7 |  | B (9/1) | 2 | 100 (> 99) 5 | 0:0:100 |
| 8 | C (9/1) | 1 | 86 (83) 5 | 100:0:0 |

| Entry | Substrate | Time (h) | 11:12 2 | GC Yield (%) 3 |
|---|---|---|---|---|
| 1 |  | 3 | 100:0 | 89 |
| 2 | 5 | 0:100 | 34 | |
| 3 |  | 0.5 | 100:0 | 92 |
| 4 | 3 | 0:100 | 63 | |
| 5 |  | 0.5 | 97:3 | 88 |
| 6 | 3 | 0:100 | 40 | |
| 7 |  | 1 | 100:0 | 83 4 |
| 8 | 2 | 0:100 5 | 80 4 | |
| 9 |  | 3 | 100:0 | 72 |
| 10 |  | 2 | 100:0 | 99 4 |
| 11 | 24 | 0:100 | 97 4 | |
| 12 6 |  | 24 | 100:0 | 78 4 |
| 13 | 24 | 0:100 | 72 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.-J.; Chiu, C.-C.; Wang, T.; Lee, D.-S.; Lu, T.-J. Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction. Catalysts 2021, 11, 8. https://doi.org/10.3390/catal11010008
Chen H-J, Chiu C-C, Wang T, Lee D-S, Lu T-J. Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction. Catalysts. 2021; 11(1):8. https://doi.org/10.3390/catal11010008
Chicago/Turabian StyleChen, Hui-Ju, Chien-Cheng Chiu, Tsui Wang, Dong-Sheng Lee, and Ta-Jung Lu. 2021. "Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction" Catalysts 11, no. 1: 8. https://doi.org/10.3390/catal11010008
APA StyleChen, H. -J., Chiu, C. -C., Wang, T., Lee, D. -S., & Lu, T. -J. (2021). Bis-NHC–Ag/Pd(OAc)2 Catalytic System Catalyzed Transfer Hydrogenation Reaction. Catalysts, 11(1), 8. https://doi.org/10.3390/catal11010008