A Review of Photocatalytic Materials for Urban NOx Remediation
Abstract
:1. Introduction
1.1. The Issue of Urban NOx Pollution
1.2. Possible NOx Removal Methods
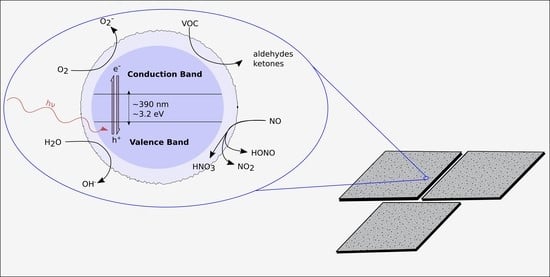
1.3. Photocatalytic Surface Principle of Operation
1.4. Previous Reviews
1.5. Scope and Method
2. Results
2.1. Laboratory Results
2.1.1. Impact of Physical Parameters
Humidity
Irradiance
Flow
Inlet Concentration
Co-Pollutants
Physical Parameters Summary
2.1.2. Durability
Durability Summary
2.1.3. Material Improvements
Doping
Support Material
Other Catalysts
Material Improvements Summary
2.2. Field Results
2.2.1. Streets
Copenhagen
Hong Kong
Hengelo
Madrid
Tsitsihar
Bergamo—Model Street Canyon
Bergamo—Concrete Paving
Streets Summary
2.2.2. Walls
Paris
Sir John Cass School, London
Artworks Elephant, London
Netherlands, Motorway Study
Gyeongbu Expressway, Korea
Gaudalupe Station, Manila
Walls Summary
2.2.3. Tunnels and Enclosed Spaces
Umberto I Tunnel
Leopold II Tunnel
Koningstunnel
Car Park Studies
Tunnels and Enclosed Spaces Summary
2.3. Modelling
Modelling Summary
3. Discussion
3.1. Impact of Physical Parameters
3.2. Durability
3.3. Selectivity
3.4. Material Improvements
3.5. Comparison of Field Studies
3.6. Comparison of Lab Studies
3.7. Modelling Results
3.8. Previous Review Efforts
4. Conclusions and Summary
5. Recommendations
- Plain anatase or rutile TiO2 should not be used as a PCO surface due to their low selectivity and likely negative impacts on air quality. Optimised PCO active components and support materials which are more selective must be developed, or existing improvements from lab studies must be proved in the field.
- A new standard for lab testing, with relevance to ambient conditions, quantification of by-products, and transferable results is necessary.
- A standardised metric is needed for the assessment of NOx abatement efficiency (separating NO and NO2), which takes into account selectivity as well activity, such as the DeNOx index.
- A standard method for field testing, which accounts for; accurate comparisons of active and control areas, sampling inlet position, averaging times for calculating abatement, seasonal changes, and durability must be developed to make studies more reliable and comparable.
- Site-specific field testing (as well as lab testing) is necessary before deployment for assessment of performance and passivation. Assessment of the prevailing humidity, rainfall, irradiance and pollution levels at a site should also be conducted before field tests are considered.
- Determination of potential by-products including VOCs and reactive nitrogen oxides is necessary for both the field and lab tests.
- When assessing PCO materials more consideration should be taken for durability in the proposed field environment, abrasive wear, poisoning and nitrate build-up should be accounted for.
- Similarly, modelling should account for loss of performance over time, as well as seasonal changes in weather.
- In future, commercial materials should be tested under standardised conditions and certified for use, to ensure that products which have a negative impact on air quality are not sold.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AQEG | Air Quality Expert Group |
| CNT | Carbon NanoTube |
| EIC | Environmental Industries Commission |
| I | Irradiance |
| NOx | reactive Nitrogen Oxides (NO and NO2) |
| NOy | all oxidised atmospheric odd-Nitrogen species |
| PCO | PhotoCatalytic Oxidation |
| PC | PhotoCatalytic |
| PhotoPAQ | Photocatalytic remediation Processes on Air Quality |
| PICADA | Photo-catalytic Innovative Coverings and Applications for De-pollution Assessment |
| PM | Particulate Matter |
| Q | Flow |
| RE | Removal Efficiency |
| rGO | reduced Graphene Oxide |
| RH | Relative Humidity |
| SA/V | Surface Area / Volume (ratio) |
| SSA | Specific Surface Area |
| T | Temperature |
| VOC | Volatile Organic Compound |
| WD | Wind Direction |
| WS | Wind Speed |
References
- Nakata, K.; Ochiai, T.; Murakami, T.; Fujishima, A. Photoenergy Conversion with TiO2 Photocatalysis: New Materials and Recent Applications. Electrochim. Acta 2012, 84, 103–111. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent Advances in Photocatalysis for Environmental Applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic Oxidation for Indoor Air Purification: A Literature Review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Cardellicchio, L. Self-Cleaning and Colour-Preserving Efficiency of Photocatalytic Concrete: Case Study of the Jubilee Church in Rome. Build. Res. Inf. 2020, 48, 160–179. [Google Scholar] [CrossRef]
- WHO. Air Quality Guidelines: Global Update 2005: Particulate Matter, Ozone, Nitrogen Dioxide, and Sulfur Dioxide; World Health Organization: Geneve, Switzerland, 2006. [Google Scholar]
- Directive 1999/30/EC of the European Parliament and the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:31999L0030&from=EN (accessed on 20 May 2021).
- Directive 2008/50/EC of the European Parliament and the Council. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32008L0050&from=en (accessed on 20 May 2021).
- London Has Already Reached Air Pollution Limits for 2018. Available online: https://www.newscientist.com/article/2159875-london-has-already-reached-air-pollution-limits-for-2018/ (accessed on 20 May 2021).
- Zhang, R.; Tie, X.; Bond, D.W. Impacts of Anthropogenic and Natural NOx Sources over the U.S. on Tropospheric Chemistry. Proc. Natl. Acad. Sci. USA 2003, 100, 1505–1509. [Google Scholar] [CrossRef] [Green Version]
- Fuentes, J.D.; Lerdau, M.; Atkinson, R.; Baldocchi, D.; Bottenheim, J.W.; Ciccioli, P.; Lamb, B.; Geron, C.; Gu, L.; Guenther, A. Biogenic Hydrocarbons in the Atmospheric Boundary Layer: A Review. Bull. Am. Meteorol. Soc. 2000, 81, 1537–1576. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.N.; Aber, J.D.; Erisman, J.W.; Seitzinger, S.P.; Howarth, R.W.; Cowling, E.B.; Cosby, B.J. The Nitrogen Cascade. BioScience 2003, 53, 341–356. [Google Scholar] [CrossRef]
- Environmental Industries Commission. Towards Purer Air: A Review of the Latest Evidence of the Effectiveness of Photocatalytic Materials and Treatments in Tackling Local Air Pollution; Technical Report; EIC: London, UK, 2017. [Google Scholar]
- Brandenberger, S.; Kröcher, O.; Tissler, A.; Althoff, R. The State of the Art in Selective Catalytic Reduction of NOx by Ammonia Using Metal-Exchanged Zeolite Catalysts. Catal. Rev. 2008, 50, 492–531. [Google Scholar] [CrossRef]
- Environmental Industries Comission. A Clear Choice for the UK: Technology Options for Tackling Air Pollution; Technical Report; EIC: London, UK, 2015. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Chen, H.; Nanayakkara, C.E.; Grassian, V.H. Titanium Dioxide Photocatalysis in Atmospheric Chemistry. Chem. Rev. 2012, 112, 5919–5948. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the Anatase to Rutile Phase Transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Luttrell, T.; Halpegamage, S.; Tao, J.; Kramer, A.; Sutter, E.; Batzill, M. Why Is Anatase a Better Photocatalyst than Rutile?—Model Studies on Epitaxial TiO2 Films. Sci. Rep. 2014, 4, 4043. [Google Scholar] [CrossRef] [Green Version]
- Ohno, T.; Sarukawa, K.; Tokieda, K.; Matsumura, M. Morphology of a TiO2 Photocatalyst (Degussa, P-25) Consisting of Anatase and Rutile Crystalline Phases. J. Catal. 2001, 203, 82–86. [Google Scholar] [CrossRef]
- Patzsch, J.; Folli, A.; Macphee, D.E.; Bloh, J.Z. On the Underlying Mechanisms of the Low Observed Nitrate Selectivity in Photocatalytic NOx Abatement and the Importance of the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2017, 19, 32678–32686. [Google Scholar] [CrossRef] [Green Version]
- Ballari, M.M.; Yu, Q.L.; Brouwers, H.J.H. Experimental Study of the NO and NO2 Degradation by Photocatalytically Active Concrete. Catal. Today 2011, 161, 175–180. [Google Scholar] [CrossRef]
- Laufs, S.; Burgeth, G.; Duttlinger, W.; Kurtenbach, R.; Maban, M.; Thomas, C.; Wiesen, P.; Kleffmann, J. Conversion of Nitrogen Oxides on Commercial Photocatalytic Dispersion Paints. Atmos. Environ. 2010, 44, 2341–2349. [Google Scholar] [CrossRef]
- Monks, P. Paints and Surfaces for the Removal of Nitrogen Oxides; Technical Report; Air Quality Expert Group: London, UK, 2016.
- Monge, M.E.; D’Anna, B.; George, C. Nitrogen Dioxide Removal and Nitrous Acid Formation on Titanium Oxide Surfaces—An Air Quality Remediation Process? Phys. Chem. Chem. Phys. 2010, 12, 8991. [Google Scholar] [CrossRef]
- Langridge, J.M.; Gustafsson, R.J.; Griffiths, P.T.; Cox, R.A.; Lambert, R.M.; Jones, R.L. Solar Driven Nitrous Acid Formation on Building Material Surfaces Containing Titanium Dioxide: A Concern for Air Quality in Urban Areas? Atmos. Environ. 2009, 43, 5128–5131. [Google Scholar] [CrossRef]
- Ndour, M.; D’Anna, B.; George, C.; Ka, O.; Balkanski, Y.; Kleffmann, J.; Stemmler, K.; Ammann, M. Photoenhanced Uptake of NO2 on Mineral Dust: Laboratory Experiments and Model Simulations. Geophys. Res. Lett. 2008, 35. [Google Scholar] [CrossRef] [Green Version]
- Monge, M.E.; George, C.; D’Anna, B.; Doussin, J.F.; Jammoul, A.; Wang, J.; Eyglunent, G.; Solignac, G.; Daële, V.; Mellouki, A. Ozone Formation from Illuminated Titanium Dioxide Surfaces. J. Am. Chem. Soc. 2010, 132, 8234–8235. [Google Scholar] [CrossRef]
- Beaumont, S.K.; Gustafsson, R.J.; Lambert, R.M. Heterogeneous Photochemistry Relevant to the Troposphere: H2O2 Production during the Photochemical Reduction of NO2 to HONO on UV-Illuminated TiO2 Surfaces. ChemPhysChem 2009, 10, 331–333. [Google Scholar] [CrossRef]
- Laboratory, L.B.N. Evaluation of Titanium Dioxide as a Photocatalyst for Removing Air Pollutants; Technical Report; California Energy Commission Public Interest Energy Research Program: Sacramento, CA, USA, 2008.
- Auvinen, J.; Wirtanen, L. The Influence of Photocatalytic Interior Paints on Indoor Air Quality. Atmos. Environ. 2008, 42, 4101–4112. [Google Scholar] [CrossRef]
- Salthammer, T.; Fuhrmann, F. Photocatalytic Surface Reactions on Indoor Wall Paint. Environ. Sci. Technol. 2007, 41, 6573–6578. [Google Scholar] [CrossRef]
- Geiss, O.; Cacho, C.; Barrero-Moreno, J.; Kotzias, D. Photocatalytic Degradation of Organic Paint Constituents-Formation of Carbonyls. Build. Environ. 2012, 48, 107–112. [Google Scholar] [CrossRef]
- Toro, C.; Jobson, B.T.; Haselbach, L.; Shen, S.; Chung, S.H. Photoactive Roadways: Determination of CO, NO and VOC Uptake Coefficients and Photolabile Side Product Yields on TiO2 Treated Asphalt and Concrete. Atmos. Environ. 2016, 139, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Kebede, M.A.; Scharko, N.K.; Appelt, L.E.; Raff, J.D. Formation of Nitrous Acid during Ammonia Photooxidation on TiO2 under Atmospherically Relevant Conditions. J. Phys. Chem. Lett. 2013, 4, 2618–2623. [Google Scholar] [CrossRef]
- Boonen, E.; Beeldens, A. Recent Photocatalytic Applications for Air Purification in Belgium. Coatings 2014, 4, 553–573. [Google Scholar] [CrossRef] [Green Version]
- Boonen, E.; Beeldens, A. Photocatalytic Roads: From Lab Tests to Real Scale Applications. Eur. Transp. Res. Rev. 2013, 5, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Macphee, D.E.; Folli, A. Photocatalytic Concretes—The Interface between Photocatalysis and Cement Chemistry. Cem. Concr. Res. 2016, 85, 48–54. [Google Scholar] [CrossRef]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 Photocatalysis in Cementitious Systems: Insights into Self-Cleaning and Depollution Chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic Air Cleaners and Materials Technologies—Abilities and Limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Chen, J.; Poon, C.S. Photocatalytic Construction and Building Materials: From Fundamentals to Applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium Dioxide Photocatalysis: Present Situation and Future Approaches. C. R. Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269. [Google Scholar] [CrossRef]
- Fujishima, A.; Hashimoto, K.; Watanabe, T. TiO2 Photocatalysis: Fundamentals and Applications; Bkc: Tokyo, Japan, 1999. [Google Scholar]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemann, D.W. Environmental Applications of Semiconductor Photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- De Richter, R.; Caillol, S. Fighting Global Warming: The Potential of Photocatalysis against CO2, CH4, N2O, CFCs, Tropospheric O3, BC and Other Major Contributors to Climate Change. J. Photochem. Photobiol. C Photochem. Rev. 2011, 12, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic Purification of Volatile Organic Compounds in Indoor Air: A Literature Review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- ISO 22197-1:2007. 2007. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data\/standard/04/07/40761.html (accessed on 20 May 2021).
- ISO/TC 206 Fine ceramics. ISO 22197-1:2016. 2016. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/54/65416.html (accessed on 20 May 2021).
- Motohashi, K.; Dehn, F.; Ohama, Y. Standardization of Testing Methods for Construction Materials with TiO2 Photocatalyst. In Applications of Titanium Dioxide Photocatalysis to Construction Materials: State-of-the-Art Report of the RILEM Technical Committee 194-TDP; Ohama, Y., Van Gemert, D., Eds.; RILEM State of the Art Reports; Springer: Dordrecht, The Netherlands, 2011; pp. 37–41. [Google Scholar] [CrossRef]
- Khanal, V.; Balayeva, N.O.; Günnemann, C.; Mamiyev, Z.; Dillert, R.; Bahnemann, D.W.; Subramanian, V.R. Photocatalytic NOx Removal Using Tantalum Oxide Nanoparticles: A Benign Pathway. Appl. Catal. B Environ. 2021, 291, 119974. [Google Scholar] [CrossRef]
- Freitag, J.; Domínguez, A.; Niehaus, T.A.; Hülsewig, A.; Dillert, R.; Frauenheim, T.; Bahnemann, D.W. Nitrogen(II) Oxide Charge Transfer Complexes on TiO2: A New Source for Visible-Light Activity. J. Phys. Chem. C 2015, 119, 4488–4501. [Google Scholar] [CrossRef]
- Balayeva, N.O.; Fleisch, M.; Bahnemann, D.W. Surface-Grafted WO3/TiO2 Photocatalysts: Enhanced Visible-Light Activity towards Indoor Air Purification. Catal. Today 2018, 313, 63–71. [Google Scholar] [CrossRef]
- Chen, M.; Chu, J.W. NOx Photocatalytic Degradation on Active Concrete Road Surface—From Experiment to Real-Scale Application. J. Clean. Prod. 2011, 19, 1266–1272. [Google Scholar] [CrossRef]
- Folli, A.; Strøm, M.; Madsen, T.P.; Henriksen, T.; Lang, J.; Emenius, J.; Klevebrant, T.; Nilsson, Å. Field Study of Air Purifying Paving Elements Containing TiO2. Atmos. Environ. 2015, 107, 44–51. [Google Scholar] [CrossRef]
- Hassan, M.; Mohammad, L.N.; Asadi, S.; Dylla, H.; Cooper, S. Sustainable Photocatalytic Asphalt Pavements for Mitigation of Nitrogen Oxide and Sulfur Dioxide Vehicle Emissions. J. Mater. Civ. Eng. 2013, 25, 365–371. [Google Scholar] [CrossRef]
- Hüsken, G.; Hunger, M.; Brouwers, H.J.H. Experimental Study of Photocatalytic Concrete Products for Air Purification. Build. Environ. 2009, 44, 2463–2474. [Google Scholar] [CrossRef]
- De Melo, J.V.S.; Trichês, G. Evaluation of the Influence of Environmental Conditions on the Efficiency of Photocatalytic Coatings in the Degradation of Nitrogen Oxides (NOx). Build. Environ. 2012, 49, 117–123. [Google Scholar] [CrossRef]
- Dillert, R.; Stötzner, J.; Engel, A.; Bahnemann, D.W. Influence of Inlet Concentration and Light Intensity on the Photocatalytic Oxidation of Nitrogen(II) Oxide at the Surface of Aeroxide® TiO2 P25. J. Hazard. Mater. 2012, 211–212, 240–246. [Google Scholar] [CrossRef]
- Herrmann, J.M.; Péruchon, L.; Puzenat, E.; Guillard, C. Photocatalysis: From fundamentals to self-cleaning glass application. In International RILEM Symposium on Photocatalysis, Environment and Construction Materials; RILEM Publications SARL: Paris, France, 2007; pp. 8–9. [Google Scholar]
- Jacoby, W.A.; Blake, D.M.; Noble, R.D.; Koval, C.A. Kinetics of the Oxidation of Trichloroethylene in Air via Heterogeneous Photocatalysis. J. Catal. 1995, 157, 87–96. [Google Scholar] [CrossRef]
- Lim, T.H.; Jeong, S.M.; Kim, S.D.; Gyenis, J. Photocatalytic Decomposition of NO by TiO2 Particles. J. Photochem. Photobiol. A Chem. 2000, 134, 209–217. [Google Scholar] [CrossRef]
- Peral, J.; Ollis, D.F. Heterogeneous Photocatalytic Oxidation of Gas-Phase Organics for Air Purification: Acetone, 1-Butanol, Butyraldehyde, Formaldehyde, and m-Xylene Oxidation. J. Catal. 1992, 136, 554–565. [Google Scholar] [CrossRef]
- Hunger, M.; Hüsken, G.; Brouwers, H. Photocatalytic Degradation of Air Pollutants—From Modeling to Large Scale Application. Cem. Concr. Res. 2010, 40, 313–320. [Google Scholar] [CrossRef]
- Gallus, M.; Akylas, V.; Barmpas, F.; Beeldens, A.; Boonen, E.; Boréave, A.; Cazaunau, M.; Chen, H.; Daële, V.; Doussin, J.F.; et al. Photocatalytic De-Pollution in the Leopold II Tunnel in Brussels: NOx Abatement Results. Build. Environ. 2015, 84, 125–133. [Google Scholar] [CrossRef]
- Zouzelka, R.; Rathousky, J. Photocatalytic Abatement of NOx Pollutants in the Air Using Commercial Functional Coating with Porous Morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Rhee, I.; Lee, J.S.; Kim, J.; Kim, J.H. Nitrogen Oxides Mitigation Efficiency of Cementitious Materials Incorporated with TiO2. Materials 2018, 11, 877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ballari, M.M.; Brouwers, H.J.H. Full Scale Demonstration of Air-Purifying Pavement. J. Hazard. Mater. 2013, 254–255, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallus, M.; Ciuraru, R.; Mothes, F.; Akylas, V.; Barmpas, F.; Beeldens, A.; Bernard, F.; Boonen, E.; Boréave, A.; Cazaunau, M.; et al. Photocatalytic Abatement Results from a Model Street Canyon. Environ. Sci. Pollut. Res. 2015, 22, 18185–18196. [Google Scholar] [CrossRef] [PubMed]
- Martinez, T.; Bertron, A.; Ringot, E.; Escadeillas, G. Degradation of NO Using Photocatalytic Coatings Applied to Different Substrates. Build. Environ. 2011, 46, 1808–1816. [Google Scholar] [CrossRef]
- Araña, J.; Garzón Sousa, D.; González Díaz, O.; Pulido Melián, E.; Doña Rodríguez, J.M. Effect of NO2 and NO3−/HNO3 Adsorption on NO Photocatalytic Conversion. Appl. Catal. B Environ. 2019, 244, 660–670. [Google Scholar] [CrossRef]
- Maggos, T.; Bartzis, J.G.; Liakou, M.; Gobin, C. Photocatalytic Degradation of NOx Gases Using TiO2-Containing Paint: A Real Scale Study. J. Hazard. Mater. 2007, 146, 668–673. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Mak, C.L.; Chan, L.Y. Photodegradation of Volatile Organic Compounds (VOCs) and NO for Indoor Air Purification Using TiO2: Promotion versus Inhibition Effect of NO. Appl. Catal. B Environ. 2003, 42, 119–129. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C.; Zou, S.C.; Mak, C.L. Inhibition Effect of SO2 on NOx and VOCs during the Photodegradation of Synchronous Indoor Air Pollutants at Parts per Billion (ppb) Level by TiO2. Appl. Catal. B Environ. 2004, 49, 187–193. [Google Scholar] [CrossRef]
- De Melo, J.V.S.; Trichês, G.; Gleize, P.J.P.; Villena, J. Development and Evaluation of the Efficiency of Photocatalytic Pavement Blocks in the Laboratory and after One Year in the Field. Constr. Build. Mater. 2012, 37, 310–319. [Google Scholar] [CrossRef]
- Osborn, D.; Hassan, M.; Asadi, S.; White, J.R. Durability Quantification of TiO2 Surface Coating on Concrete and Asphalt Pavements. J. Mater. Civ. Eng. 2014, 26, 331–337. [Google Scholar] [CrossRef]
- Jiménez-Relinque, E.; Hingorani, R.; Rubiano, F.; Grande, M.; Castillo, Á.; Castellote, M. In Situ Evaluation of the NOx Removal Efficiency of Photocatalytic Pavements: Statistical Analysis of the Relevance of Exposure Time and Environmental Variables. Environ. Sci. Pollut. Res. 2019, 26, 36088–36095. [Google Scholar] [CrossRef]
- Kaja, A.M.; Brouwers, H.J.H.; Yu, Q.L. NOx Degradation by Photocatalytic Mortars: The Underlying Role of the CH and C-S-H Carbonation. Cem. Concr. Res. 2019, 125, 105805. [Google Scholar] [CrossRef]
- Poon, C.S.; Cheung, E. NO Removal Efficiency of Photocatalytic Paving Blocks Prepared with Recycled Materials. Constr. Build. Mater. 2007, 21, 1746–1753. [Google Scholar] [CrossRef]
- Jimenez-Relinque, E.; Rodriguez-Garcia, J.R.; Castillo, A.; Castellote, M. Characteristics and Efficiency of Photocatalytic Cementitious Materials: Type of Binder, Roughness and Microstructure. Cem. Concr. Res. 2015, 71, 124–131. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Balbuena, J.; Cruz-Yusta, M.; Sánchez, L.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Photocatalytic NOx Abatement by Calcium Aluminate Cements Modified with TiO2: Improved NO2 Conversion. Cem. Concr. Res. 2015, 70, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Gauvin, F.; Caprai, V.; Yu, Q.L.; Brouwers, H.J.H. Effect of the Morphology and Pore Structure of Porous Building Materials on Photocatalytic Oxidation of Air Pollutants. Appl. Catal. B Environ. 2018, 227, 123–131. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.; Zhang, K.; Ning, Z.; Leung, M.K.H. Solar Photocatalytic Asphalt for Removal of Vehicular NOx: A Feasibility Study. Appl. Energy 2018, 225, 535–541. [Google Scholar] [CrossRef]
- Witkowski, H.; Jackiewicz-Rek, W.; Chilmon, K.; Jarosławski, J.; Tryfon-Bojarska, A.; Gąsiński, A. Air Purification Performance of Photocatalytic Concrete Paving Blocks after Seven Years of Service. Appl. Sci. 2019, 9, 1735. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.; Wang, P.; Li, W.; Bai, Y.; Xie, H.; Zhou, Y.; Ye, L. Change in Photocatalytic NO Removal Mechanisms of Ultrathin BiOBr/BiOI via NO3- Adsorption. Appl. Catal. B Environ. 2019, 243, 322–329. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Bai, H. Photocatalytic Removal of NO and NO2 Using Titania Nanotubes Synthesized by Hydrothermal Method. J. Environ. Sci. 2014, 26, 1180–1187. [Google Scholar] [CrossRef]
- Sheng, Z.; Wu, Z.; Liu, Y.; Wang, H. Gas-Phase Photocatalytic Oxidation of NO over Palladium Modified TiO2 Catalysts. Catal. Commun. 2008, 9, 1941–1944. [Google Scholar] [CrossRef]
- Boonen, E.; Akylas, V.; Barmpas, F.; Boréave, A.; Bottalico, L.; Cazaunau, M.; Chen, H.; Daële, V.; De Marco, T.; Doussin, J.F.; et al. Construction of a Photocatalytic De-Polluting Field Site in the Leopold II Tunnel in Brussels. J. Environ. Manag. 2015, 155, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Liu, Y. NOx Removal from Vehicle Emissions by Functionality Surface of Asphalt Road. J. Hazard. Mater. 2010, 174, 375–379. [Google Scholar] [CrossRef]
- Castellote, M. Device for Determining Photocatalytic Properties of Materials. WO Patent 2017085342 A1, 26 May 2017. [Google Scholar]
- Hunger, M.; Brouwers, H. Comparative Study on Cementitious Products Containing Titanium Dioxide as Photo-Catalyst. In Proceedings of the International RILEM Symposium on Photocatalysis, Environment and Construction Materials, Florence, Italy, 8–9 October 2007. [Google Scholar]
- Murata, Y.; Obata, H.; Tawara, H.; Murata, K. NOx-Cleaning Paving Block. U.S. Patent US5861205A, 19 January 1999. [Google Scholar]
- Cassar, L.; Cucitore, R.; Pepe, C. Cement-Based Paving Blocks for Photocatalytic Paving for the Abatement of Urban Pollutants. U.S. Patent US7960042B2, 14 June 2011. [Google Scholar]
- Ma, J.; Wu, H.; Liu, Y.; He, H. Photocatalytic Removal of NOx over Visible Light Responsive Oxygen-Deficient TiO2. J. Phys. Chem. C 2014, 118, 7434–7441. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx Removal: Study of Cement Mortars with Iron- and Vanadium-Doped TiO2 as Visible Light–Sensitive Photocatalysts. Constr. Build. Mater. 2017, 149, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent Advances in TiO2-Based Photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Martinez-Oviedo, A. Enhancement of NOx Photo-Oxidation by Fe- and Cu-Doped Blue TiO2. Environ. Sci. Pollut. Res. 2020, 27, 26702–26713. [Google Scholar] [CrossRef]
- Ma, J.; He, H.; Liu, F. Effect of Fe on the Photocatalytic Removal of NOx over Visible Light Responsive Fe/TiO2 Catalysts. Appl. Catal. B Environ. 2015, 179, 21–28. [Google Scholar] [CrossRef]
- Balbuena, J.; Carraro, G.; Cruz, M.; Gasparotto, A.; Maccato, C.; Pastor, A.; Sada, C.; Barreca, D.; Sánchez, L. Advances in Photocatalytic NOx Abatement through the Use of Fe2O3/TiO2 Nanocomposites. RSC Adv. 2016, 6, 74878–74885. [Google Scholar] [CrossRef]
- Martinez-Oviedo, A.; Ray, S.K.; Nguyen, H.P.; Lee, S.W. Efficient Photo-Oxidation of NOx by Sn Doped Blue TiO2 Nanoparticles. J. Photochem. Photobiol. A Chem. 2019, 370, 18–25. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Du, H.L.; Shi, L. Zn-Doped TiO2 Nanoparticles with High Photocatalytic Activity Synthesized by Hydrogen–Oxygen Diffusion Flame. Appl. Catal. B Environ. 2008, 79, 208–215. [Google Scholar] [CrossRef]
- Papoulis, D.; Somalakidi, K.; Todorova, N.; Trapalis, C.; Panagiotaras, D.; Sygkridou, D.; Stathatos, E.; Gianni, E.; Mavrikos, A.; Komarneni, S. Sepiolite/TiO2 and Metal Ion Modified Sepiolite/TiO2 Nanocomposites: Synthesis, Characterization and Photocatalytic Activity in Abatement of NOx Gases. Appl. Clay Sci. 2019, 179, 105156. [Google Scholar] [CrossRef]
- Luna, M.; Gatica, J.M.; Vidal, H.; Mosquera, M.J. Au-TiO2/SiO2 Photocatalysts with NOx Depolluting Activity: Influence of Gold Particle Size and Loading. Chem. Eng. J. 2019, 368, 417–427. [Google Scholar] [CrossRef]
- Soylu, A.M.; Polat, M.; Erdogan, D.A.; Say, Z.; Yıldırım, C.; Birer, Ö.; Ozensoy, E. TiO2–Al2O3 Binary Mixed Oxide Surfaces for Photocatalytic NOx Abatement. Appl. Surf. Sci. 2014, 318, 142–149. [Google Scholar] [CrossRef]
- Fujiwara, K.; Pratsinis, S.E. Atomically Dispersed Pd on Nanostructured TiO2 for NO Removal by Solar Light. AIChE J. 2017, 63, 139–146. [Google Scholar] [CrossRef]
- Hu, Y.; Song, X.; Jiang, S.; Wei, C. Enhanced Photocatalytic Activity of Pt-Doped TiO2 for NOx Oxidation Both under UV and Visible Light Irradiation: A Synergistic Effect of Lattice Pt4+ and Surface PtO. Chem. Eng. J. 2015, 274, 102–112. [Google Scholar] [CrossRef]
- Hernández Rodríguez, M.J.; Pulido Melián, E.; García Santiago, D.; González Díaz, O.; Navío, J.A.; Doña Rodríguez, J.M. NO Photooxidation with TiO2 Photocatalysts Modified with Gold and Platinum. Appl. Catal. B Environ. 2017, 205, 148–157. [Google Scholar] [CrossRef]
- Xu, M.; Wang, Y.; Geng, J.; Jing, D. Photodecomposition of NOx on Ag/TiO2 Composite Catalysts in a Gas Phase Reactor. Chem. Eng. J. 2017, 307, 181–188. [Google Scholar] [CrossRef]
- Tobaldi, D.M.; Hortigüela Gallo, M.J.; Otero-Irurueta, G.; Singh, M.K.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Purely Visible-Light-Induced Photochromism in Ag–TiO2 Nanoheterostructures. Langmuir 2017, 33, 4890–4902. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, C.; He, H. Enhanced Photocatalytic Oxidation of NO over G-C3N4-TiO2 under UV and Visible Light. Appl. Catal. B Environ. 2016, 184, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Papailias, I.; Todorova, N.; Giannakopoulou, T.; Yu, J.; Dimotikali, D.; Trapalis, C. Photocatalytic Activity of Modified G-C3N4/TiO2 Nanocomposites for NOx Removal. Catal. Today 2017, 280, 37–44. [Google Scholar] [CrossRef]
- Trapalis, A.; Todorova, N.; Giannakopoulou, T.; Boukos, N.; Speliotis, T.; Dimotikali, D.; Yu, J. TiO2/Graphene Composite Photocatalysts for NOx Removal: A Comparison of Surfactant-Stabilized Graphene and Reduced Graphene Oxide. Appl. Catal. B Environ. 2016, 180, 637–647. [Google Scholar] [CrossRef]
- Lu, X.; Song, C.; Jia, S.; Tong, Z.; Tang, X.; Teng, Y. Low-Temperature Selective Catalytic Reduction of NOx with NH3 over Cerium and Manganese Oxides Supported on TiO2–Graphene. Chem. Eng. J. 2015, 260, 776–784. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, D.; Hu, X.; Qian, Y.; Li, D. Preparation of TiO2/Carbon Nanotubes/Reduced Graphene Oxide Composites with Enhanced Photocatalytic Activity for the Degradation of Rhodamine B. Nanomaterials 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Todorova, N.; Giannakopoulou, T.; Karapati, S.; Petridis, D.; Vaimakis, T.; Trapalis, C. Composite TiO2/Clays Materials for Photocatalytic NOx Oxidation. Appl. Surf. Sci. 2014, 319, 113–120. [Google Scholar] [CrossRef]
- Folli, A. Properties and Photochemistry of Valence-Induced-Ti3+ Enriched (Nb,N)-Codoped Anatase TiO2 Semiconductors. Phys. Chem. Chem. Phys. 2015, 17, 4849–4853. [Google Scholar] [CrossRef]
- Folli, A.; Bloh, J.Z.; Beukes, E.P.; Howe, R.F.; Macphee, D.E. Photogenerated Charge Carriers and Paramagnetic Species in (W,N)-Codoped TiO2 Photocatalysts under Visible-Light Irradiation: An EPR Study. J. Phys. Chem. C 2013, 117, 22149–22155. [Google Scholar] [CrossRef]
- Folli, A.; Bloh, J.; Macphee, D. Band Structure and Charge Carrier Dynamics in (W,N)-Codoped TiO2 Resolved by Electrochemical Impedance Spectroscopy Combined with UV–Vis and EPR Spectroscopies. J. Electroanal. Chem. 2016, 780, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Bloh, J.Z.; Folli, A.; Macphee, D.E. Adjusting Nitrogen Doping Level in Titanium Dioxide by Codoping with Tungsten: Properties and Band Structure of the Resulting Materials. J. Phys. Chem. C 2014, 118, 21281–21292. [Google Scholar] [CrossRef]
- Fujiwara, K.; Pratsinis, S.E. Single Pd Atoms on TiO2 Dominate Photocatalytic NOx Removal. Appl. Catal. B Environ. 2018, 226, 127–134. [Google Scholar] [CrossRef]
- Fujiwara, K.; Müller, U.; Pratsinis, S.E. Pd Subnano-Clusters on TiO2 for Solar-Light Removal of NO. ACS Catal. 2016, 6, 1887–1893. [Google Scholar] [CrossRef]
- Nava-Núñez, M.Y.; Jimenez-Relinque, E.; Grande, M.; Martínez-de la Cruz, A.; Castellote, M. Photocatalytic BiOX Mortars under Visible Light Irradiation: Compatibility, NOx Efficiency and Nitrate Selectivity. Catalysts 2020, 10, 226. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Ji, T.; Su, W.; Yang, B.; Zhang, Y.; Yang, Z. Photocatalytic NOx Abatement and Self-Cleaning Performance of Cementitious Composites with g-C3N4 Nanosheets under Visible Light. Constr. Build. Mater. 2019, 225, 120–131. [Google Scholar] [CrossRef]
- Jin, Q.; Saad, E.M.; Zhang, W.; Tang, Y.; Kurtis, K.E. Quantification of NOx Uptake in Plain and TiO2-Doped Cementitious Materials. Cem. Concr. Res. 2019, 122, 251–256. [Google Scholar] [CrossRef]
- Fan, W.; Chan, K.Y.; Zhang, C.; Leung, M.K.H. Advanced Solar Photocatalytic Asphalt for Removal of Vehicular NOx. Energy Procedia 2017, 143, 811–816. [Google Scholar] [CrossRef]
- Tawari, A.; Einicke, W.D.; Gläser, R. Photocatalytic Oxidation of NO over Composites of Titanium Dioxide and Zeolite ZSM-5. Catalysts 2016, 6, 31. [Google Scholar] [CrossRef]
- Ao, C.H.; Lee, S.C. Combination Effect of Activated Carbon with TiO2 for the Photodegradation of Binary Pollutants at Typical Indoor Air Level. J. Photochem. Photobiol. A Chem. 2004, 161, 131–140. [Google Scholar] [CrossRef]
- Folli, A.; Pochard, I.; Nonat, A.; Jakobsen, U.H.; Shepherd, A.M.; Macphee, D.E. Engineering Photocatalytic Cements: Understanding TiO2 Surface Chemistry to Control and Modulate Photocatalytic Performances. J. Am. Ceram. Soc. 2010, 93, 3360–3369. [Google Scholar] [CrossRef]
- Final Report Summary—LIGHT2CAT (Visible LIGHT Active PhotoCATalytic Concretes for Air Pollution Treatment)|Report Summary|FP7. 2015. Available online: https://cordis.europa.eu/project/id/283062/reporting (accessed on 20 May 2021).
- Folli, A.; Bloh, J.Z.; Strøm, M.; Pilegaard Madsen, T.; Henriksen, T.; Macphee, D.E. Efficiency of Solar-Light-Driven TiO2 Photocatalysis at Different Latitudes and Seasons. Where and When Does TiO2 Really Work? J. Phys. Chem. Lett. 2014, 5, 830–832. [Google Scholar] [CrossRef]
- Poulsen, S.; Svec, O.; Kaasgaard, M.; Folli, A. Visible LIGHT Active PhotoCATalytic Concretes for Air Pollution Treatment; Technical Report Version 2; DTI: Taastrup, Denmark, 2016. [Google Scholar]
- Flassak, T. Numerical simulation of the depollution effectiveness of photocatalytic coverings in street canyons. In Proceedings of the Photocatalysis: Science and Application for Urban Air Quality, The LIFE+ PhotoPaq Conference, Corse, France, 14–17 May 2012. [Google Scholar]
- Bolte, G.; Flassak, T. Numerical simulation of the effectiveness of photocatalytically active concrete surfaces. Int. Build. Mater. Conf. 2012, 18, 548–558. [Google Scholar]
- Guerrini, G.L.; Peccati, E. Photocatalytic Cementitious Roads for Depollution. In Proceedings of the International RILEM Symposium on Photocatalysis, Environment and Construction Materials, Florence, Italy, 8–9 October 2007. [Google Scholar]
- Suárez, S.; Portela, R.; Hernández-Alonso, M.D.; Sánchez, B. Development of a Versatile Experimental Setup for the Evaluation of the Photocatalytic Properties of Construction Materials under Realistic Outdoor Conditions. Environ. Sci. Pollut. Res. 2014, 21, 11208–11217. [Google Scholar] [CrossRef]
- Borlaza, L.J.S. Evaluation of the Efficiency of an Ultrafine Titanium Dioxide—Based Paint for Removing Nitrogen Oxides in an Indoor and Outdoor Environment. Master’s Thesis, Manila University, Metro Manila, Philippines, 2012. [Google Scholar]
- Kim, Y.K.; Hong, S.J.; Kim, H.B.; Lee, S.W. Evaluation of In-Situ NOx Removal Efficiency of Photocatalytic Concrete in Expressways. KSCE J. Civ. Eng. 2018, 22, 2274–2280. [Google Scholar] [CrossRef]
- Tremper, A.; Green, D. Artworks D-NOX Paint Trial Report; Technical Report; King’s College London: London, UK, 2016. [Google Scholar]
- IPL. Dutch Air Quality Innovation Programme Concluded; Technical Report; Rijkswaterstaat: Utrecht, The Netherlands, 2010. [Google Scholar]
- Maggos, T.; Plassais, A.; Bartzis, J.G.; Vasilakos, C.; Moussiopoulos, N.; Bonafous, L. Photocatalytic Degradation of NOx in a Pilot Street Canyon Configuration Using TiO2-Mortar Panels. Environ. Monit. Assess. 2008, 136, 35–44. [Google Scholar] [CrossRef]
- Barratt, B. CoL De-Nox Paint Statistical Report; Technical Report; King’s College London: London, UK, 2007. [Google Scholar]
- Barratt, B.; Carslaw, D.; Green, D. High Holborn D-NOx Paint Trial –Report 3 (Updated); Client: London Borough of Camden Report 3; King’s College London: London, UK, 2012. [Google Scholar]
- Ifang, S.; Gallus, M.; Liedtke, S.; Kurtenbach, R.; Wiesen, P.; Kleffmann, J. Standardization Methods for Testing Photo-Catalytic Air Remediation Materials: Problems and Solution. Atmos. Environ. 2014, 91, 154–161. [Google Scholar] [CrossRef]
- Colvile, R.; Barmpas, P.; Ossanlis, I.; Moussiopoulos, N. Assessment of the Effectiveness of NOx Absorbing Paint at the Sir John Cass Primary School; Technical Report; Imperial College: London, UK, 2007. [Google Scholar]
- Guerrini, G.L. Photocatalytic Performances in a City Tunnel in Rome: NOx Monitoring Results. Constr. Build. Mater. 2012, 27, 165–175. [Google Scholar] [CrossRef]
- Kerrod, J.; McIntyre, R. The Effectiveness of CristalACTiV™ for Depollution in Tunnels with Low Levels of Light; Technical Report; Cristal R&D: Lincolnshire, UK, 2014. [Google Scholar]
- Guerrini, G.L.; Peccati, E. TUNNEL “UMBERTO I”, IN ROME Monitoring Program Results; Technical Report 24; CTG Italcementi Group: Bergamo, Italy, 2008. [Google Scholar]
- Dylla, H.; Asadi, S.; Hassan, M.; Mohammad, L.N. Evaluating Photocatalytic Asphalt Pavement Effectiveness in Real-World Environments through Developing Models: A Statistical and Kinetic Study. Road Mater. Pavement Des. 2013, 14, 92–105. [Google Scholar] [CrossRef]
- Jiang, Z.; Yu, X.B. Impact of Visible-Solar-Light-Driven Photocatalytic Pavement on Air Quality Improvement. Transp. Res. Part D Transp. Environ. 2020, 84, 102341. [Google Scholar] [CrossRef]
- De O.B. Lira, J.; Padoin, N.; Vilar, V.J.P.; Soares, C. Photocatalytic NOx Abatement: Mathematical Modeling, CFD Validation and Reactor Analysis. J. Hazard. Mater. 2019, 372, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ballari, M.M.; Hunger, M.; Hüsken, G.; Brouwers, H.J.H. Modelling and Experimental Study of the NOx Photocatalytic Degradation Employing Concrete Pavement with Titanium Dioxide. Catal. Today 2010, 151, 71–76. [Google Scholar] [CrossRef]
- Moussiopoulos, N.; Barmpas, P.; Ossanlis, I.; Bartzis, J. Comparison of Numerical and Experimental Results for the Evaluation of the Depollution Effectiveness of Photocatalytic Coverings in Street Canyons. Environ. Model. Assess. 2008, 13, 357–368. [Google Scholar] [CrossRef]
- Simmons, A.J.; Willett, K.M.; Jones, P.D.; Thorne, P.W.; Dee, D.P. Low-Frequency Variations in Surface Atmospheric Humidity, Temperature, and Precipitation: Inferences from Reanalyses and Monthly Gridded Observational Data Sets. J. Geophys. Res. 2010, 115, D01110. [Google Scholar] [CrossRef]
- Carslaw, D. Evidence of an Increasing NO2/NOx Emissions Ratio from Road Traffic Emissions. Atmos. Environ. 2005, 39, 4793–4802. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.C.; Poon, C.S. Photocatalytic Cement-Based Materials: Comparison of Nitrogen Oxides and Toluene Removal Potentials and Evaluation of Self-Cleaning Performance. Build. Environ. 2011, 46, 1827–1833. [Google Scholar] [CrossRef]
- Bloh, J.Z.; Folli, A.; Macphee, D.E. Photocatalytic NOx Abatement: Why the Selectivity Matters. RSC Adv. 2014, 4, 45726–45734. [Google Scholar] [CrossRef]
- Ângelo, J.; Andrade, L.; Mendes, A. Highly Active Photocatalytic Paint for NOx Abatement under Real-Outdoor Conditions. Appl. Catal. A Gen. 2014, 484, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Sofianou, M.V.; Psycharis, V.; Boukos, N.; Vaimakis, T.; Yu, J.; Dillert, R.; Bahnemann, D.; Trapalis, C. Tuning the Photocatalytic Selectivity of TiO2 Anatase Nanoplates by Altering the Exposed Crystal Facets Content. Appl. Catal. B Environ. 2013, 142–143, 761–768. [Google Scholar] [CrossRef]
- Shelimov, B.N.; Tolkachev, N.N.; Tkachenko, O.P.; Baeva, G.N.; Klementiev, K.V.; Stakheev, A.Y.; Kazansky, V.B. Enhancement Effect of TiO2 Dispersion over Alumina on the Photocatalytic Removal of NOx Admixtures from O2–N2 Flow. J. Photochem. Photobiol. A Chem. 2008, 195, 81–88. [Google Scholar] [CrossRef]
- Devahasdin, S.; Fan, C.; Li, K.; Chen, D.H. TiO2 Photocatalytic Oxidation of Nitric Oxide: Transient Behavior and Reaction Kinetics. J. Photochem. Photobiol. A Chem. 2003, 156, 161–170. [Google Scholar] [CrossRef]
- Folli, A.; Campbell, S.B.; Anderson, J.A.; Macphee, D.E. Role of TiO2 Surface Hydration on NO Oxidation Photo-Activity. J. Photochem. Photobiol. A Chem. 2011, 220, 85–93. [Google Scholar] [CrossRef]
- Ohko, Y.; Nakamura, Y.; Negishi, N.; Matsuzawa, S.; Takeuchi, K. Photocatalytic Oxidation of Nitrogen Monoxide Using TiO2 Thin Films under Continuous UV Light Illumination. J. Photochem. Photobiol. A Chem. 2009, 205, 28–33. [Google Scholar] [CrossRef]
- Wu, Q.; van de Krol, R. Selective Photoreduction of Nitric Oxide to Nitrogen by Nanostructured TiO2 Photocatalysts: Role of Oxygen Vacancies and Iron Dopant. J. Am. Chem. Soc. 2012, 134, 9369–9375. [Google Scholar] [CrossRef]
- Polat, M.; Soylu, A.M.; Erdogan, D.A.; Erguven, H.; Vovk, E.I.; Ozensoy, E. Influence of the Sol–Gel Preparation Method on the Photocatalytic NO Oxidation Performance of TiO2/Al2O3 Binary Oxides. Catal. Today 2015, 241, 25–32. [Google Scholar] [CrossRef] [Green Version]
- Lewis, R.J.; Sax, N. Sax’s Dangerous Properties of Industrial Materials; John Wiley & Sons: New York, NY, USA, 1996; Volume 8. [Google Scholar]
- Pepin, L. Etude In Situ des Propriétés Purificatrices de Revêtements Photocatalytiques sur la Pollution Atmosphérique; Technical Report 1; TERA Environment: Rhône-Alpes, France, 2009. [Google Scholar]
- Jimenez-Relinque, E.; Castellote, M. Quick Assessment of the Photocatalytic Activity of TiO2 Construction Materials by Nitroblue Tetrazolium (NBT) Ink. Constr. Build. Mater. 2019, 214, 1–8. [Google Scholar] [CrossRef]
- Gandolfo, A.; Bartolomei, V.; Gomez Alvarez, E.; Tlili, S.; Gligorovski, S.; Kleffmann, J.; Wortham, H. The Effectiveness of Indoor Photocatalytic Paints on NOx and HONO Levels. Appl. Catal. B Environ. 2015, 166–167, 84–90. [Google Scholar] [CrossRef]

| Material | Test Setup | UV Irradiance | Inlet Concentration | Removal | T (C) | RH (%) | Reference |
|---|---|---|---|---|---|---|---|
| Protectam FN2 (Evonik P25) | ISO standard, two flow reactors (laminar flow, ideally-mixed flow) | 1.0 mW cm−2 | 0.1–1.0 ppm NO or 0.1 ppm NO2 | NO: 75 mol m−2 h−1, NO2: 50 mol m−2 h−1 | 25 | 50 | Zouzelka et al. (2017) [67] |
| Anatase TiO2 (N-400) | ISO standard photoreactor | 10 W m−2 | 1 ± 0.015 ppm NO | Mortar with 5–10% NP-400: ∼15% NO, mortar with 20% NP-400: 70% NO | 25 ± 2 | 50 ± 5 | Rhee et al. (2018) [68] |
| Concrete block with 0.59 wt% TiO2 | ISO standard planar reactor | 10 W m−2 | 1 ppm NO | 39% NOx under ISO conditions, 45% Under optimal conditions in the field (high sun (890 W m−2), low RH (40–55%), low wind (1.9 m s−1)). Negligible removal if dew covered or raining. | 25 | 50 | Ballari et al. (2013) [69] |
| Italcementi, TX-Active photocatalytic mortar | Field tests in a tunnel and ISO standard testing | 0.6–1.6 W m−2 | 50–850 ppb NOx | 0–2% NOx, UVA irradiance was below the targeted values (above 4 W m−2), de-activation and reduction in photocatalytic activity were recorded | - | 70–90 | Gallus et al. (2015) [70] |
| Anatase TiO2 nanoparticles | Modified testing from JIS TR Z 0018 standard [51] | 0.5–2.4 mW cm−2 | 430 ppb NO | NOx removal 8–65% dependent on flow, RH levels and light intensity | 24 ± 2 | 20–80 | Hassan et al. (2013) [57] |
| Anatase TiO2 | Flow reactor adapted from ISO standard | 5.8 W m−2 | 100–2000 ppb NO | 15–50% NOx, NO2 removal negative under certain conditions | 25 | 0–75 | Martinez et al. (2011) [71] |
| Commercial paving stone with TiO2 | ISO standard flow reactor | 0–15 W m−2 | 0.1–1 ppm NO | 0–68.4% NO, dependent on parameters | - | 10–80 | Husken et al. (2009) [58] |
| Concrete with TiO2 | ‘Similar’ to ISO standard | 2–11 W m−2 | 0–1 ppm NO and NO2 NO/NO2 ratio from 0 to 1 | More NO2 produced when conditions are less favourable | - | 10–70 | Ballari et al. (2011) [22] |
| Mortar with rutile TiO2 rods | Flow reactor (not ISO standard) | 10–40 W m−2 | 20 ppm NO | 24–69 mg hr−1 m−2 NOx | - | 30–70 | Staub de Melo and Trichês (2012) [59] |
| Active Material | Test Setup | Duration | Activity | Selectivity | Reasoning | Reference |
|---|---|---|---|---|---|---|
| Commercial P25 TiO2 and lab synthesised HT-ET TiO2 | Lab: borosilicate reactor, 30 mL min−1 airflow, total NO flow: 2.21 mol h−1, lamp nm, irradiance: 9 mW cm−2 | 18 h | NO conversion fell from 100% to 68% | Conversion of NO to NO2 increases rapidly from 0 to 50% | HNO and NO formed on surface deactivate TiO2 | J. Araa et al. (2019) [72] |
| ‘Nano-TiO2’ | Lab and field: bicycle lane in Poland and novel lab setup, 100 ppb inlet NO, 70 and 300 W lamps, flow of 120 L hr−1 | 7 years | Only measured after wear, 4–45% NO RE depending on test conditions | NO2 production was equal to NO removal for dirty samples under 70 W light | TiO2 still present in samples after 7 years | Witkowski et al. (2019) [85] |
| Ultrathin BiOBr/BiOI | Lab: continuous flow reactor | 360 min | Negligible change | Increased NO2 production as NO on surface increases | Adsorbed NO alters the surface environment, changes oxidation process | Shi et al. (2019) [86] |
| Concrete with TiO2 and activated carbon spray coating | Lab: stainless steel reactor, RH 48–53%, T = 25 C, light intensity 0.3 mW cm−2 | ‘multiple tests’, followed by regeneration and accelerated wear from polishing | Quoted for NO and NO2 separately, ranging from 78.2% to 25.84%, reduced RE after repeat tests, partially regained after regeneration, and further lowered after wear | - | Regeneration by washing with water removes nitrates from the surface. Some RE capability is retained after wear as the coating is able to penetrate the surface | Chen et al. (2011) [55] |
| Asphalt and concrete with spray TiO2 coating | Lab and field: tested according to JIS R 1701 [51] in the lab and with nitrate extraction method on pavements in the field | 5 months of ‘light wear’ | Loss of 50% in RE over 2.5 months, calculated lifetimes of 6–11 months for concrete and 10–16 months for asphalt | - | Sprays coatings on the surface are subject to wear | Osborn et al. (2014) [77] |
| Blocks of Portland cement containing pozzolanic material with anatase or rutile TiO2 at varying thicknesses | Lab and field: lab studies conducted on blocks before and after 1 year field exposure | 1 year of wear on roads and pavements | Loss of 79–87% of initial RE after 1 year, 21–29% of initial RE regained after washing | - | The blocks showed considerable loss of PC area due to abrasion and wear. Blocks with a thicker PC layer are recommended. Textured surface increases initial RE but is more susceptible to wear | Staub de Melo et al. (2012) [76] |
| Concrete blocks with 0.59 wt% TiO2 content | Field: paving blocks in residential street | 20 months cumulative for all materials | Activity lost after 2.5 months and then 11 months | - | Coating lost due to normal wearing via vehicles, weathering, and deposition of soils | Ballari et al. (2013) [69] |
| Material | Test Setup | Dopant | Support | Method of Incorporation | Activity | Selectivity | Reasoning | Reference |
|---|---|---|---|---|---|---|---|---|
| BiOX Mortars | ISO | - | 1:3 ratio of sand to Portland cement | Powder incorporated in cement at 10 wt% | BiOX Mortars: 7.6% NO, 4% NO2, P25: 4.3% NO, 1% NO2 | BiOX Mortars: 83% P25: 24% | Presence of oxygen vacancies together with a strong oxidation potential | Nava-Núñez et al. (2020) [123] |
| P25, KRONOclean 7000 and lab synthesised SiO2-TiO2 composite | ISO | C | Microsilica (pozzolanic material) in Portland cement | PC as 5 wt% of the binder | Microsilica addition imporoves post-carbonation activity | Microsilica addition imporoves post-carbonation selectivity | High SSA, maintained after carbonation due to high pH | Kaja et al. (2019) [79] |
| Blue TiO2 | ISO | Fe and Cu | Sample disk | Hydrothermal-assisted sol-gel method | NO oxidation: Blue Fe-TiO2: 70% Blue Cu-TiO2: 57.71% Blue TiO2: 54.57% P25: 34.96%. | NO2 selectivity: P25 TiO2:36.08% Blue TiO2: 21.7% Blue Cu-TiO2: 4.3% Blue Fe-TiO2: 11.65% | Oxygen vacancies and smaller band-gap | Martinez-Oviedo et al. (2020) [98] |
| Commercial TiO2 | Continuous flow reactor | g-C3N4 | Portland cement | Suspension Mixed into cement before casting | 2% g-C3N4 addition increases NOx removal from 37.5 to 227.3 mol m−2 h−1 | - | Smaller band gap | Yang et al. (2019) [124] |
| KRONOClean 7050 TiO2 | ISO | - | Portland cement | Liquidized powder incorporated in cement | 360% NOx removal compared to plain cement | - | Addition of TiO2 alters microstructure as well as adding PC effect | Jin et al. (2019) [125] |
| P25 TiO2 | Continuous flow reactor, similar to ambient conditions | Sn | Sample disk | Dispersed in acetone and distributed on a sample disk | Blue Sn-TiO2: 72% NOx removal, compared with 42% for P25 | Blue Sn-TiO2: 29.42% NO2 formation, compared with 125.19% for P25 | Multiple reasons including: band gap reduction, increased SSA, and efficient e−/h+ separation | Martinez-Oviedo et al. (2019) [101] |
| KRONOClean 7000 TiO2 | Chamber test | C | Asphalt | PC powder suspended in water, sprayed onto asphalt, and heated | For 2 g m−2, after 3 h C-TiO2: 90% NOx RE, compared with 55% for P25 | - | Smaller band gap | Fan et al. (2017) [126] |
| TiO2 | RhB solution | - | CNT and rGO composite | Solvothermal method | 1.5× the rate of pure TiO2 | - | Decreased e−/h+ recombination, increased hydroxyl content at surface | Huang et al. (2018) [115] |
| Aeroxide P25 TiO2, Evonik | Continuous flow reactor | V and Fe | Selection of cement matrices, including high alumina cement (HAC) and air lime | Binder:aggregate ratio of 1:3 and TiO2 as 0.5, 1 and 2.5 wt% of binder | Fe showed better improvement than V, even though V harvested more visible light | High NO selectivity with low release of NO2 for air lime and HAC systems | Selectivity values for NO degradation were high (>60%), yielding calcium nitrates that are easily removed from the photocatalyst surface, thus enhancing the PCO reaction | Pérez-Nicolás et al. (2017) [96] |
| Range of commercial TiO2 products | Specific ‘PHOTONSITE’ setup, validated against ISO standard | - | Asphalt and concrete tiles | Slurries, emulsions and precast into bulk | Faster RE loss on asphalt than concrete, RH of preceding days impacts RE | - | Substrate hygro-inertia alters acute impact of RH | Jiménez-Relinque et al. (2019) [78] |
| Commercial anatase, Evonik Aerodisp W740X | Flow reactor adapted from ISO standard | - | Glass and mortar | Coating made with acrylic binder | - | Significantly lower selectivity on glass, also falling with exposure | On mortar NO2 produced is absorbed, meaning less competition for adsorptive sites | Martinez et al. (2011) [71] |
| Commercial TiO2 and carbon doped TiO2 | ISO | Carbon | Concrete with varying surface roughness | Dry powder or suspension | Increased RE for: rougher surfaces, doped TiO2, suspension method and finer powders | - | Rougher surface improves deposition, increased SSA and distribution improve uptake, dopants shift absorbance toward visible | Husken et al. (2009) [58] |
| Nanostructured TiO2 | ISO | Atomically dispersed Pd | Glass fibre | Annealing and spray drying | Increase in NOx RE by 10 times with 1 wt% Pd, relative to P25 | - | Single Pd atoms on TiO2 dominate NOx removal | Fujiwara et al. (2017) [106] |
| Fe2O3/TiO2 nanocomposite | Similar to ISO but 100 ppb NO and 0.3 L min−1 | Fe2O3 | - | ‘Original low- temperature plasma assisted strategy’ | Activity of composite lower than Degussa P25 | 63% for composite, compared with 25% for P25 | Nanocomposite has a lower band gap and improved electron-hole separation | Balbuena et al. (2016) [100] |
| Aeroxide P25 TiO2, Evonik | ISO | g-C3N4 and CaCO3 | - | Suspended in water, then annealed | 5 times greater than pure TiO2 under visible light | Alters with composition, moved DeNOx index from negative to positive | CaCO3 reduces NO2 production, composite is synergistic and shifts bandgap toward visible region | Papailias et al. (2017) [112] |
| P25, anatase and rutile TiO2 | Self-designed flow reactor | - | Concrete with varying cement, glass and sand aggregrate ratios | Wet-mixed, compacted and cured | NO removal in mg hr−1 m−2 increased with porosity and glass content | - | Porosity increases the area available for reaction and glass increases transmission of light | Poon and Cheung (2007) [80] |
| Aeroxide P25 TiO2, Evonik and Hombikat | ISO | - | Zeolite ZSM-5 | Sol-Gel synthesis | Composite TiO2: 41% NO conversion, P25: 45% | Composite TiO2: 19% NO2 selecticity, P25: 65% | Zeolite gives extra available SSA and act as sink/reservoir for HNO3 | Tawari et al. (2016) [127] |
| Location | Test Setup | Light | Removal | WS | T | RH | Comment | Ref |
|---|---|---|---|---|---|---|---|---|
| Copenhagen, Denmark | 2 × 200 m stretches, each 100 m with and 100 m without TiO2, sampling at 2 m height | Sunlight, 0–1500 kJ m−2 day−1 | 22% NO, but negligible for NO2 | - | 18–25 | 25–83 | NOx conversion decreases with RH, increases with T, selectivity is an issue | Folli et al. (2015) [56] |
| Sheung Shui District, Hong Kong | 30 × 3 m, 15 m coated, 15 m uncoated | Sunlight | 100% NOx in 1.5 h in lab, NO2 removal negligible in field | - | - | - | NOx source: two cars driven continuously, poor selectivity and durability | Fan et al. (2018) [84] |
| Hengelo, Netherlands | 150 m coated street, 100 m control street | 44–745 w m−2 with UV-A 3% of total | 19% NOx (daytime) | 0.6–2.69 | 2.4–27.9 | 37.7–86.6 | Sampled at 5, 30 and 150 cm, rapid decay in performance, negligible removal when wet | Ballari et al. (2013) [69] |
| Madrid, Spain | Pilot scale, 9 materials tested over 17 months, using a specially developed ‘PHOTONSITE DEVICE’ | Ambient or 10 W m−2 from 365 nm LEDS | 1–32% NOx | 3 L min−1 | - | 25 | Lab tests also conducted under ISO conditions to validate results, conclusions are that the RH of the preceding days is key to RE, due to hygro-inertia. Recorded overall degradation in RE over time, more on asphalt than concrete | Jiménez-Relinque et al. (2019) [78] |
| Tsitsihar, China | Experimental area of road divided into test and control sections, with synchronised sampling, 0.5 m from surface | Sunlight | 12.35–24.1% NOx (daytime) | <0.8 | - | - | Far lower RE recorded for outdoor than indoor (lab) tests. Different rates in summer and winter | Chen et al. (2011) [55] |
| Bergamo, Italy | PC concrete blocks in central Bergamo street, 500 m long, 12,000 m2 active area. 2 measurement campaigns, each two weeks | Sunlight | 26–66% NOx RE | - | - | - | Analysis is contested in Gallus et al. [70] and Flassak and Bolte [133,134] | Guerrini and Peccati (2007) [135] |
| Bergamo, Italy | 5 × 5 × 53 m, Walls and ground coated with Italcementi, TX-Active | Sunlight, UV-A up to 40 W m−2 | ≤2% NOx RE | <1 inside, 1.5 above | - | - | SA/V Ratio: 0.6 m−1 Material did not decay after field test | Gallus et al. (2015) [70] |
| Location | Test Setup | Removal | WS | T | RH | Comment | Reference |
|---|---|---|---|---|---|---|---|
| Gyeongbu expressway, Korea | TiO2 paint added to retaining wall, coated section: 150 m × 1.9 m, control section: 200 m × 1.9 m | 13% NOx (daytime) | 1–3 | 4–18 | 35–99 | Quantity of sunlight, was a key variable. Measurements made directly above PC surface | Kim et al. (2018) [138] |
| Artworks Elephant, London, United Kingdom | TiO2 painted on walls in a courtyard | Negligible | - | - | - | Seasonal variation and changes in traffic complicated the analysis | Tremper and Green (2016) [139] |
| A1 and A28 motorways, Netherlands | PC coating added to noise barrier on A1 and porous air quality barrier on A28 | Negligible | - | - | - | Negligible performance attributed to short contact times and unfavourable meteorology, including high RH and low T | Dutch Air Quality Innovation Programme (2010) [140] |
| Guerville (near Paris), France | Model street canyons built at a 1:5 scale, reference canyon compared to canyon with walls clad in TiO2 mortar panels, artificial pollution source applied | 36.7–82.0% NOx | 0.1–4.8 | - | - | Conclude that variation is due to differences in wall orientation, wind direction and source emissions. Unrealistic SA/V ratio of around 1 m−1 is used. | Maggos et al. (2008) [141] |
| Gaudalupe MRT station, Manila, Philipines | 6000 m2 of wall coated in PC paint, NOx measured at 18 sites with Ogawa passive samplers. Enclosed car park also coated with 9000 m2 of paint | Outdoor: 10% NO2, indoor: −51% NO2 | Outdoor: 2.4, indoor: 0.34 | - | Outdoor: 82, indoor: 48 | Questionable method of RE quantification. UV irradiance far lower indoor than outdoor | Borlaza (2012) [137] |
| Sir John Cass School, London, United Kingdom | Courtyard wall coated with TiO2 paint, measurement at 2.5 m | Negligible | - | - | - | Measurement length was not optimal, meteorology may have impacted results | Barratt et al. (2007) [142] |
| Location | Test Setup/Metric | Test area dimensions | Light | Removal | WS | RH | SA/V Ratio | Ref |
|---|---|---|---|---|---|---|---|---|
| Umberto Tunnel, Rome | Whole tunnel (9000 m) painted, 4 NOx analysers in tunnel, compared to monitoring stations in the city | l = 348 m, w = 17 m, h = 8.5 m | UV and visible: 20 W m−2, UV: 2 mW cm−2 | 23% NOx, ‘real’ effect > 50% | 0.38 | 40–70 | 0.23 m−1 | Guerrini (2012) [146] |
| Leopold II Tunnel, Brussels | NOx removal normalised with NOx:CO2 ratio, comparison of measurements before and after application, up and down wind of active section and in active section with UV on and off | 2.5 km long city tunnel, Test section: l = 160 m, w = 8.4 m, h = 4.2 m | UV-A: 1.6 W m−2 | <2% NOx | 3 | 70–90 | 0.4 m−1 | Gallus et al. (2015) [66] |
| Koningstunnel, Hague, Netherlands | 650 m long tunnel, KNOxOUT paint added to walls and ceiling, chemiluminescent monitors at the beginning and end of active section, as well as nitrate accumulation strips | 150 m test section | UV: 1 W m−2 (wall) and 0.6 W m−2 (ceiling) | ‘significant’ NO removal, at around 20%, ‘inconsistent’ NO2 results | 0.01–3.5 | - | - | Kerrod and McIntyre (2004) [147] |
| Car park | 322 m2 ceiling covered with TiO2 paint and car exhaust connected to a sealed section of the car park, photocatalytic rates recorded in g m2 s−1 | 917 m3 closed area | Total UV: 1 Wm−2 | 0.09–0.16 g m−2 s−1 NO2 removal | - | ‘unstable’ | 0.35 m−1 | Maggos et al. (2007) [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russell, H.S.; Frederickson, L.B.; Hertel, O.; Ellermann, T.; Jensen, S.S. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts 2021, 11, 675. https://doi.org/10.3390/catal11060675
Russell HS, Frederickson LB, Hertel O, Ellermann T, Jensen SS. A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts. 2021; 11(6):675. https://doi.org/10.3390/catal11060675
Chicago/Turabian StyleRussell, Hugo Savill, Louise Bøge Frederickson, Ole Hertel, Thomas Ellermann, and Steen Solvang Jensen. 2021. "A Review of Photocatalytic Materials for Urban NOx Remediation" Catalysts 11, no. 6: 675. https://doi.org/10.3390/catal11060675
APA StyleRussell, H. S., Frederickson, L. B., Hertel, O., Ellermann, T., & Jensen, S. S. (2021). A Review of Photocatalytic Materials for Urban NOx Remediation. Catalysts, 11(6), 675. https://doi.org/10.3390/catal11060675