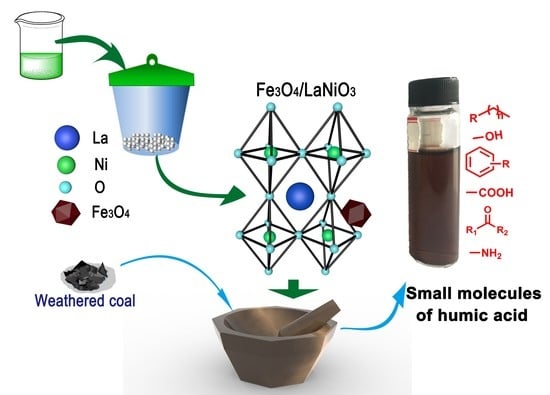
Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Catalysts
2.1.1. Crystal Structure and Morphology
2.1.2. XPS Analysis
2.1.3. N2 Adsorption−Desorption
2.2. Catalytic Performance Evaluation
2.3. Mechanism Analysis
3. Materials and Methods
3.1. Materials and Characterizations
3.1.1. Materials
3.1.2. Characterizations
3.2. Catalyst Preparation
3.2.1. Synthesis of LaNiO3 Catalysts
3.2.2. Synthesis of Fe3O4/LaNiO3 Catalysts
3.3. Catalytic Activity Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Song, J.-J.; Deng, J.; Zhao, J.-Y.; Zhang, Y.-N.; Shu, C.-M. Comparative analysis of exothermic behaviour of fresh and weathered coal during low-temperature oxidation. Fuel 2021, 289, 119942. [Google Scholar] [CrossRef]
- Kim, J.H.; Chang, B.; Kim, B.J.; Park, C.; Goo, J.Y.; Lee, Y.J.; Lee, S. Applicability of weathered coal waste as a reactive material to prevent the spread of inorganic contaminants in groundwater. Environ. Sci. Pollut. Res. 2020, 27, 45297–45310. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Li, Y.; Wang, Q.; Zhao, W.; Jia, J.; Lv, J.; Liu, S.; Xia, D. Feasibility analysis of the in situ conversion of biomethane in surface weathered coal. Fuel 2020, 268, 117273. [Google Scholar] [CrossRef]
- Yenilmez, F.; Kuter, N.; Emil, M.K.; Aksoy, A. Evaluation of pollution levels at an abandoned coal mine site in Turkey with the aid of GIS. Int. J. Coal Geol. 2011, 86, 12–19. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Cajthaml, T.; Brus, J.; Frouz, J. Humus accumulation, humification, and humic acid composition in soils of two post-mining chronosequences after coal mining. J. Soil. Sediments 2012, 13, 491–500. [Google Scholar] [CrossRef]
- Sarlaki, E.; Sharif Paghaleh, A.; Kianmehr, M.H.; Asefpour Vakilian, K. Valorization of lignite wastes into humic acids: Process optimization, energy efficiency and structural features analysis. Renew. Energy 2021, 163, 105–122. [Google Scholar] [CrossRef]
- Zhang, S.; Su, J.; Ali, A.; Zheng, Z.; Sun, Y. Enhanced denitrification performance of strain YSF15 by different molecular weight of humic acid: Mechanism based on the biological products and activity. Bioresour. Technol. 2021, 325, 124709. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-Q.; Yuan, L.; Li, W.; Lin, Z.-A.; Li, Y.-T.; Hu, S.-W.; Zhao, B.-Q. Effects of urea enhanced with different weathered coal-derived humic acid components on maize yield and fate of fertilizer nitrogen. J. Integr. Agr. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Pittarello, M.; Busato, J.G.; Carletti, P.; Dobbss, L.B. Possible developments for ex situ phytoremediation of contaminated sediments, in tropical and subtropical regions—Review. Chemosphere 2017, 182, 707–719. [Google Scholar] [CrossRef]
- Sun, Z.; Tang, B.; Xie, H. Treatment of Waste Gases by Humic Acid. Energy Fuels 2015, 29, 1269–1278. [Google Scholar]
- Salati, S.; Papa, G.; Adani, F. Perspective on the use of humic acids from biomass as natural surfactants for industrial applications. Biotechnol. Adv. 2011, 29, 913–922. [Google Scholar] [PubMed]
- Xiao, Z.; Zhou, H.; Hao, J.; Hong, H.; Song, Y.; He, R.; Zhi, K.; Liu, Q. A novel and highly efficient Zr-containing catalyst based on humic acids for the conversion of biomass-derived ethyl levulinate into gamma-valerolactone. Fuel 2017, 193, 322–330. [Google Scholar] [CrossRef]
- Zhumanova, M.O.; Usanboev, N.; Namazov, S.S.; Beglov, B.M. Oxidation of brown coal of Angren deposit with a mixture of nitric and sulfuric acids. Russ. J. Appl. Chem. 2010, 82, 2223–2229. [Google Scholar] [CrossRef]
- Fong, S.S.; Seng, L.; Majri, N.B.; Mat, H.B. A Comparative Evaluation on the Oxidative Approaches for Extraction of Humic Acids from Low Rank Coal of Mukah. J. Braz. Chem. Soc. 2007, 18, 34–40. [Google Scholar] [CrossRef]
- Doskočil, L.; Grasset, L.; Válková, D.; Pekař, M. Hydrogen peroxide oxidation of humic acids and lignite. Fuel 2014, 134, 406–413. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, Y.C. Value-Added Humic Acid Derived from Lignite Using Novel Solid-Phase Activation Process with Pd/CeO2 Nanocatalyst: A Physiochemical Study. ACS Sustain. Chem. Eng. 2017, 5, 10099–10110. [Google Scholar] [CrossRef]
- Tang, Y.; Hou, S.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, Y.C.; Yao, Y.; Zhang, S.; Xie, J. Activation of Humic Acid in Lignite Using Molybdate-Phosphorus Hierarchical Hollow Nanosphere Catalyst Oxidation: Molecular Characterization and Rice Seed Germination-Promoting Performances. J. Agric. Food Chem. 2020, 68, 13620–13631. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Hou, S.; Cheng, D.; Yao, Y.; Zhang, S.; Xie, J.; Wang, X.; Ma, X.; Yu, Z.; et al. Multifunctional Iron–Humic Acid Fertilizer from Ball Milling Double-Shelled Fe–N-doped Hollow Mesoporous Carbon Microspheres with Lignite. ACS Sustain. Chem. Eng. 2021, 9, 717–731. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, Y.; Cheng, D.; Gao, B.; Wan, Y.; Li, Y.C.; Yao, Y.; Xie, J.; Liu, L. Multifunctional Slow-Release Fertilizer Prepared from Lignite Activated by a 3D-Molybdate-Sulfur Hierarchical Hollow Nanosphere Catalyst. ACS Sustain. Chem. Eng. 2019, 7, 10533–10543. [Google Scholar]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, W.; Weng, X.; Liu, Y.; Wang, H.; Wu, Z. Active Oxygen Species in Lan+1NinO3n+1 Layered Perovskites for Catalytic Oxidation of Toluene and Methane. J. Phys. Chem. C 2016, 120, 3259–3266. [Google Scholar] [CrossRef]
- Li, W.; Wang, S.; Li, J. Highly Effective Ru/BaCeO3 Catalysts on Supports with Strong Basic Sites for Ammonia Synthesis. Chem. Asian J. 2019, 14, 2815–2821. [Google Scholar] [PubMed]
- Zhu, J.; Li, H.; Zhong, L.; Xiao, P.; Xu, X.; Yang, X.; Zhao, Z.; Li, J. Perovskite Oxides: Preparation, Characterizations, and Applications in Heterogeneous Catalysis. ACS Catal. 2014, 4, 2917–2940. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Xu, C.-M.; Duan, A.-J.; Jiang, G.-Y. The Structures, Adsorption Characteristics of La−Rb−Cu−O Perovskite-like Complex Oxides, and Their Catalytic Performances for the Simultaneous Removal of Nitrogen Oxides and Diesel Soot. J. Phys. Chem. C 2008, 112, 5930–5941. [Google Scholar] [CrossRef]
- Nair, M.M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 Perovskites as Precursors for the Preparation of Coke-Resistant Dry Reforming Catalysts. ACS Catal. 2014, 4, 3837–3846. [Google Scholar] [CrossRef]
- Xiao, G.; Xin, S.; Wang, H.; Zhang, R.; Wei, Q.; Lin, Y. Catalytic Oxidation of Styrene over Ce-Substituted La1–xCexMnO3 Catalysts. Ind. Eng. Chem. Res. 2019, 58, 5388–5396. [Google Scholar] [CrossRef]
- Zhang, C.; Guo, Y.; Guo, Y.; Lu, G.; Boreave, A.; Retailleau, L.; Baylet, A.; Giroir-Fendler, A. LaMnO3 perovskite oxides prepared by different methods for catalytic oxidation of toluene. Appl. Catal. B: Environ. 2014, 148, 490–498. [Google Scholar] [CrossRef]
- Liu, J.; Jia, E.; Wang, L.; Stoerzinger, K.A.; Zhou, H.; Tang, C.S.; Yin, X.; He, X.; Bousquet, E.; Bowden, M.E.; et al. Tuning the Electronic Structure of LaNiO3 through Alloying with Strontium to Enhance Oxygen Evolution Activity. Adv. Sci. 2019, 6, 1901073. [Google Scholar] [CrossRef] [Green Version]
- Kubota, K.; Pang, Y.D.; Miura, A.; Ito, H. Redox reactions of small organic molecules using ball milling and piezoelectric materials. Science 2019, 366, 1500–1504. [Google Scholar] [CrossRef]
- Ohtomo, A.; Hwang, H.Y. A high-mobility electron gas at the LaAlO3/SrTiO3 heterointerface. Nature 2004, 427, 423–4266. [Google Scholar] [CrossRef] [PubMed]
- Arandiyan, H.; Li, J.; Ma, L.; Hashemnejad, S.M.; Mirzaei, M.Z.; Chen, J.; Chang, H.; Liu, C.; Wang, C.; Chen, L. Methane reforming to syngas over LaNixFe1−xO3 (0 ≤ x ≤ 1) mixed-oxide perovskites in the presence of CO2 and O2. J. Ind. Eng. Chem. 2012, 18, 2103–2114. [Google Scholar] [CrossRef]
- Mickevičius, S.; Grebinskij, S.; Bondarenka, V.; Vengalis, B.; Šliužienė, K.; Orlowski, B.A.; Osinniy, V.; Drube, W. Investigation of epitaxial LaNiO3−x thin films by high-energy XPS. J. Alloys Compd. 2006, 423, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Rao, C.; Ji, Y.; Zhang, L.; Liu, W.; Wang, X.; Xu, X.; Wang, Z.; Zhang, N.; Peng, H. Double-shelled hollow LaNiO3 nanocage as nanoreactors with remarkable catalytic performance: Illustrating the special morphology and performance relationship. Mol. Catal. 2018, 455, 57–67. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, K.; Wang, H.; Wang, Y.; Tian, D.; Wei, Y.; Zhu, X.; Zeng, C.; Luo, Y. Structure dependence and reaction mechanism of CO oxidation: A model study on macroporous CeO2 and CeO2-ZrO2 catalysts. J. Catal. 2016, 344, 365–377. [Google Scholar] [CrossRef]
- Fu, C.; He, D.; Wang, Y.; Zhao, X. Facile synthesis and microwave absorption performance of coated carbon nanotubes by porous Fe3O4@ C nanorods. Synthetic Met. 2019, 248, 76–80. [Google Scholar] [CrossRef]
- Zheng, T.; Liang, Y.; Ye, S.; He, Z. Superabsorbent hydrogels as carriers for the controlled-release of urea: Experiments and a mathematical model describing the release rate. Biosyst. Eng. 2009, 102, 44–50. [Google Scholar] [CrossRef]
- Oliveira, L.K.; Molina, E.F.; Moura, A.L.; de Faria, E.H.; Ciuffi, K.J. Synthesis, Characterization, and Environmental Applications of Hybrid Materials Based on Humic Acid Obtained by the Sol-Gel Route. ACS Appl. Mater. Inter. 2016, 8, 1478–1485. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Lövgren, L.; Tysklind, M.; Haglund, P. Multivariate assessment of barriers materials for treatment of complex groundwater rich in dissolved organic matter and organic and inorganic contaminants. J. Environ. Chem. Eng. 2017, 5, 3075–3082. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Skripkina, T.S.; Bychkov, A.L.; Tikhova, V.D.; Lomovsky, O.I. Mechanochemical Solid-Phase Reactions of Humic Acids from Brown Coal with Sodium Percarbonate. Solid Fuel Chem. 2019, 52, 356–360. [Google Scholar] [CrossRef]
- Kozyatnyk, I.; Latham, K.G.; Jansson, S. Valorization of Humic Acids by Hydrothermal Conversion into Carbonaceous Materials: Physical and Functional Properties. ACS Sustain. Chem. Eng. 2018, 7, 2585–2592. [Google Scholar] [CrossRef]
- Baglieri, A.; Vindrola, D.; Gennari, M.; Negre, M. Chemical and spectroscopic characterization of insoluble and soluble humic acid fractions at different pH values. Chem. Biol. Technol. Agric. 2014, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Monteil-Rivera, F.; Brouwer, E.B.; Masset, S.; Deslandes, Y.; Dumonceau, J. Combination of X-ray photoelectron and solid-state 13C nuclear magnetic resonance spectroscopy in the structural characterisation of humic acids. Anal. Chim. Acta 2000, 424, 243–255. [Google Scholar] [CrossRef]
- Doskočil, L.; Burdíková-Szewieczková, J.; Enev, V.; Kalina, L.; Wasserbauer, J. Spectral characterization and comparison of humic acids isolated from some European lignites. Fuel 2018, 213, 123–132. [Google Scholar] [CrossRef]
- Jing, Q.; Li, H. Hierarchical nickel cobalt oxide spinel microspheres catalyze mineralization of humic substances during wet air oxidation at atmospheric pressure. Appl. Catal. B Environ. 2019, 256, 117858. [Google Scholar] [CrossRef]
- Wang, M.; Cui, S.; Yang, X.; Bi, W. Synthesis of g-C3N4/Fe3O4 nanocomposites and application as a new sorbent for solid phase extraction of polycyclic aromatic hydrocarbons in water samples. Talanta 2015, 132, 922–928. [Google Scholar] [CrossRef]
- Campitelli, P.A.; Velasco, M.I.; Ceppi, S.B. Chemical and physicochemical characteristics of humic acids extracted from compost, soil and amended soil. Talanta 2006, 69, 1234–1239. [Google Scholar] [CrossRef]












| Sample | Elemental Composition (wt.%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C ad | H ad | O ad | N ad | H/C | O/C | TOC a | THA b | E4/E6 c | |
| WC | 45.26 | 3.73 | 26.28 | 0.83 | 0.08 | 0.58 | 43.48 | 32.14 | 2.38 |
| AWC | 40.62 | 3.31 | 24.89 | 0.81 | 0.08 | 0.61 | 42.34 | 37.65 | 3.52 |
| ALC | 41.46 | 3.52 | 23.98 | 0.80 | 0.08 | 0.58 | 40.01 | 49.46 | 5.02 |
| AFC | 37.61 | 2.96 | 27.85 | 0.80 | 0.07 | 0.74 | 35.14 | 48.40 | 4.94 |
| Weathered Coal | Aromatic | Ether/Alcohol | Carboxylic | π-π * | ||||
|---|---|---|---|---|---|---|---|---|
| BE a | RP b | BE a | RP b | BE a | RP b | BE a | RP b | |
| WC | 284.6 | 58.98 | 285.4 | 31.31 | 288.8 | 8.15 | 290.5 | 1.56 |
| AWC | 284.6 | 63.18 | 285.5 | 26.54 | 289.0 | 7.63 | 290.8 | 2.65 |
| ALC | 284.7 | 74.48 | 286.3 | 12.90 | 288.8 | 12.51 | 290.4 | 1.89 |
| AFC | 284.6 | 68.32 | 286.0 | 19.72 | 288.9 | 10.58 | 290.6 | 1.38 |
| Weathered Coa | Oxygen | Nitrogen | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C-O- | C=O | H2O | Pyrrole | Ammonium | ||||||
| BE a | RP b | BE a | RP b | BE a | RP b | BE a | RP b | BE a | RP b | |
| WC | 531.8 | 54.78 | 533.2 | 39.53 | 534.9 | 5.69 | 399.8 | 60.37 | 401.4 | 39.63 |
| AWC | 531.8 | 44.47 | 533.3 | 54.76 | 535.5 | 0.77 | 400.0 | 70.12 | 401.1 | 29.88 |
| ALC | 531.6 | 44.14 | 533.2 | 55.29 | 535.8 | 0.58 | 399.9 | 73.82 | 400.9 | 26.18 |
| AFC | 531.7 | 47.73 | 533.3 | 49.58 | 535.0 | 2.68 | 400.0 | 72.36 | 400.8 | 27.64 |
| Functional Groups | Chemical Shift (ppm) | Area (%) | |
|---|---|---|---|
| AFC | WC | ||
| Aliphatic C | 0–50 | 7.45 | 6.86 |
| O-Alkyl | 50–100 | 13.83 | 11.42 |
| Aromatic C | 100–148 | 53.19 | 57.14 |
| Aromatic C-O | 148–170 | 3.72 | 4.57 |
| C=O/N-C=O | 170–220 | 21.81 | 20.0 |
| No. | Molecular Formula | Chemicals (Name) a | WC | AFC |
|---|---|---|---|---|
| 1 | C2H4O2 | Acetic acid | ✓ | ✓ |
| 2 | C2H4O2 | Methyl formate | ✓ | |
| 3 | C10H14N2O4 | 2-(2,5-dimethoxy-4-nitrophenyl)ethan-1-amine | ✓ | |
| 4 | C3H7NO | Acetaldehyde, O-methyloxime | ✓ | |
| 5 | C6H11NO3 | 2-Aminoethanol, N,O-diacetyl- | ✓ | |
| 6 | C7H12N2 | 1-Butylimidazole | ✓ | |
| 7 | C3H5NO | Propanenitrile, 2-hydroxy- | ✓ | |
| 8 | C17H34O2 | Hexadecanoic acid, methyl ester | ✓ | |
| 9 | C16H32O | 2-Hexadecanone | ✓ | |
| 10 | C16H22O4 | Dibutyl phthalate | ✓ | |
| 11 | C19H38O2 | Methyl stearate | ✓ | ✓ |
| 12 | C5H8N2 | 3,5-Dimethylpyrazole | ✓ | |
| 13 | C6H8O | 4-Methyl-2H-pyran | ✓ | |
| 14 | C18H33N | 9-Octadecenenitrile, (Z)- | ✓ | |
| 15 | C6H10N2O | 3,5-Dimethylpyrazole-1-methanol | ✓ | |
| 16 | C16H32O2 | n-Hexadecanoic acid | ✓ | |
| 17 | C23H44O2 | 13-Docosenoic acid, methyl ester, (Z)- | ✓ | |
| 18 | C22H41N | (Z)-Docos-9-enenitrile | ✓ | |
| 19 | C17H32O | 13-Heptadecyn-1-ol | ✓ | |
| 20 | C18H31N | 11-Octadecynenitrile | ✓ | |
| 21 | C20H38O2 | Ethanol, 2-(9,12-octadecadienyloxy)-, (Z,Z)- | ✓ | |
| 22 | C20H39N | Eicosanenitrile | ✓ | |
| 23 | C24H38O4 | Bis(2-ethylhexyl) phthalate | ✓ | |
| 24 | C18H33N | 9-Octadecenenitrile, (Z)- | ✓ | |
| 25 | C24H38O4 | 1,4-Benzenedicarboxylic acid, bis(2-ethylhexyl) ester | ✓ | |
| 26 | C22H43NO | 13-Docosenamide, (Z)- | ✓ | |
| 27 | C35H62O3 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, octadecyl ester | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, M.; Wang, G.; Suo, Y.; Wu, Z.; Zhan, H.; Liu, W. Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions. Catalysts 2022, 12, 392. https://doi.org/10.3390/catal12040392
Song M, Wang G, Suo Y, Wu Z, Zhan H, Liu W. Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions. Catalysts. 2022; 12(4):392. https://doi.org/10.3390/catal12040392
Chicago/Turabian StyleSong, Manrong, Gang Wang, Yanli Suo, Zhiqiang Wu, Haijuan Zhan, and Wanyi Liu. 2022. "Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions" Catalysts 12, no. 4: 392. https://doi.org/10.3390/catal12040392
APA StyleSong, M., Wang, G., Suo, Y., Wu, Z., Zhan, H., & Liu, W. (2022). Conversion of Weathered Coal into High Value-Added Humic Acid by Magnetically Recoverable Fe3O4/LaNiO3 Nanocatalysts under Solid-Phase Grinding Conditions. Catalysts, 12(4), 392. https://doi.org/10.3390/catal12040392