Efficient Synthesis of Dihydropyrimidines Using a Highly Ordered Mesoporous Functionalized Pyridinium Organosilica
Abstract
:1. Introduction
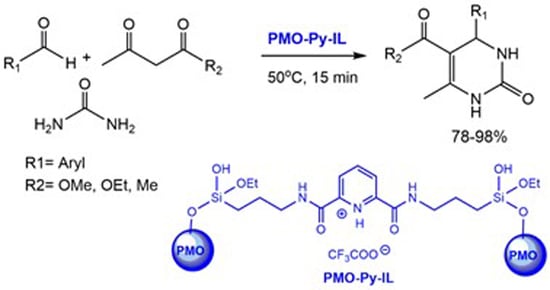
2. Results and Discussion
3. Experimental Section
3.1. General Remarks
3.2. Synthesis of PMO Materials Bearing Protic Pyridinium Ionic Liquid (PMO-Py-IL)
3.3. General Procedure for the Preparation of 3,4-dihydropyrimidin-2(1H)-Ones Using PMO-Py-IL Nanocatalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Busse, W.-D.; Garthoff, B.; Seuter, F. Dihydropyridines Progress in Pharmacology and Therapy; Springer: Berlin/Heidelberg, Germany, 1993. [Google Scholar]
- Lagoja, I.M. Pyrimidine as constituent of natural biologically active compounds. Chem. Biodivers. 2005, 2, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Kimura, H.; Katoh, T.; Kajimoto, T.; Node, M.; Hisaki, M.; Sugimoto, Y.; Majima, T.; Uehara, Y.; Yamori, T. Modification of pyrimidine derivatives from antiviral agents to antitumor agents. Anticancer Res. 2006, 26, 91–97. [Google Scholar] [PubMed]
- Santana, M.L.H.; Masson, F.T.; Simeoni, L.A.; Homem-de-Mello, M. Biological activity of dihydropyrimidinone (DHPM) derivatives: A systematic review. Eur. J. Med. Chem. 2018, 143, 1779–1789. [Google Scholar]
- Fargualy, A.M.; Habib, N.S.; Ismail, K.A.; Hassan, A.M.M.; Sarg, M.T.M. Synthesis, biological evaluation and molecular docking studies of some pyrimidine derivatives. Eur. J. Med. Chem. 2013, 66, 276–295. [Google Scholar] [CrossRef]
- Amir, M.; Javed, S.A.; Kumar, H. Pyrimidine as antiinflammatory agent: A Review. Indian J. Pharm. Sci. 2007, 69, 337. [Google Scholar] [CrossRef]
- Mayer, T.U.; Kapoor, T.M.; Haggarty, S.J.; King, R.W.; Schreiber, S.L.; Mitchison, T.J. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 1999, 286, 971–974. [Google Scholar] [CrossRef]
- Nagarajaiah, H.; Khazi, I.M.; Begum, N.S. Synthesis, characterization and biological evaluation of thiazolopyrimidine derivatives. J. Chem. Sci. 2012, 124, 847–855. [Google Scholar] [CrossRef]
- Rovnyak, G.C.; Kimball, S.D.; Beyer, B.; Cucinotta, G.; DiMarco, J.D.; Gougoutas, J.; Hedberg, A.; Malley, M.; McCarthy, J.P. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995, 38, 119–129. [Google Scholar] [CrossRef]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—a literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Chorlton, A.P. Pyrimidine and pyrimidine derivatives. In Ullmann, F. Ullmann’s Encyclopedia of Industrial Chemistry, 1st ed.; Wiley-VCH: Weinheim, Germany, 1999. [Google Scholar]
- Biginelli, P. Ueber aldehyduramide des acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319. [Google Scholar] [CrossRef]
- Biginelli, P. Synthesis of 3,4-Dihydropyrimidin-2(1H)-Ones. Gazz. Chim. Ital. 1893, 23, 360–416. [Google Scholar]
- Kampe, O.C. The Biginelli Reaction. In Multicomponent Reactions; Zhu, J., Bienaymé, H., Eds.; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Kappe, C.O.; Stadler, A. The Biginelli Dihydropyrimidine Synthesis. Org. React. 2004, 63, 1–116. [Google Scholar]
- Jin, T.; Zhang, S.; Li, T. p-Toluenesulfonic acid-catalyzed efficient synthesis of dihydropyrimidines: Improved high yielding protocol for the biginelli reaction. Synth. Commun. 2002, 32, 1847–1851. [Google Scholar] [CrossRef]
- Gangu, K.K.; Maddila, S.; Mukkamala, S.B.; Jonnalagadda, S.B. Catalytic activity of supra molecular self-assembled Nickel (II) coordination complex in synthesis of indeno-pyrimidine derivatives. Polyhedron 2019, 158, 464–470. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, D.; Liu, C.; Luo, G. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using chloroacetic acid as catalyst. Bioorganic. Med. Chem. Lett. 2007, 17, 3508–3510. [Google Scholar] [CrossRef]
- Nagawade, R.R.; Kotharkar, S.A.; Shinde, D.B. Titanium (IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones and thiones. Mendeleev Commun. 2005, 15, 150–151. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. Ruthenium (III) chloride-catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2-(1H)-ones under solvent-free conditions. Synthesis 2005, 2005, 1748–1750. [Google Scholar] [CrossRef]
- De, S.K.; Gibbs, R.A. Scandium (III) Triflate as an Efficient and Reusable Catalyst for Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones. Synth. Commun. 2005, 35, 2645–2651. [Google Scholar] [CrossRef]
- Pasha, A.; Puttaramegowda, J.V. Co(OAc)2-catalyzed tandem reaction: Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones from β-keto ester. Heterocycl. Commun. 2006, 12, 61–66. [Google Scholar]
- Reddy, B.M.; Sreekanth, P.M.; Lakshmanan, P. Sulfated zirconia as an efficient catalyst for organic synthesis and transformation reactions. J. Mol. Catal. A Chem. 2005, 237, 93–100. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Bolourtchian, M.; Hosseini, M. Microwave-assisted efficient synthesis of dihydropyrimidines in solvent-free condition. Synth. Commun. 2004, 34, 3335–3341. [Google Scholar] [CrossRef]
- Salehi, H.; Guo, Q.-X. A facile and efficient one-pot synthesis of dihydropyrimidinones catalyzed by magnesium bromide under solvent-free conditions. Synth. Commun. 2004, 34, 171–179. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S.; Srinivas, R.; Venugopal, C.; Ramalingam, T. LiClO4-catalyzed one-pot synthesis of dihydropyrimidinones: An improved protocol for Biginelli reaction. Synthesis 2001, 2001, 1341–1345. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.; Wang, L.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium (III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef]
- Gohain, M.; Prajapati, D.; Sandhu, J.S. A novel Cu-catalysed three-component one-pot synthesis of dihydropyrimidin-2(1H)-ones using microwaves under solvent-free conditions. Synlett 2004, 2004, 235–238. [Google Scholar]
- Russowsky, D.; Lopes, F.A.; da Silva, V.S.S.; Canto, K.F.S.; D’Oca, M.G.M.; Godoi, M.N. Multicomponent Biginelli’s synthesis of 3, 4-dihydropyrimidin-2(1H)-ones promoted by SnCl2. 2H2O. J. Braz. Chem. Soc. 2004, 15, 165–169. [Google Scholar] [CrossRef]
- Hu, E.H.; Sidler, D.R.; Dolling, U.-H. Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Rodriguez-Domínguez, J.C.; Bernardi, D.; Kirsch, G. ZrCl4 or ZrOCl2 under neat conditions: Optimized green alternatives for the Biginelli reaction. Tetrahedron Lett. 2007, 48, 5777–5780. [Google Scholar] [CrossRef]
- Pasha, M.A.; Swamy, N.R.; Jayashankara, V.P. One pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones-thiones catalysed by zinc chloride: An improved procedure for the Biginelli reaction using microwaves under solvent free condition. Indian. J. Chem. 2005, 44B, 823–826. [Google Scholar]
- Bose, D.S.; Kumar, R.K.; Fatima, L. A remarkable rate acceleration of the one-pot three-component cyclocondensation reaction at room temperature: An expedient synthesis of mitotic kinesin Eg5 inhibitor monastrol. Synlett 2004, 2004, 279–282. [Google Scholar] [CrossRef]
- Narsaiah, A.V.; Basak, A.K.; Nagaiah, K. Cadmium chloride: An efficient catalyst for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synthesis 2004, 2004, 1253–1256. [Google Scholar] [CrossRef]
- Jin, T.-S.; Wang, H.-X.; Xing, C.-Y.; Li, X.-L.; Li, T.-S. An efficient one-pot synthesis of 3,4-dihydropyrimidin-2-ones catalyzed by methanesulfonic acid. Synth. Commun. 2004, 34, 3009–3016. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Novel Biginelli-like three-component cyclocondensation reaction: Efficient synthesis of 5-unsubstituted 3, 4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2004, 45, 7951–7953. [Google Scholar] [CrossRef]
- Han, X.; Xu, F.; Luo, Y.; Shen, Q. An Efficient One-Pot Synthesis of Dihydropyrimidinones by a Samarium Diiodide Catalyzed Biginelli Reaction Under Solvent-Free Conditions. Eur. J. Org. Chem. 2005, 2005, 1500–1503. [Google Scholar] [CrossRef]
- Kolosov, M.A.; Orlov, V.D.; Beloborodov, D.A.; Dotsenko, V.V. A chemical placebo: NaCl as an effective, cheapest, non-acidic and greener catalyst for Biginelli-type 3, 4-dihydropyrimidin-2(1H)-ones (-thiones) synthesis. Mol. Divers. 2009, 13, 5–25. [Google Scholar] [CrossRef] [PubMed]
- Khakyzadeh, V.; Moosavi-Zare, A.R.; Sheikhaleslami, S.; Ehsani, A.; Sediqi, S.; Rezaei-Gohar, M.; Jalilian, Z. Boric acid in magnetized water: Clean and powerful media for synthesis of 3,4-dihydropyrimidin-2(1H)-ones. RSC Adv. 2021, 11, 22751–22755. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Parallel synthesis of dihydropyrimidinones using Yb (III)-resin and polymer-supported scavengers under solvent-free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett. 2001, 42, 7975–7978. [Google Scholar] [CrossRef]
- Ahn, B.J.; Gang, M.S.; Chae, K.; Oh, Y.; Shin, J.; Chang, W. A microwave-assisted synthesis of 3,4-dihydro-pyrimidin-2-(1H)-ones catalyzed by FeCl3-supported Nanopore Silica under solvent-free conditions. J. Ind. Eng. Chem. 2008, 14, 401–405. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Liu, C.; Wang, J. An efficient synthesis of 4-substituted pyrazolyl-3, 4-dihydropyrimidin-2(1H)-(thio) ones catalyzed by Mg (ClO4)2 under ultrasound irradiation. J. Mol. Catal. A Chem. 2006, 253, 207–211. [Google Scholar] [CrossRef]
- Foroughifar, N.; Mobinikhaledi, A.; Fathinejad Jirandehi, H. Synthesis of Some Biginelli Compounds in Solvent Medium Using a Photochemistry Method, Phosphorus. Sulfur Silicon Relat. Elem. 2003, 178, 495–500. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, X.; Xu, H.; Du, J. Brønsted Acidic Ionic Liquid: An Efficient and Reusable Catalyst for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones. Synth. Commun. 2006, 36, 1503–1513. [Google Scholar] [CrossRef]
- Khodamorady, M.; Sohrabnezhad, S.; Bahrami, K. Efficient one-pot synthetic methods for the preparation of 3,4-dihydropyrimidinones and 1,4-dihydropyridine derivatives using BNPs@ SiO2(CH2)3NHSO3H as a ligand and metal free acidic heterogeneous nano-catalyst. Polyhedron 2020, 178, 114340. [Google Scholar] [CrossRef]
- Patil, R.V.; Chavan, J.U.; Dalal, D.S.; Shinde, V.S.; Beldar, A.G. Biginelli reaction: Polymer supported catalytic approaches. ACS Comb. Sci. 2019, 21, 105–148. [Google Scholar] [CrossRef]
- Shi, X.-L.; Xing, X.; Lin, H.; Zhang, W. Synthesis of Polyacrylonitrile Fiber-Supported Poly (ammonium methanesulfonate) s as Active and Recyclable Heterogeneous Brønsted Acid Catalysts. Adv. Synth. Catal. 2014, 356, 2349–2354. [Google Scholar] [CrossRef]
- Wang, J.-H.; Tang, G.-M.; Yan, S.-C.; Wang, Y.-T.; Zhan, S.-J.; Zhang, E.; Sun, Y.; Jiang, Y.; Cui, Y.-Z. Cobalt-based metal coordination polymers with 4, 4′-bipyridinyl groups: Highly efficient catalysis for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Appl. Organomet. Chem. 2016, 30, 1009–1021. [Google Scholar] [CrossRef]
- Diarjani, E.S.; Rajabi, F.; Yahyazadeh, A.; Puente-Santiago, A.R.; Luque, R. Copper tridentate Schiff base complex supported on SBA-15 as efficient nanocatalyst for three-component reactions under solvent less conditions. Materials 2018, 11, 2458. [Google Scholar] [CrossRef]
- Xie, O.-B.; Wang, N.; Wu, W.-X.; Le, Z.-G.; Yu, X.-Q. Trypsin-catalyzed tandem reaction: One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones by in situ formed acetaldehyde. J. Biotechnol. 2014, 170, 1–5. [Google Scholar] [CrossRef]
- Van der Voort, P.; Esquivel, D.; De Canck, E.; Goethals, F.; Van Driessche, I.; Romero-Salguero, F.J. Periodic mesoporous organosilicas: From simple to complex bridges; a comprehensive overview of functions, morphologies and applications. Chem. Soc. Rev. 2013, 42, 3913–3955. [Google Scholar] [CrossRef]
- Hoffmann, F.; Cornelius, M.; Morell, J.; Fröba, M. Silica-based mesoporous organic--inorganic hybrid materials. Angew. Chem. Int. Ed. 2006, 45, 3216–3251. [Google Scholar] [CrossRef] [PubMed]
- Asefa, T.; MacLachlan, M.J.; Coombs, N.; Ozin, G.A. Periodic mesoporous organosilicas with organic groups inside the channel walls. Nature 1999, 402, 867–871. [Google Scholar] [CrossRef]
- Melde, B.J.; Holland, B.T.; Blanford, C.F.; Stein, A.A. Mesoporous sieves with unified hybrid inorganic/organic frameworks. Chem. Mater. 1999, 11, 3302–3308. [Google Scholar] [CrossRef]
- Inagaki, S.; Guan, S.; Fukushima, Y.; Ohsuna, T.; Terasaki, O. Novel mesoporous materials with a uniform distribution of organic groups and inorganic oxide in their frameworks. J. Am. Chem. Soc. 1999, 121, 9611–9614. [Google Scholar] [CrossRef]
- Waki, M.; Inagaki, S. Periodic mesoporous organosilicas possessing molecularly mixed pyridine and benzene moieties in the frameworks. Microporous Mesoporous Mater. 2019, 284, 10–15. [Google Scholar] [CrossRef]
- Rajabi, F.; Pinilla-de Dios, M.; Abdollahi, M.; Luque, R. Aqueous synthesis of 1,8-dioxo-octahydroxanthenes using supported cobalt nanoparticles as a highly efficient and recyclable nanocatalyst. Catal. Commun. 2019, 120, 95–100. [Google Scholar] [CrossRef]
- Rajabi, F.; Ebrahimi, A.Z.; Rabiee, A.; Pineda, A.; Luque, R. Synthesis and characterization of novel pyridine periodic mesoporous organosilicas and its catalytic activity in the Knoevenagel condensation reaction. Materials 2020, 13, 1097. [Google Scholar] [CrossRef]
- Rajabi, F.; Luque, R. Highly ordered mesoporous functionalized pyridinium protic ionic liquids framework as efficient system in esterification reactions for biofuels production. Mol. Catal. 2020, 498, 111238. [Google Scholar] [CrossRef]
- Chopda, L.V.; Dave, P.N. 12-Tungstosilicic Acid H4[W12SiO40] Over Natural Bentonite as a Heterogeneous Catalyst for the Synthesis of 3,4-dihydropyrimidin-2(1H)-Ones. ChemistrySelect 2020, 5, 2395–2400. [Google Scholar] [CrossRef]
- Dong, F.; Jun, L.; Xinli, Z.; Zhiwen, Y.; Zuliang, L. One-pot green procedure for Biginelli reaction catalyzed by novel task-specific room temperature ionic liquids. J. Mol. Catal. A Chem. 2007, 274, 208–211. [Google Scholar] [CrossRef]
- Saikia, M.; Bhuyan, D.; Saikia, L. Keggin type phosphotungstic acid encapsulated chromium (III) terephthalate metal organic framework as active catalyst for Biginelli condensation. Appl. Catal. Gen. 2015, 505, 501–506. [Google Scholar] [CrossRef]
- Aher, D.S.; Khillare, K.R.; Chavan, L.D.; Shankarwa, S.G. Tungsten-substituted molybdophosphoric acid impregnated with kaolin: Effective catalysts for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones via biginelli reaction. RSC Adv. 2021, 11, 2783–2792. [Google Scholar] [CrossRef]
- Liberto, N.A.; Silva, S.D.P.; Fatima, A.D.; Fernandes, S.A. β-Cyclodextrin-assisted synthesis of Biginelli adducts under solvent-free conditions. Tetrahedron 2013, 69, 8245–8249. [Google Scholar] [CrossRef]
- Tayebee, R.; Ghadamgahi, M. Solvent free one-pot multi-component synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by mesoporous NH4H2PO4/MCM-41 as an environmentally friendly, cheap, and effective catalyst. Arab. J. Chem. 2017, 10, S757–S764. [Google Scholar] [CrossRef]
- Bosica, G.; Cachia, F.; De Nittis, R.; Mariotti, N. Efficient One-Pot Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones via a Three-Component Biginelli Reaction. Molecules 2021, 26, 3753. [Google Scholar] [CrossRef]
- Bamoniri, A.; Mirjalili, B.B.F.; Mahmoodi Fard Chegeni, M. Synthesis of 3,4-Dihydropyrimidinones using Nano γ-Al2O3/BF3/Fe3O4 as an Efficient Magnetic Nanocatalyst under Solvent-free Conditions. J. Nanostruct. 2020, 10, 751–759. [Google Scholar]




| Entry | PMO-Py-IL (mg) | Solvent | Temp. (°C) | Time (min) | Yield (%) a |
|---|---|---|---|---|---|
| 1 | - | - | 100 | 120 | 20 |
| 2 | - | C2H5OH | Reflux | 120 | 28 |
| 3 | 10 | C2H5OH | Reflux | 120 | 95 |
| 4 | 10 | CH2Cl2 | Reflux | 120 | 42 |
| 5 | 10 | THF | Reflux | 120 | 54 |
| 6 | 10 | H2O | Reflux | 120 | 82 |
| 8 | 10 | CH3CN | Reflux | 120 | 58 |
| 9 | 10 | - | 80 | 120 | 99 |
| 10 | 8 | - | 80 | 120 | 88 |
| 11 | 10 | - | 70 | 120 | 84 |
| 12 | 10 | - | 60 | 120 | 87 |
| 13 | 10 | - | 50 | 120 | 99 |
| 14 | 10 | - | 40 | 120 | 70 |
| 15 | 10 | - | 50 | 60 | 98 |
| 16 | 10 | - | 50 | 30 | 98 |
| 17 | 10 | - | 50 | 15 | 98 |
| 18 | 10 | - | 50 | 10 | 91 |
| Entry | R1 | R2 | Product | Yield (%) a | M.P(°C) [Ref.] |
|---|---|---|---|---|---|
| 1 | C6H5 | OEt | 4a | 98 | 201–203 [16] |
| 2 | 4-NO2-C6H4 | OEt | 4b | 94 | 211–213 [16] |
| 3 | 4-Cl-C6H4 | OEt | 4c | 92 | 210–212 [16] |
| 4 | 2-OH-C6H4 | OEt | 4d | 80 | 217–219 [16] |
| 5 | 2-Cl-C6H4 | OEt | 4e | 82 | 220–223 [16] |
| 6 | 4-OCH3-C6H4 | OEt | 4f | 84 | 201–203 [16] |
| 7 | C6H5 | OMe | 4g | 96 | 221–223 [16] |
| 8 | 4-NO2-C6H4 | OMe | 4h | 92 | 233–235 [16] |
| 9 | 4-Cl-C6H4 | OMe | 4i | 85 | 154–156 [16] |
| 10 | 2-OH-C6H4 | OMe | 4j | 78 | 243–244 [45] |
| 11 | 2-Cl-C6H4 | OMe | 4k | 82 | 249–252 [16] |
| 12 | 4-OCH3-C6H4 | OMe | 4l | 80 | 232–233 [16] |
| 13 | C6H5 | Me | 4m | 98 | 231–233 [45] |
| 14 | 4-NO2-C6H4 | Me | 4n | 93 | 229–230 [45] |
| 15 | 4-Cl-C6H4 | Me | 4o | 90 | 204–206 [45] |
| 16 | 2-OH-C6H4 | Me | 4p | 82 | 215–217 [45] |
| 17 | 2-Cl-C6H4 | Me | 4q | 85 | 201–203 [45] |
| 18 | 4-OCH3-C6H4 | Me | 4r | 84 | 172–174 [45] |
| Entry | Catalyst | T (°C) | Time | Conversion (%) | Ref. |
|---|---|---|---|---|---|
| 1 | PMO-Py-IL (0.002 g) | 50 | 15 min | 98 | This work |
| 2 | Cu@SBA-15 (0.01 g) | 100 | 5 min | 94 | [50] |
| 3 | TSA/bent (0.09 g) | 80 | 5 h | 86 | [61] |
| 4 | TSILS (ionic liquids) | 90 | 10 min | 94 | [62] |
| 5 | PTA@MIL-101 (0.6 mol%) | 100 | 60 min | 90 | [63] |
| 6 | PMo7W5/kaolin (20%) | 100 | 8 min | 95 | [64] |
| 7 | β-Cyclodexterin (0.5 mol%) | 100 | 180 min | 85 | [65] |
| 8 | NH4H2PO4/MCM-41 (0.04 g) | 100 | 6 h | 72 | [66] |
| 9 | 40% w/w WSi/A-15 (0.05 g) | 92 | 4.5 | 88 | [67] |
| 10 | Nano-γ-Al2O3/BF3/Fe3O4 (0.008 g) | 80 | 30 min | 95 | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajabi, F.; Sillanpää, M.; Len, C.; Luque, R. Efficient Synthesis of Dihydropyrimidines Using a Highly Ordered Mesoporous Functionalized Pyridinium Organosilica. Catalysts 2022, 12, 350. https://doi.org/10.3390/catal12030350
Rajabi F, Sillanpää M, Len C, Luque R. Efficient Synthesis of Dihydropyrimidines Using a Highly Ordered Mesoporous Functionalized Pyridinium Organosilica. Catalysts. 2022; 12(3):350. https://doi.org/10.3390/catal12030350
Chicago/Turabian StyleRajabi, Fatemeh, Mika Sillanpää, Christophe Len, and Rafael Luque. 2022. "Efficient Synthesis of Dihydropyrimidines Using a Highly Ordered Mesoporous Functionalized Pyridinium Organosilica" Catalysts 12, no. 3: 350. https://doi.org/10.3390/catal12030350
APA StyleRajabi, F., Sillanpää, M., Len, C., & Luque, R. (2022). Efficient Synthesis of Dihydropyrimidines Using a Highly Ordered Mesoporous Functionalized Pyridinium Organosilica. Catalysts, 12(3), 350. https://doi.org/10.3390/catal12030350