Facile Synthesis of Nanosheet-Stacked Hierarchical ZSM-5 Zeolite for Efficient Catalytic Cracking of n-Octane to Produce Light Olefins
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Crystalline Structure of the ZSM-5 Catalysts
2.2. The Morphology of the ZSM-5 Catalysts
2.3. The Textural Properties of the ZSM-5 Catalysts
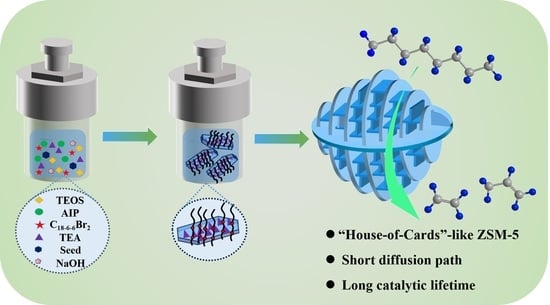
2.4. The Formation Mechanism of Hierarchical ZSM-5 Nanosheets
2.5. The Acid Properties of ZSM-5 Catalysts
3. Catalytic Cracking of n-Octane over the As-Prepared ZSM-5 Catalysts
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Synthesis of Hierarchical Layered ZSM-5
4.3. Catalyst Characterization
4.4. Catalytic Activity Measurement
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alabdullah, M.; Rodriguez-Gomez, A.; Shoinkhorova, T.; Dikhtiarenko, A.; Chowdhury, A.D.; Hita, I.; Kulkarni, S.R.; Vittenet, J.; Sarathy, S.M.; Castaño, P.; et al. One-step conversion of crude oil to light olefins using a multi-zone reactor. Nat. Catal. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Palčić, A.; Catizzone, E. Application of nanosized zeolites in methanol conversion processes: A short review. Curr. Opin. Green Sustain. Chem. 2021, 27, 100393. [Google Scholar] [CrossRef]
- Sadrameli, S.M. Thermal/catalytic cracking of hydrocarbons for the production of olefins: A state-of-the-art review I: Thermal cracking review. Fuel 2015, 140, 102–115. [Google Scholar] [CrossRef]
- Akah, A.; Williams, J.; Ghrami, M. An Overview of Light Olefins Production via Steam Enhanced Catalytic Cracking. Catal. Surv. Asia 2019, 23, 265–276. [Google Scholar] [CrossRef]
- Rahimi, N.; Karimzadeh, R. Catalytic cracking of hydrocarbons over modified ZSM-5 zeolites to produce light olefins: A review. Appl. Catal. A Gen. 2011, 398, 1–17. [Google Scholar] [CrossRef]
- Blay, V.; Louis, B.; Miravalles, R.; Yokoi, T.; Peccatiello, K.A.; Clough, M.; Yilmaz, B. Engineering Zeolites for Catalytic Cracking to Light Olefins. ACS Catal. 2017, 7, 6542–6566. [Google Scholar] [CrossRef]
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Papanikolaou, G.; Lanzafame, P.; Giorgianni, G.; Abate, S.; Perathoner, S.; Centi, G. Highly selective bifunctional Ni zeo-type catalysts for hydroprocessing of methyl palmitate to green diesel. Catal. Today 2020, 345, 14–21. [Google Scholar] [CrossRef]
- Blay, V.; Epelde, E.; Miravalles, R.; Perea, L.A. Converting olefins to propene: Ethene to propene and olefin cracking. Catal. Rev. 2018, 60, 278–335. [Google Scholar] [CrossRef]
- Alipour, S.M. Recent advances in naphtha catalytic cracking by nano ZSM-5: A review. Chin. J. Catal. 2016, 37, 671–680. [Google Scholar] [CrossRef]
- Konno, H.; Tago, T.; Nakasaka, Y.; Ohnaka, R.; Nishimura, J.-I.; Masuda, T. Effectiveness of nano-scale ZSM-5 zeolite and its deactivation mechanism on catalytic cracking of representative hydrocarbons of naphtha. Microporous Mesoporous Mater. 2013, 175, 25–33. [Google Scholar] [CrossRef]
- Mochizuki, H.; Yokoi, T.; Imai, H.; Watanabe, R.; Namba, S.; Kondo, J.N.; Tatsumi, T. Facile control of crystallite size of ZSM-5 catalyst for cracking of hexane. Microporous Mesoporous Mater. 2011, 145, 165–171. [Google Scholar] [CrossRef]
- Sazama, P.; Sobalik, Z.; Dedecek, J.; Jakubec, I.; Parvulescu, V.; Bastl, Z.; Rathousky, J.; Jirglova, H. Enhancement of activity and selectivity in acid-catalyzed reactions by dealuminated hierarchical zeolites. Angew. Chem. Int. Ed. Engl. 2013, 52, 2038–2041. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wang, R.; Wang, P.; Zheng, A.; Guo, Y.; Chen, Y.; Jiang, Q.; Lin, W. Hierarchical hollow Al-rich nano ZSM-5 crystals for highly selective production of light olefins from naphthenes. Catal. Sci. Technol. 2021, 11, 6089–6095. [Google Scholar] [CrossRef]
- Abildstrøm, J.O.; Kegnæs, M.; Hytoft, G.; Mielby, J.; Kegnæs, S. Synthesis of mesoporous zeolite catalysts by in situ formation of carbon template over nickel nanoparticles. Microporous Mesoporous Mater. 2016, 225, 232–237. [Google Scholar] [CrossRef] [Green Version]
- Tago, T.; Konno, H.; Nakasaka, Y.; Masuda, T. Size-Controlled Synthesis of Nano-Zeolites and Their Application to Light Olefin Synthesis. Catal. Surv. Asia 2012, 16, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Dai, W.; Kouvatas, C.; Tai, W.; Wu, G.; Guan, N.; Li, L.; Valtchev, V. Platelike MFI Crystals with Controlled Crystal Faces Aspect Ratio. J. Am. Chem. Soc. 2021, 143, 1993–2004. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Roth, W.J.; Nachtigall, P.; Morris, R.E.; Cejka, J. Two-dimensional zeolites: Current status and perspectives. Chem. Rev. 2014, 114, 4807–4837. [Google Scholar] [CrossRef]
- Xu, L.; Sun, J. Recent Advances in the Synthesis and Application of Two-Dimensional Zeolites. Adv. Energy Mater. 2016, 6, 1600441. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, M.Y.; Stottrup, B.L.; Tsapatsis, M. para-Xylene Ultra-selective Zeolite MFI Membranes Fabricated from Nanosheet Monolayers at the Air-Water Interface. Angew. Chem. Int. Ed. Engl. 2018, 57, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Cheng, D..; Chen, F.; Zhan, X. n-Heptane catalytic cracking on ZSM-5 zeolite nanosheets: Effect of nanosheet thickness. Microporous Mesoporous Mater. 2021, 310, 110647. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, B.; Liang, H.; Hou, X.; Wang, L.; Zhang, X.; Liu, G. Synthesis and performance of pillared HZSM-5 nanosheet zeolites for n-decane catalytic cracking to produce light olefins. Appl. Catal. A Gen. 2019, 572, 24–33. [Google Scholar] [CrossRef]
- Xiao, X.; Zhang, Y.Y.; Jiang, G.Y.; Liu, J.; Han, S.L.; Zhao, Z.; Wang, R.P.; Li, C.; Xu, C.M.; Duan, A.J.; et al. Simultaneous realization of high catalytic activity and stability for catalytic cracking of n-heptane on highly exposed (010) crystal planes of nanosheet ZSM-5 zeolite. Chem. Commun. 2016, 52, 10068–10071. [Google Scholar] [CrossRef] [PubMed]
- Seo, Y.; Cho, K.; Jung, Y.; Ryoo, R. Characterization of the Surface Acidity of MFI Zeolite Nanosheets by 31P NMR of Adsorbed Phosphine Oxides and Catalytic Cracking of Decalin. ACS Catal. 2013, 3, 713–720. [Google Scholar] [CrossRef]
- Li, S.; Li, J.; Dong, M.; Fan, S.; Zhao, T.; Wang, J.; Fan, W. Strategies to control zeolite particle morphology. Chem. Soc. Rev. 2019, 48, 885–907. [Google Scholar] [CrossRef]
- Olafson, K.N.; Li, R.; Alamani, B.G.; Rimer, J.D. Engineering Crystal Modifiers: Bridging Classical and Nonclassical Crystallization. Chem. Mater. 2016, 28, 8453–8465. [Google Scholar] [CrossRef]
- Lupulescu, A.I.; Kumar, M.; Rimer, J.D. A facile strategy to design zeolite L crystals with tunable morphology and surface architecture. J. Am. Chem. Soc. 2013, 135, 6608–6617. [Google Scholar] [CrossRef]
- Lupulescu, A.I.; Rimer, J.D. Tailoring silicalite-1 crystal morphology with molecular modifiers. Angew. Chem. Int. Ed. Engl. 2012, 51, 3345–3349. [Google Scholar] [CrossRef]
- Qin, W.; Agarwal, A.; Choudhary, M.K.; Palmer, J.C.; Rimer, J.D. Molecular Modifiers Suppress Nonclassical Pathways of Zeolite Crystallization. Chem. Mater. 2019, 31, 3228–3238. [Google Scholar] [CrossRef]
- Kumar, M.; Luo, H.; Roman-Leshkov, Y.; Rimer, J.D. SSZ-13 Crystallization by Particle Attachment and Deterministic Pathways to Crystal Size Control. J. Am. Chem. Soc. 2015, 137, 13007–13017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Mayoral, A.; Terasaki, O.; Zhang, Q.; Ma, B.; Zhao, C.; Yang, G.; Yu, J. Amino Acid-Assisted Construction of Single-Crystalline Hierarchical Nanozeolites via Oriented-Aggregation and Intraparticle Ripening. J. Am. Chem. Soc. 2019, 141, 3772–3776. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Yang, X.; Zhang, T.; Zhang, H.; Zhang, Q.; Hu, D.; Chang, X.; Li, Y.; Chen, Z.; Jia, M.; et al. Controlling the Morphology and Titanium Coordination States of TS-1 Zeolites by Crystal Growth Modifier. Inorg. Chem. 2020, 59, 13201–13210. [Google Scholar] [CrossRef]
- Emeis, C.A. Determination of integrated molar extinction coefficients for infrared-absorption bands of pyridine adsorbed on solid acid catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Tian, Y.; Zhu, T.; Duan, H.; He, X.; Qiao, C.; Liu, G. Catalytic cracking of n-heptane over Fe modified HZSM-5 nanosheet to produce light olefins. Fuel 2021, 306, 121725–121735. [Google Scholar] [CrossRef]
- Vuong, G.-T.; Do, T.-O. A new route for the synthesis of uniform nanozeolites with hydrophobic external surface in organic solvent medium. J. Am. Chem. Soc. 2007, 129, 3810–3811. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Chen, Z.; Li, Z.; Yang, Q.; Hu, L.; Jiang, G.; Xu, C.; Wang, Y.; Zhao, Z. Hierarchical ZSM-5 Zeolites with Tunable Sizes of Building Blocks for Efficient Catalytic Cracking of i-Butane. Ind. Eng. Chem. Res. 2018, 57, 10327–10335. [Google Scholar] [CrossRef]
- Wei, R.; Yang, H.; Scott, J.A.; Aguey-Zinsou, K.-F.; Zhang, D. Synthesis of 2D MFI zeolites in the form of self-interlocked nanosheet stacks with tuneable structural and chemical properties for catalysis. Appl. Mater. Today 2018, 11, 22–33. [Google Scholar] [CrossRef]
- Shen, X.; Mao, W.; Ma, Y.; Xu, D.; Wu, P.; Terasaki, O.; Han, L.; Che, S. A Hierarchical MFI Zeolite with a Two-Dimensional Square Mesostructure. Angew. Chem. Int. Ed. Engl. 2018, 57, 724–728. [Google Scholar] [CrossRef]
- Wang, R.; Peng, Z.; Wu, P.; Lu, J.; Rood, M.J.; Sun, H.; Zeng, J.; Wang, Y.; Yan, Z. Direct Synthesis of Nanosheet-Stacked Hierarchical “Honey Stick-like” MFI Zeolites by an Aromatic Heterocyclic Dual-Functional Organic Structure-Directing Agent. Chemistry 2021, 27, 8694–8697. [Google Scholar] [CrossRef]
- Bonilla, G.; Diaz, I.; Tsapatsis, M.; Jeong, H.K.; Lee, Y.; Vlachos, D.G. Zeolite (MFI) crystal morphology control using organic structure-directing agents. Chem. Mater. 2004, 16, 5697–5705. [Google Scholar] [CrossRef]
- Chaikittisilp, W.; Suzuki, Y.; Mukti, R.R.; Suzuki, T.; Sugita, K.; Itabashi, K.; Shimojima, A.; Okubo, T. Formation of hierarchically organized zeolites by sequential intergrowth. Angew. Chem. Int. Ed. Engl. 2013, 52, 3355–3359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, D.; Xu, D.; Asahina, S.; Cychosz, K.A.; Agrawal, K.V.; Al Wahedi, Y.; Bhan, A.; Al Hashimi, S.; Terasaki, O.; et al. Synthesis of self-pillared zeolite nanosheets by repetitive branching. Science 2012, 336, 1684–1687. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Qiang, W.; Ji, T.; Zhang, M.; Li, M.; Lu, J. Uniform hierarchical MFI nanosheets prepared via anisotropic etching for solution-based sub-100-nm-thick oriented MFI layer fabrication. Sci. Adv. 2020, 6, eaay5993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, A.; Yang, T.C.; Chen, M.Y.; Hsiao, H.M.; Yang, C.M. Hierarchical zeolites comprising orthogonally stacked bundles of zeolite nanosheets for catalytic and adsorption applications. J. Hazard. Mater. 2020, 400, 123241. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; Garcia-Martinez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Liu, B.; Duan, Q.; Li, C.; Zhu, Z.; Xi, H.; Qian, Y. Template synthesis of the hierarchically structured MFI zeolite with nanosheet frameworks and tailored structure. New J. Chem. 2014, 38, 4380–4387. [Google Scholar] [CrossRef]
- Pérez-Ramírez, J.; Verboekend, D.; Bonilla, A.; Abelló, S. Zeolite Catalysts with Tunable Hierarchy Factor by Pore-Growth Moderators. Adv. Funct. Mater. 2009, 19, 3972–3979. [Google Scholar] [CrossRef]
- Choi, M.; Cho, H.S.; Srivastava, R.; Venkatesan, C.; Choi, D.H.; Ryoo, R. Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat. Mater. 2006, 5, 718–723. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Y.; Song, K.; Zhang, Y.; Tang, Y. Nano-crystallite oriented self-assembled ZSM-5 zeolite and its LDPE cracking properties: Effects of accessibility and strength of acid sites. J. Catal. 2013, 302, 115–125. [Google Scholar] [CrossRef]
- Davis, T.M.; Drews, T.O.; Ramanan, H.; He, C.; Dong, J.; Schnablegger, H.; Katsoulakis, M.A.; Kokkoli, E.; McCormick, A.V.; Penn, R.L.; et al. Mechanistic principles of nanoparticle evolution to zeolite crystals. Nat. Mater. 2006, 5, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Qiu, C.; Zhuo, Z.; Zhang, D.; Zhao, S.; Wu, H.; Liu, Y.; He, M. Acid strength controlled reaction pathways for the catalytic cracking of 1-butene to propene over ZSM-5. J. Catal. 2014, 309, 136–145. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, Z.; Xu, C.; Zhang, P.; Duan, A. FeHZSM-5 molecular sieves—Highly active catalysts for catalytic cracking of isobutane to produce ethylene and propylene. Catal. Commun. 2006, 7, 199–203. [Google Scholar] [CrossRef]
- Jin, F.; Li, Y. A FTIR and TPD examination of the distributive properties of acid sites on ZSM-5 zeolite with pyridine as a probe molecule. Catal. Today 2009, 145, 101–107. [Google Scholar] [CrossRef]
- Zhang, X.; Cheng, D.; Chen, F.; Zhan, X. n-Heptane catalytic cracking on hierarchical ZSM-5 zeolite: The effect of mesopores. Chem. Eng. Sci. 2017, 168, 352–359. [Google Scholar] [CrossRef]
- Gong, T.; Zhang, X.; Bai, T.; Zhang, Q.; Tao, L.; Qi, M.; Duan, C.; Zhang, L. Coupling Conversion of Methanol and C4 Hydrocarbon to Propylene on La-Modified HZSM-5 Zeolite Catalysts. Ind. Eng. Chem. Res. 2012, 51, 13589–13598. [Google Scholar] [CrossRef]
- Yan, X.; Liu, B.; Huang, J.; Wu, Y.; Chen, H.; Xi, H. Dual Template Preparation of MFI Zeolites with Tuning Catalytic Properties in Alkylation of Mesitylene with Benzyl Alcohol. Ind. Eng. Chem. Res. 2019, 58, 2924–2932. [Google Scholar] [CrossRef]
- Schallmoser, S.; Ikuno, T.; Wagenhofer, M.F.; Kolvenbach, R.; Haller, G.L.; Sanchez-Sanchez, M.; Lercher, J.A. Impact of the local environment of Brønsted acid sites in ZSM-5 on the catalytic activity in n-pentane cracking. J. Catal. 2014, 316, 93–102. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, B.; Wang, P.; Fan, X.; Kong, L.; Xie, Z.; Liu, B.; Zhao, Z. Tuning the density of Brønsted acid sites on mesoporous ZSM-5 zeolite for enhancing light olefins selectivity in the catalytic cracking of n-octane. Microporous Mesoporous Mater. 2022, 330, 111621–111635. [Google Scholar] [CrossRef]
- Xue, Y.F.; Li, J.F.; Wang, P.F.; Cui, X.J.; Zheng, H.Y.; Niu, Y.L.; Dong, M.; Qin, Z.F.; Wang, J.G.; Fan, W.B. Regulating Al distribution of ZSM-5 by Sn incorporation for improving catalytic properties in methanol to olefins. Appl. Catal. B Environ. 2021, 280, 119391. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, L.; Chen, L.; Wu, H.; Wu, P. Postsynthesis of mesoporous ZSM-5 zeolite by piperidine-assisted desilication and its superior catalytic properties in hydrocarbon cracking. J. Mater. Chem. A 2015, 3, 3511–3521. [Google Scholar] [CrossRef]
- Xiao, X.; Xu, Z.; Wang, P.; Liu, X.; Fan, X.; Kong, L.; Xie, Z.; Zhao, Z. Solvent-Free Synthesis of SAPO-34 Zeolite with Tunable SiO2/Al2O3 Ratios for Efficient Catalytic Cracking of 1-Butene. Catalysts 2021, 11, 835. [Google Scholar] [CrossRef]
- Arudra, P.; Bhuiyan, T.I.; Akhtar, M.N.; Aitani, A.M.; Al-Khattaf, S.S.; Hattori, H. Silicalite-1 As Efficient Catalyst for Production of Propene from 1-Butene. ACS Catal. 2014, 4, 4205–4214. [Google Scholar] [CrossRef]













| Samples | Si/Al 1 | SBET(m2/g) 2 | Sext(m2/g) 3 | Vtol(cm3/g) 4 | Vmicro(cm3/g) 5 | HF’ 6 |
|---|---|---|---|---|---|---|
| HZ5-0/0 | - | 275 | 128 | 0.11 | 0.08 | 0.19 |
| HZ5-2.5/0 | 55 | 421 | 222 | 0.32 | 0.10 | 1.16 |
| HZ5-2.5/5 | 57 | 416 | 222 | 0.42 | 0.10 | 1.71 |
| HZ5-2.5/10 | 59 | 412 | 219 | 0.42 | 0.10 | 1.73 |
| HZ5-2.5/20 | 56 | 447 | 261 | 0.38 | 0.10 | 1.68 |
| HZ5-2.5/40 | 57 | 432 | 208 | 0.41 | 0.12 | 1.21 |
| CZSM-5 | 48 | 320 | 94 | 0.20 | 0.10 | 0.29 |
| Samples | Concentration of B Acid Sites (μmol/g) | Concentration of L Acid Sites (μmol/g) | CB/CL | ||||
|---|---|---|---|---|---|---|---|
| Weak and Med. (200 °C) | Strong (350 °C) | Total | Weak and Med. (200 °C) | Strong (350 °C) | Total | ||
| HZ5-2.5/0 | 82.3 | 75.9 | 158.2 | 6.4 | 4.6 | 11.0 | 14.4 |
| HZ5-2.5/5 | 81.4 | 80.7 | 162.1 | 9.5 | 6.3 | 15.8 | 10.2 |
| HZ5-2.5/10 | 84.6 | 78.6 | 163.2 | 16.9 | 7.6 | 24.5 | 6.7 |
| HZ5-2.5/20 | 103.6 | 102.7 | 206.3 | 13.9 | 9.9 | 23.8 | 8.7 |
| HZ5-2.5/40 | 62.0 | 54.3 | 116.3 | 6.2 | 4.8 | 11.0 | 10.6 |
| CZSM-5 | 105.0 | 99.3 | 204.3 | 8.6 | 4.4 | 13.0 | 15.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Xiao, X.; Pan, Y.; Zhao, Z.; Jiang, G.; Zhang, Z.; Meng, F.; Li, Y.; Fan, X.; Kong, L.; et al. Facile Synthesis of Nanosheet-Stacked Hierarchical ZSM-5 Zeolite for Efficient Catalytic Cracking of n-Octane to Produce Light Olefins. Catalysts 2022, 12, 351. https://doi.org/10.3390/catal12030351
Wang P, Xiao X, Pan Y, Zhao Z, Jiang G, Zhang Z, Meng F, Li Y, Fan X, Kong L, et al. Facile Synthesis of Nanosheet-Stacked Hierarchical ZSM-5 Zeolite for Efficient Catalytic Cracking of n-Octane to Produce Light Olefins. Catalysts. 2022; 12(3):351. https://doi.org/10.3390/catal12030351
Chicago/Turabian StyleWang, Peng, Xia Xiao, Yutong Pan, Zhen Zhao, Guiyuan Jiang, Zhongdong Zhang, Fanfang Meng, Yuming Li, Xiaoqiang Fan, Lian Kong, and et al. 2022. "Facile Synthesis of Nanosheet-Stacked Hierarchical ZSM-5 Zeolite for Efficient Catalytic Cracking of n-Octane to Produce Light Olefins" Catalysts 12, no. 3: 351. https://doi.org/10.3390/catal12030351
APA StyleWang, P., Xiao, X., Pan, Y., Zhao, Z., Jiang, G., Zhang, Z., Meng, F., Li, Y., Fan, X., Kong, L., & Xie, Z. (2022). Facile Synthesis of Nanosheet-Stacked Hierarchical ZSM-5 Zeolite for Efficient Catalytic Cracking of n-Octane to Produce Light Olefins. Catalysts, 12(3), 351. https://doi.org/10.3390/catal12030351