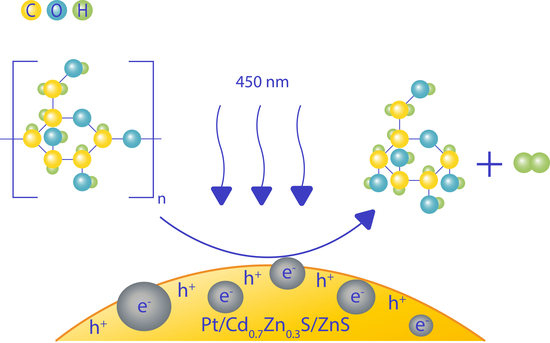
Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalyst Characterization
2.2. Photocatalytic Activity
3. Materials and Methods
3.1. Photocatalyst Synthesis
3.2. Photocatalyst Characterization
3.3. Photocatalytic Activity Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acar, C.; Bicer, Y.; Demir, M.E.; Dincer, I. Transition to A New Era with Light-Based Hydrogen Production for A Carbon-Free Society: An Overview. Int. J. Hydrogen Energy 2019, 44, 25347–25364. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen Energy, Economy and Storage: Review and Recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and Evaluation of Hydrogen Production Methods for Better Sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- Nikolaidis, P.; Poullikkas, A. A Comparative Overview of Hydrogen Production Processes. Renew. Sustain. Energy Rev. 2017, 67, 597–611. [Google Scholar] [CrossRef]
- Wang, M.; Liu, M.; Lu, J.; Wang, F. Photo Splitting of Bio-Polyols and Sugars to Methanol and Syngas. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Peng, S.-Q.; Peng, Y.-J.; Li, Y.-X.; Lu, G.-X.; Li, S.-B. Photocatalytic Hydrogen Generation Using Glucose as Electron Donor over Pt/CdxZn1−xS Solid Solutions. Res. Chem. Intermed. 2009, 35, 739–749. [Google Scholar] [CrossRef]
- Yasuda, M.; Matsumoto, T.; Yamashita, T. Sacrificial Hydrogen Production over TiO2-Based Photocatalysts: Polyols, Carboxylic Acids, and Saccharides. Renew. Sustain. Energy Rev. 2018, 81, 1627–1635. [Google Scholar] [CrossRef]
- Ramis, G.; Bahadori, E.; Rossetti, I. Design of Efficient Photocatalytic Processes for the Production of Hydrogen from Biomass Derived Substrates. Int. J. Hydrogen Energy 2020, 46, 12105–12116. [Google Scholar] [CrossRef]
- Puga, A.V. Photocatalytic Production of Hydrogen from Biomass-Derived Feedstocks. Coord. Chem. Rev. 2016, 315, 1–66. [Google Scholar] [CrossRef]
- Nguyen, V.-C.; Ke, N.-J.; Nam, L.D.; Nguyen, B.-S.; Xiao, Y.-K.; Lee, Y.-L.; Teng, H. Photocatalytic Reforming of Sugar and Glucose into H2 over Functionalized Graphene Dots. J. Mater. Chem. A 2019, 7, 8384–8393. [Google Scholar] [CrossRef]
- Kondarides, D.I.; Daskalaki, V.M.; Patsoura, A.; Verykios, X.E. Hydrogen Production by Photo-Induced Reforming of Biomass Components and Derivatives at Ambient Conditions. Catal. Lett. 2008, 122, 26–32. [Google Scholar] [CrossRef]
- Kandiel, T.A.; Ivanova, I.; Bahnemann, D.W. Long-Term Investigation of the Photocatalytic Hydrogen Production on Platinized TiO2: An Isotopic Study. Energy Environ. Sci. 2014, 7, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Puga, A.V.; Forneli, A.; García, H.; Corma, A. Production of H 2 by Ethanol Photoreforming on Au/TiO2. Adv. Funct. Mater. 2014, 24, 241–248. [Google Scholar] [CrossRef]
- Taboada, E.; Angurell, I.; Llorca, J. Dynamic Photocatalytic Hydrogen Production from Ethanol-Water Mixtures in An Optical Fiber Honeycomb Reactor Loaded with Au/TiO2. J. Catal. 2014, 309, 460–467. [Google Scholar] [CrossRef]
- Patsoura, A.; Kondarides, D.I.; Verykios, X.E. Photocatalytic Degradation of Organic Pollutants with Simultaneous Production of Hydrogen. Catal. Today 2007, 124, 94–102. [Google Scholar] [CrossRef]
- Panagiotopoulou, P.; Karamerou, E.E.; Kondarides, D.I. Kinetics and Mechanism of Glycerol Photo-Oxidation and Photo-Reforming Reactions in Aqueous TiO2 and Pt/TiO2 Suspensions. Catal. Today 2013, 209, 91–98. [Google Scholar] [CrossRef]
- Beltram, A.; Romero-Ocaña, I.; Josè Delgado Jaen, J.; Montini, T.; Fornasiero, P. Photocatalytic Valorization of Ethanol and Glycerol over TiO2 Polymorphs for Sustainable Hydrogen Production. Appl. Catal. A Gen. 2016, 518, 167–175. [Google Scholar] [CrossRef]
- Fujita, S.-i.; Kawamori, H.; Honda, D.; Yoshida, H.; Arai, M. Photocatalytic Hydrogen Production from Aqueous Glycerol Solution Using NiO/TiO2 Catalysts: Effects of Preparation and Reaction Conditions. Appl. Catal. B Environ. 2016, 181, 818–824. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Reisner, E. Solar Hydrogen Generation from Lignocellulose. Angew. Chem. Int. Ed. 2018, 57, 3290–3296. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-Based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Wu, X.; Fan, X.; Xie, S.; Lin, J.; Cheng, J.; Zhang, Q.; Chen, L.; Wang, Y. Solar Energy-Driven Lignin-First Approach to Full Utilization of Lignocellulosic Biomass under Mild Conditions. Nat. Catal. 2018, 1, 772–780. [Google Scholar] [CrossRef]
- Wakerley, D.W.; Kuehnel, M.F.; Orchard, K.L.; Ly, K.H.; Rosser, T.E.; Reisner, E. Solar-Driven Reforming of Lignocellulose to H2 with a CdS/CdOx Photocatalyst. Nat. Energy 2017, 2, 17021. [Google Scholar] [CrossRef] [Green Version]
- Speltini, A.; Gualco, F.; Maraschi, F.; Sturini, M.; Dondi, D.; Malavasi, L.; Profumo, A. Photocatalytic Hydrogen Evolution Assisted by Aqueous (Waste)Biomass under Simulated Solar Light: Oxidized g-C3N4 Vs. P25 Titanium Dioxide. Int. J. Hydrogen Energy 2019, 44, 4072–4078. [Google Scholar] [CrossRef]
- Kasap, H.; Achilleos, D.S.; Huang, A.; Reisner, E. Photoreforming of Lignocellulose into H2 Using Nanoengineered Carbon Nitride under Benign Conditions. J. Am. Chem. Soc. 2018, 140, 11604–11607. [Google Scholar] [CrossRef] [Green Version]
- Pichler, C.M.; Uekert, T.; Reisner, E. Photoreforming of Biomass in Metal Salt Hydrate Solutions. Chem. Commun. 2020, 56, 5743–5746. [Google Scholar] [CrossRef] [PubMed]
- Jaswal, R.; Shende, R.; Nan, W.; Shende, A. Photocatalytic Reforming of Pinewood (Pinus ponderosa) Acid Hydrolysate for Hydrogen Generation. Int. J. Hydrogen Energy 2017, 42, 2839–2848. [Google Scholar] [CrossRef]
- Speltini, A.; Sturini, M.; Dondi, D.; Annovazzi, E.; Maraschi, F.; Caratto, V.; Profumo, A.; Buttafava, A. Sunlight-Promoted Photocatalytic Hydrogen Gas Evolution from Water-Suspended Cellulose: A Systematic Study. Photochem. Photobiol. Sci. 2014, 13, 1410–1419. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Shen, S.; Song, T.; Chen, X.; Zhang, A.; Dou, H. Insights into the Structure and Conformation of Potato Resistant Starch (Type 2) Using Asymmetrical Flow Field-Flow Fractionation Coupled with Multiple Detectors. Food Chem. 2021, 349, 129168. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, Q.; Liu, J.; Liu, X.; Zhao, S.; Hu, Q.; Song, W.; Liu, B.; Liu, J.; Ding, C. Effect of Starch Multi-Scale Structure Alteration on Japonica Rice Flour Functionality under Infrared Radiation Drying and Storage. LWT 2021, 143, 111126. [Google Scholar] [CrossRef]
- Shimura, K.; Yoshida, H. Heterogeneous photocatalytic hydrogen production from water and biomass derivatives. Energy Environ. Sci. 2011, 4, 2467–2481. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Parmon, V.N. Heterogeneous Semiconductor Photocatalysts for Hydrogen Production from Aqueous Solutions of Electron Donors. Russ. Chem. Rev. 2017, 86, 870–906. [Google Scholar] [CrossRef]
- Stavitskaya, A.V.; Kozlova, E.A.; Kurenkova, A.Y.; Glotov, A.P.; Selischev, D.S.; Ivanov, E.V.; Kozlov, D.V.; Vinokurov, V.A.; Fakhrullin, R.F.; Lvov, Y.M. Ru/CdS Quantum Dots Templated on Clay Nanotubes as Visible-Light-Active Photocatalysts: Optimization of S/Cd Ratio and Ru Content. Chem. Eur. J. 2020, 26, 13085–13092. [Google Scholar] [CrossRef]
- Tahir, M.; Tasleem, S.; Tahir, B. Recent Development in Band Engineering of Binary Semiconductor Materials for Solar Driven Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 15985–16038. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Markovskaya, D.V.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlova, E.A. New Insights into the Mechanism of Photocatalytic Hydrogen Evolution from Aqueous Solutions of Saccharides over CdS-Based Photocatalysts under Visible Light. Int. J. Hydrogen Energy 2020, 45, 30165–30177. [Google Scholar] [CrossRef]
- Kurenkova, A.Y.; Kozlova, E.A. CdS-Based Photocatalyst for Hydrogen Evolution from the Cellulose Aqueous Suspension. AIP Conf. Proc. 2020, 2301, 040007. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Gribov, E.N.; Kurenkova, A.Y.; Cherepanova, S.V.; Gerasimov, E.Y.; Kozlov, D.V. Synthesis of Multiphase Au/Cd0. 6Zn0.4S/ZnS Photocatalysts for Improved Photocatalytic Performance. Int. J. Hydrogen Energy 2019, 44, 23589–23599. [Google Scholar] [CrossRef]
- Christoforidis, K.C.; Fornasiero, P. Photocatalytic Hydrogen Production: A Rift into the Future Energy Supply. ChemCatChem 2017, 9, 1523–1544. [Google Scholar] [CrossRef] [Green Version]
- Kumaravel, V.; Mathew, S.; Bartlett, J.; Pillai, S.C. Photocatalytic Hydrogen Production Using Metal Doped TiO2: A Review of Recent Advances. Appl. Catal. B Environ. 2019, 244, 1021–1064. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Rodríguez-Castellón, E.; Conesa, J.C.; Fernández-García, M.; Kubacka, A. Measuring and Interpreting Quantum Efficiency for Hydrogen Photo-Production Using Pt-titania Catalysts. J. Catal. 2017, 347, 157–169. [Google Scholar] [CrossRef]
- Imizcoz, M.; Puga, A.V. Assessment of Photocatalytic Hydrogen Production from Biomass or Wastewaters Depending on the Metal Co-Catalyst and Its Deposition Method on TiO2. Catalysts 2019, 9, 584. [Google Scholar] [CrossRef] [Green Version]
- Cherepanova, S.; Markovskaya, D.; Kozlova, E. Identification of A Deleterious Phase in Photocatalyst Based on Cd1-xZnxS/Zn(OH)2 by Simulated XRD Patterns. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2017, 73, 360–368. [Google Scholar] [CrossRef]
- Croy, J.R.; Mostafa, S.; Hickman, L.; Heinrich, H.; Cuenya, B.R. Bimetallic Pt-Metal Catalysts for the Decomposition of Methanol: Effect of Secondary Metal on the Oxidation State, Activity, and Selectivity of Pt. Appl. Catal. A Gen. 2008, 350, 207–216. [Google Scholar] [CrossRef]
- Chetyrin, I.A.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Khudorozhkov, A.K.; Bukhtiyarov, V.I. In Situ XPS and MS Study of Methane Oxidation on the Pd–Pt/Al2O3 Catalysts. Top. Catal. 2020, 63, 66–74. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Lyulyukin, M.N.; Markovskaya, D.V.; Bukhtiyarov, A.V.; Prosvirin, I.P.; Cherepanova, S.V.; Kozlov, D.V. Photocatalytic CO2 Reduction over Ni-Modified Cd1−xZnxS-Based Photocatalysts: Effect of Phase Composition of Photocatalyst and Reaction Media on Reduction Rate and Product Distribution. Top. Catal. 2020, 63, 121–129. [Google Scholar] [CrossRef]
- Sanders, A.F.H.; De Jong, A.M.; De Beer, V.H.J.; Van Veen, J.A.R.; Niemantsverdriet, J.W. Formation of Cobalt-Molybdenum Sulfides in Hydrotreating Catalysts: A Surface Science Approach. Appl. Surf. Sci. 1999, 144–145, 380–384. [Google Scholar] [CrossRef]
- Li, C.; Yang, X.; Yang, B.; Yan, Y.; Qian, Y. Growth of Microtubular Complexes as Precursors to Synthesize Nanocrystalline ZnS and CdS. J. Cryst. Growth 2006, 291, 45–51. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Kozlova, E.A.; Gerasimov, E.Y.; Bukhtiyarov, A.V.; Kozlov, D.V. New Photocatalysts Based on Cd0.3Zn0.7S and Ni(OH)2 for Hydrogen Production from Ethanol Aqueous Solutions under Visible Light. Appl. Catal. A Gen. 2018, 563, 170–176. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium Dioxide Photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Markovskaya, D.V.; Cherepanova, S.V.; Saraev, A.A.; Gerasimov, E.Y.; Kozlova, E.A. Photocatalytic Hydrogen Evolution from Aqueous Solutions of Na2S/Na2SO3 under Visible Light Irradiation on CuS/Cd0. 3Zn0.7S and NizCd0.3Zn0.7S1+z. Chem. Eng. J. 2015, 262, 146–155. [Google Scholar] [CrossRef]
- Preethi, V.; Kanmani, S. Photocatalytic hydrogen Production Using Fe2O3-Based Core Shell Nano Particles with ZnS and CdS. Int. J. Hydrogen Energy 2014, 39, 1613–1622. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Awatani, T.; Dobson, K.D.; McQuillan, A.J.; Ohtani, B.; Uosaki, K. In Situ Infrared Spectroscopic Studies of Adsorption of Lactic Acid and Related Compounds on the TiO2 and CdS Semiconductor Photocatalyst Surfaces from Aqueous Solutions. Chem. Lett. 1998, 27, 849–850. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, X.; Han, H.; Fan, Y.; Zhang, S.; Meng, S.; Chen, S. Photocatalytic Hydrogen Evolution from Biomass (Glucose Solution) on Au/CdS Nanorods with Au3+ Self-Reduction. J. Solid State Chem. 2020, 289, 121495. [Google Scholar] [CrossRef]
- Fu, X.; Long, J.; Wang, X.; Leung, D.Y.C.; Ding, Z.; Wu, L.; Zhang, Z.; Li, Z.; Fu, X. Photocatalytic Reforming of Biomass: A Systematic Study of Hydrogen Evolution from Glucose Solution. Int. J. Hydrogen Energy 2008, 33, 6484–6491. [Google Scholar] [CrossRef]
- Kampouri, S.; Stylianou, K.C. Dual-Functional Photocatalysis for Simultaneous Hydrogen Production and Oxidation of Organic Substances. ACS Catal. 2019, 9, 4247–4270. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Murcia, J.J.; Rizzo, L.; Ventre, G.; Pepe, G.; Campiglia, P.; Hidalgo, M.C.; Navío, J.A.; Sannino, D. Photocatalytic Hydrogen Production from Degradation of Glucose over Fluorinated and Platinized TiO2 Catalysts. J. Catal. 2016, 339, 47–56. [Google Scholar] [CrossRef]
- Gomathisankar, P.; Yamamoto, D.; Katsumata, H.; Suzuki, T.; Kaneco, S. Photocatalytic Hydrogen Production with Aid of Simultaneous Metal Deposition Using Titanium Dioxide from Aqueous Glucose Solution. Int. J. Hydrogen Energy 2013, 38, 5517–5524. [Google Scholar] [CrossRef]
- Li, Y.; Gao, D.; Peng, S.; Lu, G.; Li, S. Photocatalytic Hydrogen Evolution over Pt/Cd0.5Zn0.5S from Saltwater Using Glucose as Electron Donor: An Investigation of the Influence of Electrolyte NaCl. Int. J. Hydrogen Energy 2011, 36, 4291–4297. [Google Scholar] [CrossRef]
- Kozlova, E.A.; Kurenkova, A.Y.; Gerasimov, E.Y.; Gromov, N.V.; Medvedeva, T.B.; Saraev, A.A.; Kaichev, V.V. Comparative Study of Photoreforming of Glycerol on Pt/TiO2 and CuOx/TiO2 Photocatalysts under UV Light. Mater. Lett. 2021, 283, 128901. [Google Scholar] [CrossRef]







| Photocatalyst | Substrate | Light Source | W, μmol H2·h−1·g−1 | AQE, % | Reference |
|---|---|---|---|---|---|
| CNx | xylan | AM 1.5G, 100 mW cm–2 | 137 | - | [24] |
| lignin | 40.8 | - | |||
| 0.32%Pt/TiO2 | rice husks | Natural sunlight, 45 mW cm–2 | 95 | - | [27] |
| 5%Pt/TiO2 | potato | Xe, 500 mW cm–2 | 13 | - | [9] |
| seaweed | 25 | - | |||
| chlorella | 90 | - | |||
| rice plant | 8 | - | |||
| 0.1%Pt/TiO2 | starch | Solar Box 1500e, 500 W m–2 | 806 | 3.28 | [23] |
| 0.25%Pt/o-g-C3N4 | 593 | 0.54 | |||
| CdS/CdOx | lignin | AM 1.5G, 100 mW cm–2 | 260 | - | [22] |
| bagasse | 650 | - | |||
| 3%Au/CdS | soluble starch | Xe, cut filter (λ > 450 nm) | 20 | - | [53] |
| PtOx/Cd0.7Zn0.3S/ZnS | corn starch | LED 450 nm, 14 mW cm−2 | 730 | 1.8 | This study |
| N of Filtrate | С0 NaOH, M | Heating Temperature | Organic Compounds Detected | Concentration, mM·L−1 | TOC, g L−1 |
|---|---|---|---|---|---|
| 1 * | 0.1 | - | glucose | trace | 0.10 |
| 1 | 0.1 | - | glucose | 0.38 | 0.21 |
| acetate | 0.42 | ||||
| glycolate | 0.04 | ||||
| lactate | 0.04 | ||||
| gluconate | trace | ||||
| 2 * | 0.1 | 70 °C | glucose | 0.06 | 0.80 |
| 2 | 0.1 | 70 °C | glucose | 0.4 | 1.38 |
| acetate | 0.18 | ||||
| glycolate | 0.2 | ||||
| lactate | 0.09 | ||||
| gluconate | trace | ||||
| formate | 0.81 | ||||
| 3 * | 1.0 | - | glucose | trace | 2.58 |
| 3 | 1.0 | - | glucose | 0.43 | 3.12 |
| acetate | 0.36 | ||||
| glycolate | 0.21 | ||||
| gluconate | trace | ||||
| 4 * | 1.0 | 70 °C | glucose | 0.09 | 3.19 |
| 4 | 1.0 | 70 °C | glucose | 0.54 | 3.04 |
| acetate | 0.69 | ||||
| glycolate | 0.2 | ||||
| gluconate | 0.1 | ||||
| formate | 0.96 | ||||
| 5 * | 0.1 | 105 °C | lactate | 1.01 | 2.03 |
| formate | 5.66 | ||||
| glycolate | 0.11 | ||||
| acetate | 0.37 | ||||
| 5 | 0.1 | 105 °C | lactate | 1.01 | 1.89 |
| formate | 5.23 | ||||
| glycolate | 0.10 | ||||
| acetate | 0.26 | ||||
| 6 * | 1 | 105 °C | lactate | 0.73 | 1.55 |
| formate | 2.80 | ||||
| acetate | 3.21 | ||||
| 6 | 1 | 105 °C | lactate | 0.63 | 1.14 |
| formate | 2.58 | ||||
| acetate | 1.12 | ||||
| 7 * | 0.1 | 140 °C | glucose | trace | 2.34 |
| lactate | 2.32 | ||||
| formate | 10.18 | ||||
| glycolate | 0.29 | ||||
| acetate | 0.92 | ||||
| 7 | 0.1 | 140 °C | glucose | trace | 2.02 |
| lactate | 2.30 | ||||
| formate | 10.11 | ||||
| glycolate | 0.29 | ||||
| acetate | 0.92 | ||||
| 8 * | 1 | 140 °C | lactate | 1.31 | 2.24 |
| formate | 4.96 | ||||
| glycolate | 0.4 | ||||
| acetate | 1.01 | ||||
| 8 | 1 | 140 °C | lactate | 1.05 | 1.67 |
| formate | 4.02 | ||||
| glycolate | 0.13 | ||||
| acetate | 1.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurenkova, A.Y.; Medvedeva, T.B.; Gromov, N.V.; Bukhtiyarov, A.V.; Gerasimov, E.Y.; Cherepanova, S.V.; Kozlova, E.A. Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst. Catalysts 2021, 11, 870. https://doi.org/10.3390/catal11070870
Kurenkova AY, Medvedeva TB, Gromov NV, Bukhtiyarov AV, Gerasimov EY, Cherepanova SV, Kozlova EA. Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst. Catalysts. 2021; 11(7):870. https://doi.org/10.3390/catal11070870
Chicago/Turabian StyleKurenkova, Anna Y., Tatiana B. Medvedeva, Nikolay V. Gromov, Andrey V. Bukhtiyarov, Evgeny Y. Gerasimov, Svetlana V. Cherepanova, and Ekaterina A. Kozlova. 2021. "Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst" Catalysts 11, no. 7: 870. https://doi.org/10.3390/catal11070870
APA StyleKurenkova, A. Y., Medvedeva, T. B., Gromov, N. V., Bukhtiyarov, A. V., Gerasimov, E. Y., Cherepanova, S. V., & Kozlova, E. A. (2021). Sustainable Hydrogen Production from Starch Aqueous Suspensions over a Cd0.7Zn0.3S-Based Photocatalyst. Catalysts, 11(7), 870. https://doi.org/10.3390/catal11070870