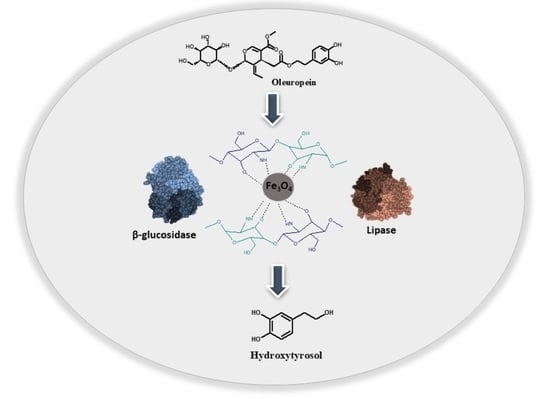
Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of the Chitosan Magnetic Nanoparticles
Atomic Force Microscopy (AFM)
2.2. Preparation of the Bi-Enzymatic Magnetic Nanobiocatalyst
2.3. Characterization of the Bio-Nanoconjugates
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. Circular Dichroism Spectroscopy (CD)
2.3.3. Fluorescence Spectroscopy
2.4. Thermal Stability of the Bi-Enzymatic Nanobiocatalyst
2.5. Kinetic Studies of Free, Individually Immobilized and Co-Immobilized Biocatalysts
2.6. Application of the Bi-Enzymatic Nanobiocatalyst to the Bioconversion of Oleuropein to Hydroxytyrosol
2.7. Reusability of the Bi-Enzymatic Nanobiocatalyst
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Functionalization of Iron Oxide Magnetic Nanoparticles (Fe3O4) with Chitosan (CS-MNPs)
3.2.2. Characterization of the Chitosan-Functionalized Magnetic Nanoparticles
XRD
AFM
3.2.3. Preparation of Bi-Enzymatic Magnetic Nanobiocatalyst
3.2.4. Characterization of the Bi-Enzymatic Magnetic Nanobiocatalyst
Fourier-Transform Infrared Spectroscopy (FTIR)
Circular Dichroism Spectroscopy (CD)
Fluorescence Spectroscopy
3.2.5. Enzyme Assays
3.2.6. Thermal Stability Studies
3.2.7. Kinetic Studies of Free, Individually Immobilized and Co-Immobilized Biocatalysts
3.2.8. Hydrolysis of Oleuropein to Hydroxytyrosol by the Βi-Enzymatic Νanobiocatalyst High-Performance Liquid Chromatography (HPLC) Analysis
3.2.9. Nuclear Magnetic Resonance (NMR) Analysis
3.2.10. Reusability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a Bioactive Compound from Olea europaea L., as a Potential Preventive and Therapeutic Agent in Non-Communicable Diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-J.; Wang, C.-Z.; Ye, J.-Z.; Tao, R.; Zhang, Y.-S. Enzymatic Hydrolysis of Oleuropein from Olea europea (Olive) Leaf Extract and Antioxidant Activities. Molecules 2015, 20, 2903–2921. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Yong, Q.; Yu, S. Efficient bioconversion of oleuropein from olive leaf extract to antioxidant hydroxytyrosol by enzymatic hydrolysis and high-temperature degradation. Biotechnol. Appl. Biochem. 2018, 65, 680–689. [Google Scholar] [CrossRef]
- De Leonardis, A.; Aretini, A.; Alfano, G.; Macciola, V.; Ranalli, G. Isolation of a hydroxytyrosol-rich extract from olive leaves (Olea Europaea L.) and evaluation of its antioxidant properties and bioactivity. Eur. Food Res. Technol. 2008, 226, 653–659. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y.; Zheng, M.; Xi, X.; Zhang, X.; Han, C. Structure Properties, Acquisition Protocols, and Biological Activities of Oleuropein Aglycone. Front. Chem. 2018, 6, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaivits, E.; Termentzi, A.; Skaltsounis, A.-L.; Fokialakis, N.; Topakas, E. Enzymatic tailoring of oleuropein from Olea europaea leaves and product identification by HRMS/MS spectrometry. J. Biotechnol. 2017, 253, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Soler-Rivas, C.; Espín, J.C.; Wichers, H.J. Oleuropein and related compounds. J. Sci. Food Agric. 2000, 80, 1013–1023. [Google Scholar] [CrossRef]
- Ramírez, E.; Brenes, M.; García, P.; Medina, E.; Romero, C. Oleuropein hydrolysis in natural green olives: Importance of the endogenous enzymes. Food Chem. 2016, 206, 204–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzikonstantinou, A.V.; Gkantzou, E.; Thomou, E.; Chalmpes, N.; Lyra, K.-M.; Kontogianni, V.G.; Spyrou, K.; Patila, M.; Gournis, D.; Stamatis, H.; et al. Enzymatic Conversion of Oleuropein to Hydroxytyrosol Using Immobilized β-Glucosidase on Porous Carbon Cuboids. Nanomaterials 2019, 9, 1166. [Google Scholar] [CrossRef] [Green Version]
- Giannakopoulou, A.; Gkantzou, E.; Polydera, A.; Stamatis, H. Multienzymatic Nanoassemblies: Recent Progress and Applications. Trends Biotechnol. 2020, 38, 202–216. [Google Scholar] [CrossRef]
- Ren, S.; Liu, X.; Jiao, X.; Jia, S.; Jiang, Y.; Bilal, M.; Cui, J. Recent progress in multienzymes co-immobilization and multienzyme system applications. Chem. Eng. J. 2019, 373, 1254–1278. [Google Scholar] [CrossRef]
- Filice, M.; Palomo, J.M. Cascade Reactions Catalyzed by Bionanostructures. ACS Catal. 2014, 4, 1588–1598. [Google Scholar] [CrossRef]
- Monteiro, R.R.C.; Lima, P.J.M.; Pinheiro, B.; Freire, T.M.; Dutra, L.M.U.; Fechine, L.; Gonçalves, L.R.B.; De Souza, M.C.M.; Dos Santos, J.C.S.; Fernandez-Lafuente, R. Immobilization of Lipase A from Candida antarctica onto Chitosan-Coated Magnetic Nanoparticles. Int. J. Mol. Sci. 2019, 20, 4018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Wang, X.; Chen, Y.; Xue, Z.; Guo, Q.; Ma, Q.; Chen, H. Preparation and characterization of a novel nanocomposite with double enzymes immobilized on magnetic Fe3O4-chitosan-sodium tripolyphosphate. Colloids Surfaces B Biointerfaces 2018, 169, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, A.M.; Patel, G.R. Analysis of variability in tensile ductility of a semi-solid metal cast A356 Al-alloy. Mater. Sci. Eng. A 2005, 392, 184–190. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Luo, G.; Dai, Y. In situ preparation of magnetic Fe3O4-chitosan nanoparticles for lipase immobilization by cross-linking and oxidation in aqueous solution. Bioresour. Technol. 2009, 100, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Hernández, A.; Gracida, J.; García-Almendárez, B.E.; Regalado, C.; Núñez, R.; Amaro-Reyes, A. Characterization of Magnetic Nanoparticles Coated with Chitosan: A Potential Approach for Enzyme Immobilization. J. Nanomater. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Vaghari, H.; Anarjan, N.; Ahmadi, O.; Berenjian, A. Chitosan magnetic nanoparticles for drug delivery systems. Crit. Rev. Biotechnol. 2016, 37, 492–509. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.T.R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, R.R.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á; da Rocha, T.N.; dos Santos, J.C.; Alcántara, A.R.; Fernandez-Lafuente, R. Biotechnological relevance of the lipase A from Candida antarctica. Catal. Today 2021, 362, 141–154. [Google Scholar] [CrossRef]
- Akın, D.; Yakar, A.; Gündüz, U. Synthesis of Magnetic Fe3O4-Chitosan Nanoparticles by Ionic Gelation and Their Dye Removal Ability. Water Environ. Res. 2015, 87, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Xiao, L.; Chen, C.; Shi, X.; Cao, Q.; Gao, L. In situ preparation of magnetic Fe3O4/chitosan nanoparticles via a novel reduction–precipitation method and their application in adsorption of reactive azo dye. Powder Technol. 2014, 260, 90–97. [Google Scholar] [CrossRef]
- López-Gallego, F.; Guisán, J.M.; Betancor, L. Glutaraldehyde-Mediated Protein Immobilization. Methods Mol. Biol. 2013, 1051, 33–41. [Google Scholar] [CrossRef]
- Giannakopoulou, A.; Patila, M.; Spyrou, K.; Chalmpes, N.; Zarafeta, D.; Skretas, G.; Gournis, D.; Stamatis, H. Development of a Four-Enzyme Magnetic Nanobiocatalyst for Multi-Step Cascade Reactions. Catalysts 2019, 9, 995. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Liu, D.; Wu, C.; Yao, K.; Li, Z.; Shi, N.; Wen, F.; Gates, I.D. Co-immobilization of cellulase and lysozyme on amino-functionalized magnetic nanoparticles: An activity-tunable biocatalyst for extraction of lipids from microalgae. Bioresour. Technol. 2018, 263, 317–324. [Google Scholar] [CrossRef]
- Sojitra, U.V.; Nadar, S.; Rathod, V.K. A magnetic tri-enzyme nanobiocatalyst for fruit juice clarification. Food Chem. 2016, 213, 296–305. [Google Scholar] [CrossRef]
- Muley, A.B.; Thorat, A.S.; Singhal, R.S.; Babu, K.H. A tri-enzyme co-immobilized magnetic complex: Process details, kinetics, thermodynamics and applications. Int. J. Biol. Macromol. 2018, 118, 1781–1795. [Google Scholar] [CrossRef]
- Pavlidis, I.V.; Vorhaben, T.; Gournis, D.; Papadopoulos, G.K.; Bornscheuer, U.T.; Stamatis, H. Regulation of catalytic behaviour of hydrolases through interactions with functionalized carbon-based nanomaterials. J. Nanopart. Res. 2012, 14, 1–10. [Google Scholar] [CrossRef]
- Tzialla, A.A.; Pavlidis, I.V.; Felicissimo, M.P.; Rudolf, P.; Gournis, D.; Stamatis, H. Lipase immobilization on smectite nanoclays: Characterization and application to the epoxidation of α-pinene. Bioresour. Technol. 2010, 101, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.L.; Rao, N.M.; Tsuzuki, T.; Barrow, C.J.; Puri, M. Suitability of Recombinant Lipase Immobilised on Functionalised Magnetic Nanoparticles for Fish Oil Hydrolysis. Catalysts 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.L.; Naebe, M.; Barrow, C.; Puri, M. Enzyme Immobilisation on Amino-Functionalised Multi-Walled Carbon Nanotubes: Structural and Biocatalytic Characterisation. PLoS ONE 2013, 8, e73642. [Google Scholar] [CrossRef]
- Chatzikonstantinou, A.V.; Polydera, A.C.; Thomou, E.; Chalmpes, N.; Baroud, T.N.; Enotiadis, A.; Estevez, L.; Patila, M.; Hammami, M.A.; Spyrou, K.; et al. Lipase immobilized on magnetic hierarchically porous carbon materials as a versatile tool for the synthesis of bioactive quercetin derivatives. Bioresour. Technol. Rep. 2020, 9, 100372. [Google Scholar] [CrossRef]
- Li, L.; Li, H.; Yan, B.; Yu, S. Preparation of a reversible soluble-insoluble β-d-Glucosidase with perfect stability and activity. J. Biotechnol. 2019, 291, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Girard-Egrot, A.P.; Godoy, S.; Blum, L.J. Enzyme association with lipidic Langmuir–Blodgett films: Interests and applications in nanobioscience. Adv. Colloid Interface Sci. 2005, 116, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Paudyal, S.; Sharma, S.K.; da Silva, R.L.; Mintz, K.J.; Liyanage, P.Y.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S.; Leblanc, R.M. Tyrosinase enzyme Langmuir monolayer: Surface chemistry and spectroscopic study. J. Colloid Interface Sci. 2020, 564, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, F.D.P.; Caseli, L. Controlling the molecular architecture of lactase immobilized in Langmuir-Blodgett films of phospholipids to modulate the enzyme activity. Colloids Surfaces B Biointerfaces 2017, 150, 8–14. [Google Scholar] [CrossRef]
- Chapman, J.; Ismail, A.E.; Dinu, C.Z. Industrial Applications of Enzymes: Recent Advances, Techniques, and Outlooks. Catalysts 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Verma, S.; Vakhlu, J. Comparative Analysis of Β- Glucosidases Thermostability: Differences in Amino Acids Com-position and Distribution among Mesostable and Thermostable Β-Glucosidases. J. Adv. Bioinform. Appl. Res. 2014, 5, 215–227. [Google Scholar]
- Shahrestani, H.; Taheri-Kafrani, A.; Soozanipour, A.; Tavakoli, O. Enzymatic clarification of fruit juices using xylanase immobilized on 1,3,5-triazine-functionalized silica-encapsulated magnetic nanoparticles. Biochem. Eng. J. 2016, 109, 51–58. [Google Scholar] [CrossRef]
- Alnadari, F.; Xue, Y.; Zhou, L.; Hamed, Y.S.; Taha, M.; Foda, M.F. Immobilization of β-Glucosidase from Thermatoga maritima on Chitin-functionalized Magnetic Nanoparticle via a Novel Thermostable Chitin-binding Domain. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-Dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Whitmore, L.; Wallace, B.A. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004, 32, W668–W673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobley, A.; Whitmore, L.; Wallace, B.A. DICHROWEB: An interactive website for the analysis of protein secondary structure from circular dichroism spectra. Bioinformatics 2002, 18, 211–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowers, G.N., Jr.; McComb, R.B.; Christensen, R.G.; Schaffer, R. High-purity 4-nitrophenol: Purification, characterization, and speci-fications for use as a spectrophotometric reference material. Clin. Chem. 1980, 26, 724–729. [Google Scholar] [CrossRef]










| Sample | α-Helix | β-Sheet | Other | |
|---|---|---|---|---|
| bgl | Buffer CS-MNPs | 28 25 | 19 23 | 53 52 |
| CalA | Buffer CS-MNPs | 32 31 | 17 22 | 51 47 |
| Sample | Half-Life Time (h) |
|---|---|
| Free CalA | 7.8 |
| Co-immobilized CalA | 44.2 |
| Individually immobilized CalA | 51.8 |
| Forms | Km (mM) | Vmax (μmol/min) |
|---|---|---|
| Free bgl | 0.72 | 9.27 |
| Co-immobilized bgl | 0.85 | 5.60 |
| Individually immobilized bgl | 1.20 | 7.64 |
| Forms | Km (mM) | Vmax (mol/min) |
|---|---|---|
| Free CalA | 0.07 | 0.07 |
| Co-immobilized CalA | 0.17 | 0.04 |
| Individually immobilized CalA | 0.22 | 0.05 |
| Sample | Initial Reaction Rate (mM h−1 mg−1 Nanobiocatalyst) | % Conversion Yield of Oleuropein | Hydroxytyrosol (mg mL−1) |
|---|---|---|---|
| Individually immobilized bgl | 0.038 | 100 | 0.150 |
| Individually immobilized CalA | 0.021 | 40 | 0.880 |
| Individually immobilized bgl and CalA | 0.032 | 90 | 1.210 |
| Co-Immobilized bgl-CalA | 0.046 | 100 | 2.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannakopoulou, A.; Chatzikonstantinou, A.V.; Chalmpes, N.; Tsapara, G.; Gournis, D.; Polydera, A.C.; Stamatis, H. Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts 2021, 11, 749. https://doi.org/10.3390/catal11060749
Giannakopoulou A, Chatzikonstantinou AV, Chalmpes N, Tsapara G, Gournis D, Polydera AC, Stamatis H. Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts. 2021; 11(6):749. https://doi.org/10.3390/catal11060749
Chicago/Turabian StyleGiannakopoulou, Archontoula, Alexandra V. Chatzikonstantinou, Nikolaos Chalmpes, Georgia Tsapara, Dimitrios Gournis, Angeliki C. Polydera, and Haralambos Stamatis. 2021. "Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol" Catalysts 11, no. 6: 749. https://doi.org/10.3390/catal11060749
APA StyleGiannakopoulou, A., Chatzikonstantinou, A. V., Chalmpes, N., Tsapara, G., Gournis, D., Polydera, A. C., & Stamatis, H. (2021). Development of a Novel Bi-Enzymatic Nanobiocatalyst for the Efficient Bioconversion of Oleuropein to Hydroxytyrosol. Catalysts, 11(6), 749. https://doi.org/10.3390/catal11060749






_Stamatis.png)