Polyaromatic Carboxylate Ligands Based Zn(II) Coordination Polymers for Ultrasound-Assisted One-Pot Tandem Deacetalization–Knoevenagel Reactions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of CP 1
2.2. Crystal Structure Analysis
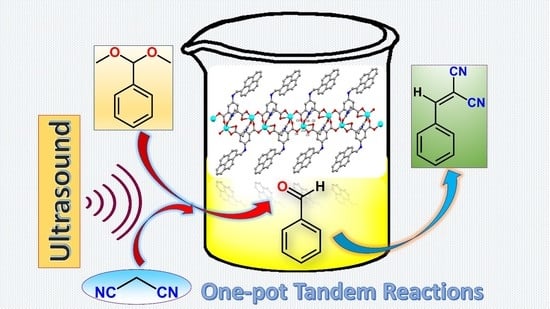
2.3. Ultrasound-Assisted One-Pot Deacetalization–Knoevenagel Tandem Reactions
3. Materials and Methods
3.1. Synthesis of the Pro-Ligands H2L1 and H2L2
3.2. Synthesis of Coordination Polymer 1
3.3. Synthesis of Coordination Polymers 2–5
3.4. Procedure for the One-Pot Tandem Deacetalization–Knoevenagel Condensation Reactions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lippi, M.; Cametti, M. Highly dynamic 1D coordination polymers for adsorption and separation applications. Coord. Chem. Rev. 2021, 430, 213661. [Google Scholar] [CrossRef]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Coordination polymers nanoparticles for bioimaging. Coord. Chem. Rev. 2021, 432, 213716. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Taddei, M.; Petit, C. Engineering metal–organic frameworks for adsorption-based gas separations: From process to atomic scale. Mol. Syst. Des. Eng. 2021, 6, 841–875. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal–organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Lu, Y.; Chen, K.; Zhao, Y.; Wang, P.; Sun, W.-Y. Metal–organic frameworks with catalytic centers: From synthesis to catalytic application. Coord. Chem. Rev. 2018, 378, 262–280. [Google Scholar] [CrossRef]
- Yang, D.; Gates, B.C. Catalysis by metal organic frameworks: Perspective and suggestions for future research. ACS Catal. 2019, 9, 1779–1798. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Pombeiro, A.J.L. Urea and thiourea based coordination polymers and metal-organic frameworks: Synthesis, structure and applications. Coord. Chem. Rev. 2022, 453, 214314. [Google Scholar] [CrossRef]
- Li, D.; Xu, H.-Q.; Jiao, L.; Jiang, H.-L. Metal-organic frameworks for catalysis: State of the art, challenges, and opportunities. EnergyChem 2019, 1, 100005. [Google Scholar] [CrossRef]
- Shen, Y.; Pan, T.; Wang, L.; Ren, Z.; Zhang, W.; Huo, F. Programmable logic in metal–organic frameworks for catalysis. Adv. Mater. 2021, 33, 2007442. [Google Scholar] [CrossRef]
- Ranocchiari, M.; van Bokhoven, J.A. Catalysis by metal–organic frameworks: Fundamentals and opportunities. Phys. Chem. Chem. Phys. 2011, 13, 6388–6396. [Google Scholar] [CrossRef]
- Karmakar, A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Zinc metal–organic frameworks: Efficient catalysts for the diastereoselective Henry reaction and transesterification. Dalton Trans. 2014, 43, 7795–7810. [Google Scholar] [CrossRef]
- Dhakshinamoorthy, A.; Garcia, H. Cascade reactions catalyzed by metal organic frameworks. ChemSusChem 2014, 7, 2392–2410. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Liang, J.; Wang, X.-S.; Cao, R. Multifunctional metal−organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar] [CrossRef]
- Mistry, S.; Sarkar, A.; Natarajan, S. New bifunctional metal–organic frameworks and their utilization in one-pot tandem catalytic reactions. Cryst. Growth Des. 2019, 19, 747–755. [Google Scholar] [CrossRef]
- Park, J.; Li, J.-R.; Chen, Y.-P.; Yu, J.; Yakovenko, A.A.; Wang, Z.U.; Sun, L.-B.; Balbuena, P.B.; Zhou, H.-C. A versatile metal organic framework for carbon dioxide capture and cooperative catalysis. Chem. Commun. 2012, 48, 9995–9997. [Google Scholar] [CrossRef]
- Toyao, T.; Fujiwaki, M.; Horiuchi, Y.; Matsuoka, M. Application of an amino-functionalised metal–organic framework: An approach to a one-pot acid–base reaction. RSC Adv. 2013, 3, 21582–21587. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Feng, P. Bifunctional heterometallic metal-organic frameworks for solvent-free heterogeneous cascade catalysis. Catalysts 2020, 10, 309. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, A.; Paul, A.; Rúbio, G.M.D.M.; Soliman, M.M.A.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Highly efficient bifunctional amide functionalized Zn and Cd metal organic frameworks for one-pot cascade Deacetalization–Knoevenagel reactions. Front. Chem. 2019, 7, 699. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, A.; Soliman, M.M.A.; Rúbio, G.M.D.M.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Synthesis and catalytic activities of a Zn(ii) based metallomacrocycle and a metal–organic framework towards one-pot Deacetalization-Knoevenagel tandem reactions under different strategies: A comparative study. Dalton Trans. 2020, 49, 8075–8085. [Google Scholar] [CrossRef]
- Banerjee, B. Recent developments on ultrasound assisted catalyst-free organic synthesis. Ultrason. Sonochem. 2017, 35, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, A.; Paul, A.; Santos, I.R.M.; Santos, P.M.R.; Pantanetti Sabatini, E.; Gurbanov, A.V.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Highly efficient adsorptive removal of organic dyes from aqueous solution using polyaromatic group containing Zn(II)-based coordination polymers. Cryst. Growth Des. 2022. to be submitted. [Google Scholar]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(i) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 9, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xi, F.-G.; Sun, W.; Yang, N.-N.; Gao, E.-Q. Amino- and Sulfo-Bifunctionalized metal−organic frameworks: One-Pot tandem catalysis and the catalytic sites. Inorg. Chem. 2016, 55, 5753–5755. [Google Scholar] [CrossRef]
- Lempers, H.E.B.; Sheldon, R.A. The stability of chromium in CrAPO-5, CrAPO-11, and CrS-1 during liquid phase oxidations. J. Catal. 1998, 175, 62–69. [Google Scholar] [CrossRef]
- Pourshojaei, Y.; Nikzad, M.; Eskandari, K.; Darijani, M.-H.; Hassanzadeh, A.; Faghih-Mirzaei, E.; Asadipour, A. Ultrasound-assisted and efficient Knoevenagel condensation reaction Catalyzed by silica sodium carbonate nanoparticles. Croat. Chem. Acta 2018, 91, 19–28. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, Z.; Hu, T.; Zhang, X. Nanochannel {InZn}–Organic framework with a high catalytic performance on CO2 chemical fixation and Deacetalization–Knoevenagel condensation. Inorg. Chem. 2021, 60, 16429–16438. [Google Scholar] [CrossRef]
- Hongming, H.; Fuxing, S.; Briana, A.; Jason, A.P.; Shengqian, M.; Guangshan, Z. A bifunctional metal–organic framework featuring the combination of open metal sites and Lewis basic sites for selective gas adsorption and heterogeneous cascade catalysis. J. Mater. Chem. A 2016, 4, 15240–15246. [Google Scholar]
- Bruker. APEX2; Bruker AXS Inc.: Madison, WI, USA, 2012. [Google Scholar]
- Sheldrick, G.M. SADABS. Program for Empirical Absorption Correction; University of Gottingen: Gottingen, Germany, 1996. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, C71, 3–8. [Google Scholar]
- Farrugia, L.J.J. WinGX and ORTEP for Windows: An update. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]







| Entry | Catalyst | Time (h) | Catalyst (mol%) | T (°C) | Solvent | Unreacted A (%) b | Yield of B (%) b | Yield of D (%) b |
|---|---|---|---|---|---|---|---|---|
| Different Catalysts | ||||||||
| 1 | 1 | 2 | 1 | 80 | DMF | 0 | 0 | >99 |
| 2 | 2 | 2 | 1 | 80 | DMF | 0 | 4 | 96 |
| 3 | 3 | 2 | 1 | 80 | DMF | 1 | 2 | 97 |
| 4 | 4 | 2 | 1 | 80 | DMF | 4 | 1 | 95 |
| 5 | 5 | 2 | 1 | 80 | DMF | 8 | 2 | 90 |
| Different Substrates | ||||||||
| 6 c | 1 | 2 | 1 | 80 | DMF | 2 | 0 | 98 |
| 7 d | 1 | 2 | 1 | 80 | DMF | 4 | 0 | 96 |
| 8 e | 1 | 2 | 1 | 80 | DMF | 8 | 1 | 91 |
| 9 f | 1 | 2 | 1 | 80 | DMF | 14 | 8 | 78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karmakar, A.; Soliman, M.M.A.; Alegria, E.C.B.A.; da Silva, M.F.C.G.; Pombeiro, A.J.L. Polyaromatic Carboxylate Ligands Based Zn(II) Coordination Polymers for Ultrasound-Assisted One-Pot Tandem Deacetalization–Knoevenagel Reactions. Catalysts 2022, 12, 294. https://doi.org/10.3390/catal12030294
Karmakar A, Soliman MMA, Alegria ECBA, da Silva MFCG, Pombeiro AJL. Polyaromatic Carboxylate Ligands Based Zn(II) Coordination Polymers for Ultrasound-Assisted One-Pot Tandem Deacetalization–Knoevenagel Reactions. Catalysts. 2022; 12(3):294. https://doi.org/10.3390/catal12030294
Chicago/Turabian StyleKarmakar, Anirban, Mohamed M. A. Soliman, Elisabete C. B. A. Alegria, Maria Fátima C. Guedes da Silva, and Armando J. L. Pombeiro. 2022. "Polyaromatic Carboxylate Ligands Based Zn(II) Coordination Polymers for Ultrasound-Assisted One-Pot Tandem Deacetalization–Knoevenagel Reactions" Catalysts 12, no. 3: 294. https://doi.org/10.3390/catal12030294
APA StyleKarmakar, A., Soliman, M. M. A., Alegria, E. C. B. A., da Silva, M. F. C. G., & Pombeiro, A. J. L. (2022). Polyaromatic Carboxylate Ligands Based Zn(II) Coordination Polymers for Ultrasound-Assisted One-Pot Tandem Deacetalization–Knoevenagel Reactions. Catalysts, 12(3), 294. https://doi.org/10.3390/catal12030294