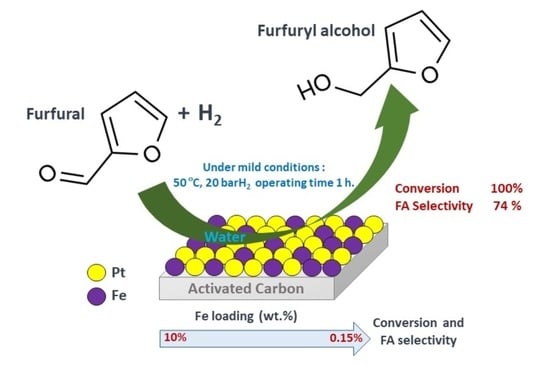
Liquid-Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol over Ferromagnetic Element (Fe, Co, Ni, Nd)-Promoted Pt Catalysts Supported on Activated Carbon
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalyst Activity
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalytic Reaction Study
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; Granados, M.L. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Yan, K.; Wu, G.; Lafleur, T.; Jarvis, C. Production, properties and catalytic hydrogenation of furfural to fuel additives and value-added chemicals. Renew. Sustain. Energy Rev. 2014, 38, 663–676. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Sun, D.; Sato, S.; Ueda, W.; Primo, A.; Garcia, H.; Corma, A. Production of C4 and C5 alcohols from biomass-derived materials. Green Chem. 2016, 18, 2579–2597. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Padmasri, A.H.; Raju, B.D.; Rao, K.R. Vapor phase selective hydrogenation of furfural to furfuryl alcohol over Cu–MgO coprecipitated catalysts. J. Mol. Catal. A Chem. 2007, 265, 90–97. [Google Scholar] [CrossRef]
- Wu, J.; Shen, Y.; Liu, C.; Wang, H.; Geng, C.; Zhang, Z. Vapor phase hydrogenation of furfural to furfuryl alcohol over environmentally friendly Cu–Ca/SiO2 catalyst. Catal. Commun. 2005, 6, 633–637. [Google Scholar] [CrossRef]
- Sharma, R.V.; Das, U.; Sammynaiken, R.; Dalai, A.K. Liquid phase chemo-selective catalytic hydrogenation of furfural to furfuryl alcohol. Appl. Catal. A Gen. 2013, 454, 127–136. [Google Scholar] [CrossRef]
- Yan, K.; Jarvis, C.; Lafleur, T.; Qiao, Y.; Xie, X. Novel synthesis of Pd nanoparticles for hydrogenation of biomass-derived platform chemicals showing enhanced catalytic performance. RSC Adv. 2013, 3, 25865–25871. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Highly active and selective NiFe/SiO2 bimetallic catalyst with optimized solvent effect for the liquid-phase hydrogenation of furfural to furfuryl alcohol. ACS Sustain. Chem. Eng. 2018, 6, 13287–13295. [Google Scholar] [CrossRef]
- Jia, P.; Lan, X.; Li, X.; Wang, T. Deactivation mechanistic studies of copper chromite catalyst for selective hydrogenation of 2-furfuraldehyde. J. Catal. 2013, 299, 336–345. [Google Scholar]
- Taylor, M.J.; Durndell, L.J.; Isaacs, M.A.; Parlett, C.M.; Wilson, K.; Lee, A.F.; Kyriakou, G. Highly selective hydrogenation of furfural over supported Pt nanoparticles under mild conditions. Appl. Catal. B Environ. 2016, 180, 580–585. [Google Scholar] [CrossRef]
- Merlo, A.B.; Vetere, V.; Ruggera, J.F.; Casella, M.L. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun. 2009, 10, 1665–1669. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, L.; Zhang, B.; Guo, X.; Mu, X. Highly selective hydrogenation of furfural to furfuryl alcohol over Pt nanoparticles supported on g-C3N4 nanosheets catalysts in water. Sci. Rep. 2016, 6, 28558. [Google Scholar] [CrossRef]
- Biradar, N.S.; Hengne, A.A.; Birajdar, S.N.; Swami, R.; Rode, C.V. Tailoring the product distribution with batch and continuous process options in catalytic hydrogenation of furfural. Org. Process Res. Dev. 2014, 18, 1434–1442. [Google Scholar] [CrossRef]
- Wang, C.; Guo, Z.; Yang, Y.; Chang, J.; Borgna, A. Hydrogenation of furfural as model reaction of bio-oil stabilization under mild conditions using multiwalled carbon nanotube (MWNT)-supported pt catalysts. Ind. Eng. Chem. Res. 2014, 53, 11284–11291. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Fei, B.; Chen, X.; Zhang, J.; Mu, X. Tunable and selective hydrogenation of furfural to furfuryl alcohol and cyclopentanone over Pt supported on biomass-derived porous heteroatom doped carbon. Faraday Discuss 2017, 202, 79–98. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, O.F.; Iqbal, S.; Miedziak, P.J.; Brett, G.L.; Jones, D.R.; Liu, X.; Edwards, J.K.; Morgan, D.J.; Knight, D.K.; Hutchings, G.J. Pd–Ru/TiO2 catalyst—An active and selective catalyst for furfural hydrogenation. Catal. Sci. Technol. 2016, 6, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Musci, J.J.; Merlo, A.B.; Casella, M.L. Aqueous phase hydrogenation of furfural using carbon-supported Ru and RuSn catalysts. Catal. Today 2017, 296, 43–50. [Google Scholar] [CrossRef]
- Garcia-Olmo, A.J.; Yepez, A.; Balu, A.M.; Prinsen, P.; Garcia, A.; Maziere, A.; Len, C.; Luque, R. Activity of continuous flow synthesized Pd-based nanocatalysts in the flow hydroconversion of furfural. Tetrahedron 2017, 73, 5599–5604. [Google Scholar] [CrossRef]
- Lee, J.; Woo, J.; Nguyen-Huy, C.; Lee, M.S.; Joo, S.H.; An, K. Highly dispersed Pd catalysts supported on various carbons for furfural hydrogenation. Catal. Today 2020, 350, 71–79. [Google Scholar] [CrossRef]
- Audemar, M.; Ciotonea, C.; De Oliveira Vigier, K.; Royer, S.; Ungureanu, A.; Dragoi, B.; Dumitriu, E.; Jérôme, D. Selective hydrogenation of furfural to furfuryl alcohol in the presence of a recyclable cobalt/SBA-15 catalyst. ChemSusChem 2015, 8, 1885–1891. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A.; Moreno-Tost, R.; Maireles-Torres, P. Selective furfural hydrogenation to furfuryl alcohol using Cu-based catalysts supported on clay minerals. Top. Catal. 2017, 60, 1040–1053. [Google Scholar] [CrossRef]
- Villaverde, M.M.; Bertero, N.M.; Garetto, T.F.; Marchi, A.J. Selective liquid-phase hydrogenation of furfural to furfuryl alcohol over Cu-based catalysts. Catal. Today 2013, 213, 87–92. [Google Scholar] [CrossRef]
- Kotbagi, T.V.; Gurav, H.R.; Nagpure, A.S.; Chilukuri, S.V.; Bakker, M.G. Highly efficient nitrogen-doped hierarchically porous carbon supported Ni nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol. RSC Adv. 2016, 6, 67662–67668. [Google Scholar] [CrossRef] [Green Version]
- Gong, W.; Chen, C.; Zhang, H.; Zhang, Y.; Zhang, Y.; Wang, G.; Zhao, H. Highly selective liquid-phase hydrogenation of furfural over N-doped carbon supported metallic nickel catalyst under mild conditions. Mol. Catal. 2017, 429, 51–59. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural over Pt based and Pd based bimetallic catalysts supported on modified multiwalled carbon nanotubes (MWNT). Appl. Catal. A Gen. 2018, 550, 1–10. [Google Scholar] [CrossRef]
- Tolek, W.; Khruechao, K.; Pongthawornsakun, B.; Mekasuwandumrong, O.; Aires, F.J.C.S.; Weerachawanasak, P.; Panpranot, J. Flame spray-synthesized Pt-Co/TiO2 catalysts for the selective hydrogenation of furfural to furfuryl alcohol. Catal. Commun. 2021, 149, 106246. [Google Scholar] [CrossRef]
- Dohade, M.G.; Dhepe, P.L. One pot conversion of furfural to 2-methylfuran in the presence of PtCo bimetallic catalyst. Clean Technol. Environ. Policy 2017, 20, 703–713. [Google Scholar] [CrossRef]
- Putro, W.S.; Hara, T.; Ichikuni, N.; Shimazu, S. Efficiently recyclable and easily separable Ni-Fe alloy catalysts for chemoselective hydrogenation of biomass-derived furfural. Chem. Lett. 2017, 46, 149–151. [Google Scholar] [CrossRef]
- Halilu, A.; Ali, T.H.; Atta, A.Y.; Sudarsanam, P.; Bhargava, S.K.; Abd Hamid, S.B. Highly Selective hydrogenation of biomass-derived furfural into furfuryl alcohol using a novel magnetic nanoparticles catalyst. Energy Fuels 2016, 30, 2216–2226. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Huang, M.; Ma, H.-L.; Zhang, Z.-Q.; Gao, J.-M.; Zhu, Y.-L.; Han, X.-L.; Guo, X.-Y. Preparation of a carbon-based solid acid catalyst by sulfonating activated carbon in a chemical reduction process. Molecules 2010, 15, 7188–7196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; He, D.; He, Q.; Jiang, P.; Zhou, G.; Fu, W. Selective hydrogenation of p-chloronitrobenzene over an Fe promoted Pt/AC catalyst. RSC Adv. 2017, 7, 29143–29148. [Google Scholar] [CrossRef] [Green Version]
- Dorniani, D.; Hussein, M.Z.B.; Kura, A.U.; Fakurazi, S.; Shaari, A.H.; Ahmad, Z. Preparation of Fe(3)O(4) magnetic nanoparticles coated with gallic acid for drug delivery. Int. J. Nanomed. 2012, 7, 5745–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, H.; Igarashi, N.; Fujita, S.I.; Panpranot, J.; Arai, M. Influence of crystallite size of TiO2 supports on the activity of dispersed pt catalysts in liquid-phase selective hydrogenation of 3-nitrostyrene, nitrobenzene, and styrene. Catal. Lett. 2014, 145, 606–611. [Google Scholar] [CrossRef]
- Corma, A.; Serna, P.; Concepción, P.; Calvino, J.J. Transforming nonselective into chemoselective metal catalysts for the hydrogenation of substituted nitroaromatics. J. Am. Chem. Soc. 2008, 130, 8748–8753. [Google Scholar] [CrossRef] [PubMed]
- Einaga, H.; Urahama, N.; Tou, A.; Teraoka, Y. CO oxidation over TiO2-supported Pt–Fe catalysts prepared by coimpregnation methods. Catal. Lett. 2014, 144, 1653–1660. [Google Scholar] [CrossRef]
- Pisduangdaw, S.; Mekasuwandumrong, O.; Fujita, S.I.; Arai, M.; Yoshida, H.; Panpranot, J. One step synthesis of Pt–Co/TiO2 catalysts by flame spray pyrolysis for the hydrogenation of 3-nitrostyrene. Catal. Commun. 2015, 61, 11–15. [Google Scholar] [CrossRef]
- Da Silva, A.B.; Jordão, E.; Mendes, M.J.; Fouilloux, P. Selective hydrogenation of cinnamaldehyde with Pt And Pt-Fe catalysts: Effects of the support. Braz. J. Chem. Eng. 1998, 15, 140–144. [Google Scholar] [CrossRef]
- Ananthan, S.A.; Narayanan, V. Liquid-phase hydrogenation of citral over Pt_TiO2 and Pt-Fe_TiO2 catalysts. Asian J. Chem. 2011, 23, 183–188. [Google Scholar]
- Von Arx, M.; Mallat, T.; Baiker, A. Unprecedented selectivity behaviour in the hydrogenation of an α,β-unsaturated ketone: Hydrogenation of ketoisophorone over alumina-supported Pt and Pd. J. Mol. Catal. A Chem. 1999, 148, 275–283. [Google Scholar] [CrossRef]
- Fuente-Hernández, A.; Lee, R.; Béland, N.; Zamboni, I.; Lavoie, J.M. Reduction of Furfural to Furfuryl Alcohol in Liquid Phase over a Biochar-Supported Platinum Catalyst. Energies 2017, 10, 286. [Google Scholar] [CrossRef]
- Chen, B.; Li, F.; Huang, Z.; Yuan, G. Tuning catalytic selectivity of liquid-phase hydrogenation of furfural via synergistic effects of supported bimetallic catalysts. Appl. Catal. A Gen. 2015, 500, 23–29. [Google Scholar] [CrossRef]
- Shchukarev, A.; Korolkov, D. XPS study of group IA carbonates. Open Chem. 2004, 2, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]








| Catalyst | ICP Results (Metal Loading, wt%) a | Atomic Ratio (Pt/M) b | N2 Physisorption Properties | The Ratio of CO Adsorption on Pt Atom at Edge Sites and Pt Terrace d | Active Sites (×1018 Molecule CO/g cat.) e | |||
|---|---|---|---|---|---|---|---|---|
| Pt | M (M = Fe, Co, or Ni) | Surface Area (m2/g) | Pore Volume (cm3/g) c | Average Pore Size (nm) c | ||||
| Pt/AC | 0.51 | - | - | 804 | 0.092 | 3.5 | 0.09 | 0.95 |
| 0.1FePt/AC | n.d. | n.d. | n.d. | 793 | 0.110 | 3.4 | n.d. | n.d. |
| 0.15FePt/AC | 0.53 | 0.23 | 0.66 | 988 | 0.102 | 3.2 | 0.47 | 5.69 |
| 0.2FePt/AC | n.d. | n.d. | n.d. | 852 | 0.106 | 3.3 | n.d. | n.d. |
| 5FePt/AC | 0.50 | 4.70 | 0.03 | 860 | 0.129 | 3.1 | n.d. | n.d. |
| 10FePt/AC | 0.42 | 9.50 | 0.013 | 755 | 0.109 | 3.9 | n.d. | n.d. |
| 0.15CoPt/AC | 0.52 | 0.10 | 1.57 | 915 | 0.104 | 3.4 | 0.21 | 2.94 |
| 0.15NiPt/AC | 0.47 | 0.16 | 0.88 | 826 | 0.110 | 3.4 | 0.34 | 0.82 |
| 0.15NdPt/AC | 0.48 | n.d. | n.d. | 780 | 0.094 | 3.5 | 0.16 | 1.85 |
| Catalyst | Solvent | Reaction Time (h) | Conversion (%) | Selectivity (%) | ||
|---|---|---|---|---|---|---|
| FA | SP | Others a | ||||
| Pt/AC | Methanol | 2 | 53.9 | 58.0 | 4.1 | 37.9 |
| 0.15FePt/AC | Methanol | 2 | 64.1 | 56.8 | 6.0 | 37.2 |
| 5FePt/AC | Methanol | 2 | 47.6 | 10.4 | 1.1 | 88.5 |
| 10FePt/AC | Methanol | 2 | 14.1 | 0 | 7.0 | 93.0 |
| 0.15CoPt/AC | Methanol | 2 | 62.9 | 35.4 | 1.5 | 63.1 |
| 0.15NiPt/AC | Methanol | 2 | 26.7 | 42.4 | 21.3 | 36.3 |
| Pt/AC | Water | 1 | 81.8 | 45.2 | - | 54.8 |
| 0.1FePt/AC | Water | 1 | 100 | 57.4 | - | 42.6 |
| 0.15FePt/AC | Water | 1 | 100 | 74.1 | - | 25.9 |
| 0.2FePt/AC | Water | 1 | 91.9 | 65.6 | - | 34.4 |
| 0.5FePt/AC | Water | 1 | 95.9 | 58.1 | - | 41.9 |
| 5FePt/AC | Water | 1 | 83.6 | 56.6 | - | 43.4 |
| 10FePt/AC | Water | 1 | 58.4 | 41.4 | - | 58.6 |
| 0.15CoPt/AC | Water | 1 | 88.1 | 47.6 | - | 52.4 |
| 0.15NiPt/AC | Water | 1 | 96.7 | 32.6 | - | 67.4 |
| 0.15NdPt/AC | Water | 1 | 75.5 | 32.6 | - | 67.4 |
| No. | Catalysts | Preparation Method | Reaction Conditions | Solvent | Reaction Time (h) | Reaction Results | Ref. | |
|---|---|---|---|---|---|---|---|---|
| Conversion (%) | Selectivity (%) | |||||||
| 1 | 0.15FePt/AC | Co-impregnation | 50 °C, 2 MPa H2 | H2O | 1 | 100 | 74.1 | This work |
| 2 | Pt-Fe/MWNT | Co-impregnation | 100 °C, 3 MPa H2 | Ethanol | 5 | 95.2 | 91.8 | [26] |
| Pt-Fe/H-AC | Co-impregnation | 100 °C, 10.3 MPa H2 | Ethanol | 5 | 35.5 | 33.4 | ||
| Pt-Fe/AC | Co-impregnation | 100 °C, 10.3 MPa H2 | Ethanol | 5 | 52.9 | 28.6 | ||
| Pt-Ni/MWNT | Co-impregnation | 100 °C, 10.3 MPa H2 | Ethanol | 5 | 95.9 | 84.1 | ||
| Pt-Co/MWNT | Co-impregnation | 100 °C, 10.3 MPa H2 | Ethanol | 5 | 86.7 | 80.3 | ||
| 3 | 5%Pt@TECN | Ultrasound-assisted reduction | 100 °C, 1 MPa H2 | H2O | 5 | >99 | >99 | [13] |
| 5%Pt@TECN | Ultrasound-assisted reduction | 100 °C, 2 MPa H2 | H2O | 1 | 98 | 98 | ||
| 4 | 3%Pt/BC | Wet impregnation | 210 °C, 10.3 MPa H2 | Toluene | 2 | 60.8 | 79.2 | [41] |
| 5 | 3%Pt/AC | Wet impregnation | 180 °C, 1 MPa H2 | Isopropanol | 8 | 100 | 71 | [28] |
| 6 | Pt/NC-BS-500 | Ultrasound-assisted reduction | 100 °C, 1 MPa H2 | H2O | 4 | >99 | >99 | [16] |
| 7 | Pt-Sn0.3/SiO2 | Controlled surface reaction | 100 °C, 1 MPa H2 | 2-propanol | 8 | >90 | 96.2 | [12] |
| 8 | Pt-Re/TiO2-ZrO2 | Co-impregnation | 130 °C, 5 MPa H2 | Ethanol | 8 | 100 | 95.7 | [42] |
| Pt-In/TiO2-ZrO2 | Co-impregnation | 130 °C, 5 MPa H2 | Ethanol | 8 | 73.3 | 74.9 | ||
| Pt-Sn/TiO2-ZrO2 | Co-impregnation | 130 °C, 5 MPa H2 | Ethanol | 8 | 98.3 | 47.8 | ||
| 0.5FePt/AC | EDX (wt%) | Ratio O/C | |||
|---|---|---|---|---|---|
| C | O | Pt | Fe | ||
| Fresh | 90.57 | 6.42 | 1.86 | 1.9 | 0.066 |
| Spent 3rd cycle | 94.2 | 3.41 | 1.64 | 0.79 | 0.036 |
| Fresh | After 3rd Cycle | |||
|---|---|---|---|---|
| Binding Energy (eV) | Mass (%) | Binding Energy (eV) | Mass (%) | |
| C 1s | 285.0 | 68.71 | 285.0 | 43.39 |
| 288.9 | 6.57 | 286.6 | 26.89 | |
| 288.7 | 12.60 | |||
| O 1s | 533.7 | 24.30 | 533.4 | 16.23 |
| Fe 2p | 711.4 | 0.42 | 711.2 | 0.90 |
| Ratio O/C | 0.323 | 0.196 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saknaphawuth, S.; Weerachawanasak, P.; Chuenchom, L.; Praserthdam, P.; Panpranot, J. Liquid-Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol over Ferromagnetic Element (Fe, Co, Ni, Nd)-Promoted Pt Catalysts Supported on Activated Carbon. Catalysts 2022, 12, 393. https://doi.org/10.3390/catal12040393
Saknaphawuth S, Weerachawanasak P, Chuenchom L, Praserthdam P, Panpranot J. Liquid-Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol over Ferromagnetic Element (Fe, Co, Ni, Nd)-Promoted Pt Catalysts Supported on Activated Carbon. Catalysts. 2022; 12(4):393. https://doi.org/10.3390/catal12040393
Chicago/Turabian StyleSaknaphawuth, Sureeporn, Patcharaporn Weerachawanasak, Laemthong Chuenchom, Piyasan Praserthdam, and Joongjai Panpranot. 2022. "Liquid-Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol over Ferromagnetic Element (Fe, Co, Ni, Nd)-Promoted Pt Catalysts Supported on Activated Carbon" Catalysts 12, no. 4: 393. https://doi.org/10.3390/catal12040393
APA StyleSaknaphawuth, S., Weerachawanasak, P., Chuenchom, L., Praserthdam, P., & Panpranot, J. (2022). Liquid-Phase Selective Hydrogenation of Furfural to Furfuryl Alcohol over Ferromagnetic Element (Fe, Co, Ni, Nd)-Promoted Pt Catalysts Supported on Activated Carbon. Catalysts, 12(4), 393. https://doi.org/10.3390/catal12040393