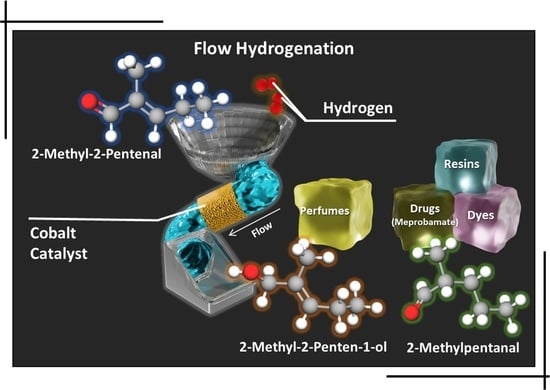
Co Loading Adjustment for the Effective Obtention of a Sedative Drug Precursor through Efficient Continuous-Flow Chemoselective Hydrogenation of 2-Methyl-2-Pentenal
Abstract
:1. Introduction
2. Results and Discussion
2.1. N2 Physisorption Results
2.2. Temperature-Programmed Reduction Studies (H2-TPR)
2.3. X-ray Diffraction Results (XRD)
2.4. Transmission Electron Microscopy (TEM) Results
2.5. Temperature-Programmed Desorption of Hydrogen (H2-TPD)
2.6. Catalytic Tests Results
3. Materials and Methods
3.1. Catalysts Preparation
3.2. Catalysts Characterization
3.2.1. N2 Physisorption
3.2.2. Temperature-Programmed Reduction
3.2.3. X-ray Diffraction
3.2.4. TEM Measurements
3.2.5. Temperature-Programmed Desorption of Hydrogen
3.2.6. Energy-Dispersive X-ray Fluorescence Analysis (EDXRF)
3.2.7. X-ray Photoelectron Spectroscopy (XPS)
3.2.8. Catalytic Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Baumann, M.; Moody, T.S.; Smyth, M.; Wharry, S. A Perspective on Continuous Flow Chemistry in the Pharmaceutical Industry. Org. Process Res. Dev. 2020, 24, 1802–1813. [Google Scholar] [CrossRef]
- Andrew Mansfield Why Perform Your Chemistry in Continuous Flow? Available online: https://blog.syrris.com/2018/05/15/why-perform-your-chemistry-in-continuous-flow/ (accessed on 1 November 2021).
- Pollak, P. The Industry and the Business, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; ISBN 978-0-470-62767-9. [Google Scholar]
- Moreno-Marrodan, C.; Liguori, F.; Barbaro, P. Continuous-Flow Processes for the Catalytic Partial Hydrogenation Reaction of Alkynes. Beilstein J. Org. Chem. 2017, 13, 734–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, B.Y.; Lim, T.; Han, M.S. A Simple and Efficient in Situ Generated Copper Nanocatalyst for Stereoselective Semihydrogenation of Alkynes. Chem. Commun. 2021, 57, 6891–6894. [Google Scholar] [CrossRef]
- Faust Akl, D.; Ruiz-Ferrando, A.; Fako, E.; Hauert, R.; Safonova, O.; Mitchell, S.; López, N.; Pérez-Ramírez, J. Precursor Nuclearity and Ligand Effects in Atomically-Dispersed Heterogeneous Iron Catalysts for Alkyne Semi-Hydrogenation. ChemCatChem 2021, 13, 3247–3256. [Google Scholar] [CrossRef]
- Ricciardi, R.; Huskens, J.; Verboom, W. Nanocatalysis in Flow. ChemSusChem 2015, 8, 2586–2605. [Google Scholar] [CrossRef] [PubMed]
- Larrã, M.D.; Lewis, R.J.; Robert, S.; Lewis, A. Hawley’s Condensed Chemical Dictionary, 6th ed.; John Wiley & Sons: New York, NY, USA, 2016. [Google Scholar] [CrossRef]
- Ramchandani, D.; López-Muñoz, F.; Alamo, C. Meprobamate–Tranquilizer or Anxiolytic? A Historical Perspective. Psychiatr. Q. 2006, 77, 43–53. [Google Scholar] [CrossRef]
- Hotta, K.; Kubomatsu, T. Liquid-Phase Selective Hydrogenation of an Aliphatic α,β-Unsaturated Aldehyde over Raney Cobalt Catalyst Modified with Ferrous Chloride. Bull. Chem. Soc. Jpn. 1969, 42, 1447–1449. [Google Scholar] [CrossRef] [Green Version]
- National Center for Biotechnology Information. PubChem Patent Summary for EP-3286290-B1, Osmanthus Odorant. Available online: https://pubchem.ncbi.nlm.nih.gov/patent/EP-3286290-B1 (accessed on 1 November 2021).
- Hu, T.; Zhang, L.; Wang, Y.; Yue, Z.; Li, Y.; Ma, J.; Xiao, H.; Chen, W.; Zhao, M.; Zheng, Z.; et al. Defect Engineering in Pd/NiCo2O4−x for Selective Hydrogenation of α,β-Unsaturated Carbonyl Compounds under Ambient Conditions. ACS Sustain. Chem. Eng. 2020, 8, 7851–7859. [Google Scholar] [CrossRef]
- Pham, T.T.; Lobban, L.L.; Resasco, D.E.; Mallinson, R.G. Hydrogenation and Hydrodeoxygenation of 2-Methyl-2-Pentenal on Supported Metal Catalysts. J. Catal. 2009, 266, 9–14. [Google Scholar] [CrossRef]
- Akbarzadeh, O.; Mohd Zabidi, N.; Abdul Wahab, Y.; Hamizi, N.; Chowdhury, Z.; Aljunid Merican, Z.; Ab Rahman, M.; Akhter, S.; Shalauddin, M.; Johan, M. Effects of Cobalt Loading, Particle Size, and Calcination Condition on Co/CNT Catalyst Performance in Fischer–Tropsch Reactions. Symmetry (Basel) 2018, 11, 7. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Jia, W.; Xu, G.; Zhang, Y.; Fu, Y. Selective Hydrodeoxygenation of Lignin-Derived Phenols to Cyclohexanols over Co-Based Catalysts. ACS Sustain. Chem. Eng. 2017, 5, 8594–8601. [Google Scholar] [CrossRef]
- Jozwiak, W.K.; Szubiakiewicz, E.; Góralski, J.; Klonkowski, A.; Paryjczak, T. Physico-Chemical and Catalytic Study of the Co/SiO2 Catalysts. Kinet. Catal. 2004, 45, 247–255. [Google Scholar] [CrossRef]
- Luo, Q.-X.; Guo, L.-P.; Yao, S.-Y.; Bao, J.; Liu, Z.-T.; Liu, Z.-W. Cobalt Nanoparticles Confined in Carbon Matrix for Probing the Size Dependence in Fischer-Tropsch Synthesis. J. Catal. 2019, 369, 143–156. [Google Scholar] [CrossRef]
- Mehrbod, M.; Martinelli, M.; Castro, J.D.; Alhraki, N.; Cronauer, D.C.; Kropf, A.J.; Marshall, C.L.; Jacobs, G. Fischer-Tropsch Synthesis: Direct Cobalt Nitrate Reduction of Promoted Co/Al2O3 Catalysts. Catal. Today 2021, 369, 129–143. [Google Scholar] [CrossRef]
- Ren, Y.; Bruce, P.G.; Ma, Z. Solid-Solid Conversion of Ordered Crystalline Mesoporous Metal Oxides under Reducing Atmosphere. J. Mater. Chem. 2011, 21, 9312–9318. [Google Scholar] [CrossRef]
- Olusola, O.J.; Sudip, M. Temperature Programme Reduction (TPR) Studies of Cobalt Phases in -Alumina Supported Cobalt Catalysts. J. Pet. Technol. Altern. Fuels 2016, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Stern, K.H. High Temperature Properties and Decomposition of Inorganic Salts Part 3, Nitrates and Nitrites. J. Phys. Chem. Ref. Data 1972, 1, 747–772. [Google Scholar] [CrossRef] [Green Version]
- Díaz, J.A.; Akhavan, H.; Romero, A.; Garcia-Minguillan, A.M.; Romero, R.; Giroir-Fendler, A.; Valverde, J.L. Cobalt and Iron Supported on Carbon Nanofibers as Catalysts for Fischer–Tropsch Synthesis. Fuel Process. Technol. 2014, 128, 417–424. [Google Scholar] [CrossRef]
- Park, J.-Y.; Lee, Y.-J.; Karandikar, P.R.; Jun, K.-W.; Ha, K.-S.; Park, H.-G. Fischer–Tropsch Catalysts Deposited with Size-Controlled Co3O4 Nanocrystals: Effect of Co Particle Size on Catalytic Activity and Stability. Appl. Catal. A Gen. 2012, 411–412, 15–23. [Google Scholar] [CrossRef]
- Chotiwan, S.; Tomiga, H.; Katagiri, M.; Yamamoto, Y.; Yamashita, S.; Katayama, M.; Inada, Y. Particle Size Effect of Redox Reactions for Co Species Supported on Silica. J. Solid State Chem. 2016, 241, 212–218. [Google Scholar] [CrossRef]
- Castner, D.G.; Watson, P.R.; Chan, I.Y. X-ray Absorption Spectroscopy, x-Ray Photoelectron Spectroscopy, and Analytical Electron Microscopy Studies of Cobalt Catalysts. 2. Hydrogen Reduction Properties. J. Phys. Chem. 2002, 94, 819–828. [Google Scholar] [CrossRef]
- Chernavskii, P.A.; Pankina, G.V.; Lermontov, A.S.; Lunin, V. V Size Distribution of Cobalt Particles in Catalysts for the Fischer-Tropsch Synthesis. Kinet. Catal. 2003, 44, 657–661. [Google Scholar] [CrossRef]
- Goring, G.E.; Curran, G.P.; Tarbox, R.P.; Gorin, E. Kinetics of Carbon Gasification by Steam. Effect of High Temperature Pretreatment on Reactivity of Low Temperature Char to Steam and Carbon Dioxide. Ind. Eng. Chem. 1952, 44, 1051–1057. [Google Scholar] [CrossRef]
- Feldkirchner, H.L.; Huebler, J. Reaction of Coal with Steam-Hydrogen Mixtures at High Temperatures and Pressures. Ind. Eng. Chem. Process Des. Dev. 1965, 4, 134–142. [Google Scholar] [CrossRef]
- Tsai, Y.-T.; Mo, X.; Campos, A.; Goodwin, J.G.; Spivey, J.J. Hydrotalcite Supported Co Catalysts for CO Hydrogenation. Appl. Catal. A Gen. 2011, 396, 91–100. [Google Scholar] [CrossRef]
- Słowik, G.; Gawryszuk-Rżysko, A.; Greluk, M.; Machocki, A. Estimation of Average Crystallites Size of Active Phase in Ceria-Supported Cobalt-Based Catalysts by Hydrogen Chemisorption vs TEM and XRD Methods. Catal. Lett. 2016, 146, 2173–2184. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, Z.; Jodzis, S.; Raróg, W.; Zieliński, J.; Pielaszek, J.; Presz, A. Carbon-Supported Ruthenium Catalyst for the Synthesis of Ammonia. The Effect of the Carbon Support and Barium Promoter on the Performance. Appl. Catal. A Gen. 1999, 184, 95–102. [Google Scholar] [CrossRef]
- Hansen, T.W.; Hansen, P.L.; Dahl, S.; Jacobsen, C.J.H. Support Effect and Active Sites on Promoted Ruthenium Catalysts for Ammonia Synthesis. Catal. Lett. 2002, 84, 7–12. [Google Scholar] [CrossRef]
- Durndell, L.J.; Parlett, C.M.A.; Hondow, N.S.; Isaacs, M.A.; Wilson, K.; Lee, A.F. Selectivity Control in Pt-Catalyzed Cinnamaldehyde Hydrogenation. Sci. Rep. 2015, 5, 9425. [Google Scholar] [CrossRef] [Green Version]
- Natesakhawat, S.; Lekse, J.W.; Baltrus, J.P.; Ohodnicki, P.R.; Howard, B.H.; Deng, X.; Matranga, C. Active Sites and Structure-Activity Relationships of Copper-Based Catalysts for Carbon Dioxide Hydrogenation to Methanol. ACS Catal. 2012, 2, 1667–1676. [Google Scholar] [CrossRef]
- Rioux, R.M.; Song, H.; Hoefelmeyer, J.D.; Yang, P.; Somorjai, G.A. High-Surface-Area Catalyst Design: Synthesis, Characterization, and Reaction Studies of Platinum Nanoparticles in Mesoporous SBA-15 Silica†. J. Phys. Chem. B 2004, 109, 2192–2202. [Google Scholar] [CrossRef]
- Gallezot, P.; Richard, D. Selective Hydrogenation of α,β-Unsaturated Aldehydes. Catal. Rev. 2006, 40, 81–126. [Google Scholar] [CrossRef]
- Ghogia, A.C.; Nzihou, A.; Serp, P.; Soulantica, K.; Pham Minh, D. Cobalt Catalysts on Carbon-Based Materials for Fischer-Tropsch Synthesis: A Review. Appl. Catal. A Gen. 2021, 609, 117906. [Google Scholar] [CrossRef]
- Kim, M.; Bertram, M.; Pollmann, M.; Von Oertzen, A.; Mikhailov, A.S.; Rotermund, H.H.; Ertl, G. Controlling Chemical Turbulence by Global Delayed Feedback: Pattern Formation in Catalytic CO Oxidation on Pt(110). Science 2001, 292, 1357–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-C.; Kim, W.K.; Dzubiella, J. Coverage Fluctuations and Correlations in Nanoparticle-Catalyzed Diffusion-Influenced Bimolecular Reactions. J. Phys. Chem. C 2020, 124, 24204–24214. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., King, R.C., Jr., Eds.; Physical Electronics, Inc.: Chanhassen, MN, USA, 1995. [Google Scholar]
- NIST X-ray Photoelectron Spectroscopy Database 20, Version 4.1. Available online: http://srdata.nist.gov/xps/ (accessed on 1 November 2021).
- Beamson, G.; Brighs, D. High Resolution XPS of Organic Polymers: The Scienta ESCA300 Database; John Wiley & Sons, Ltd.: Chichester, UK, 1992. [Google Scholar]
- Reuel, R.C.; Bartholomew, C.H. The Stoichiometries of H2 and CO Adsorptions on Cobalt: Effects of Support and Preparation. J. Catal. 1984, 85, 63–77. [Google Scholar] [CrossRef]
- Borodziński, A.; Bonarowska, M. Relation between Crystallite Size and Dispersion on Supported Metal Catalysts. Langmuir 1997, 13, 5613–5620. [Google Scholar] [CrossRef]











| Samples | Specific Surface Area (m2 g−1) | Total Pore Volume (cm3 g−1) | Pore Size (Å) |
|---|---|---|---|
| 2 wt.% Co/CNR115 | 1585± 10.4 | 0.34 | 27.8 |
| 6 wt.% Co/CNR115 | 1417 ± 9.0 | 0.32 | 28.2 |
| 10 wt.% Co/CNR115 | 1367± 9.1 | 0.31 | 28.4 |
| 14 wt.% Co/CNR115 | 1064 ± 5.9 | 0.31 | 24.9 |
| Catalyst | Co Dispersion [%] | dCo [nm] | SH2 [m2∙gCo−1] |
|---|---|---|---|
| 2 wt.% Co/CNR115 | 13.7 | 9 | 73 |
| 6 wt.% Co/CNR115 | 8.4 | 15 | 45 |
| 10 wt.% Co/CNR115 | 4.3 | 25 | 23 |
| 14 wt.% Co/CNR115 | 3.2 | 40 | 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Ropero, A.J.; Zawadzki, B.; Matus, K.; Patkowski, W.; Krawczyk, M.; Lisovytskiy, D.; Raróg-Pilecka, W.; Śrębowata, A. Co Loading Adjustment for the Effective Obtention of a Sedative Drug Precursor through Efficient Continuous-Flow Chemoselective Hydrogenation of 2-Methyl-2-Pentenal. Catalysts 2022, 12, 19. https://doi.org/10.3390/catal12010019
Fernández-Ropero AJ, Zawadzki B, Matus K, Patkowski W, Krawczyk M, Lisovytskiy D, Raróg-Pilecka W, Śrębowata A. Co Loading Adjustment for the Effective Obtention of a Sedative Drug Precursor through Efficient Continuous-Flow Chemoselective Hydrogenation of 2-Methyl-2-Pentenal. Catalysts. 2022; 12(1):19. https://doi.org/10.3390/catal12010019
Chicago/Turabian StyleFernández-Ropero, Antonio Jesús, Bartosz Zawadzki, Krzysztof Matus, Wojciech Patkowski, Mirosław Krawczyk, Dmytro Lisovytskiy, Wioletta Raróg-Pilecka, and Anna Śrębowata. 2022. "Co Loading Adjustment for the Effective Obtention of a Sedative Drug Precursor through Efficient Continuous-Flow Chemoselective Hydrogenation of 2-Methyl-2-Pentenal" Catalysts 12, no. 1: 19. https://doi.org/10.3390/catal12010019
APA StyleFernández-Ropero, A. J., Zawadzki, B., Matus, K., Patkowski, W., Krawczyk, M., Lisovytskiy, D., Raróg-Pilecka, W., & Śrębowata, A. (2022). Co Loading Adjustment for the Effective Obtention of a Sedative Drug Precursor through Efficient Continuous-Flow Chemoselective Hydrogenation of 2-Methyl-2-Pentenal. Catalysts, 12(1), 19. https://doi.org/10.3390/catal12010019