Highly Efficient Asymmetric Morita–Baylis–Hillman Reaction Promoted by Chiral Aziridine-Phosphines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Chiral Catalysts 1–8
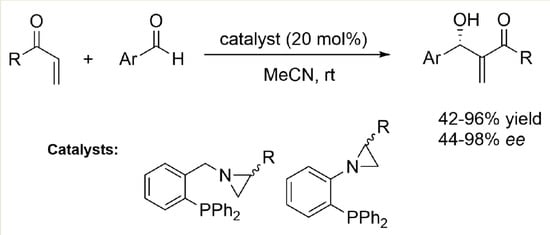
2.2. Asymmetric Morita–Baylis–Hillman Reaction Promoted by Aziridine-Phosphines 1–8
2.3. Organocatalytic Asymmetric Morita–Baylis–Hillman Reaction Catalyzed by Aziridine-Phosphine 6—Scope of the Substrates
3. Materials and Methods
3.1. Materials
3.2. Methods
Asymmetric Morita–Baylis–Hillman Reaction—General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kananovich, D.; Elek, G.Z.; Lopp, M.; Borovkov, V. Aerobic Oxidations in Asymmetric Synthesis: Catalytic Strategies and Recent Developments. Front. Chem. 2021, 9, 614944. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.-H.; Tan, B. Advances in asymmetric organocatalysis over the last 10 years. Nat. Commun. 2020, 11, 3786. [Google Scholar] [CrossRef] [PubMed]
- Krištofíková, D.; Modrocká, V.; Mečiarová Šebesta, R. Green Asymmetric Organocatalysis. ChemSusChem 2020, 13, 2828–2858. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Wei, Y. Recent Advances in Organocatalytic Asymmetric Morita-Baylis-Hillman/aza-Morita-Baylis-Hillman Reactions. Chem. Rev. 2013, 113, 6659–6690. [Google Scholar] [CrossRef]
- Angamuthu, V.; Lee, C.-H.; Tai, D.-F. Brucine Diol-Catalyzed Enantioselective Morita-Baylis-Hillman Reaction in the Presence of Brucine N-Oxide. Catalysts 2021, 11, 237. [Google Scholar] [CrossRef]
- Menkudle, M.S.; Pendalwar, S.S.; Goswami, S.V.; Jadhav, W.N.; Bhusare, S.R. Asymmetric Baylis-Hillman reaction catalyzed by pyrrolidine based organocatalyst. SN Appl. Sci. 2020, 2, 672. [Google Scholar] [CrossRef] [Green Version]
- Aydin, A.E. Chiral thiourea derivatives as organocatalysts in the enantioselective Morita-Baylis-Hillman reactions. Arkivoc 2020, part vi, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Gergelitsová, I.; Tauchman, J.; Císařová, I.; Veselý, J. Bifunctional (Thio)urea-Phosphine Organocatalysts Derived from D-Glucose and α-Amino Acids and Their Application to the Enantioselective Morita-Baylis-Hillman Reaction. Synlett 2015, 26, 2690–2696. [Google Scholar] [CrossRef]
- Yoshimura, H.; Ishihara, J.; Hatakeyama, S. Stereoselective Construction of Entire Diastereomeric Stereotetrads Based on an Asymmetric Morita-Baylis-Hillman Reaction. Eur. J. Org. Chem. 2017, 2017, 2719–2729. [Google Scholar] [CrossRef]
- Ni, H.; Chan, W.-L.; Lu, Y. Phosphine-Catalyzed Asymmetric Organic Reactions. Chem. Rev. 2018, 118, 9344–9411. [Google Scholar] [CrossRef]
- Xue, J.-W.; Song, J.; Manion, I.C.K.; He, Y.-H.; Guan, Z. Asymmetric Morita-Baylis-Hillman reaction catalyzed by pepsin. J. Mol. Catal. B: Enzym. 2016, 124, 62–69. [Google Scholar] [CrossRef]
- Shairgojray, B.A.; Dar, A.A.; Bhat, B.A. Cationic chiral surfactant based micelle-guided asymmetric Morita-Baylis-Hillman reaction. Catal. Commun. 2016, 83, 58–61. [Google Scholar] [CrossRef]
- Hu, Y.-H.; Liu, C.-X.; Wang, J.-C.; Ren, X.-H.; Kan, X.; Dong, Y.-B. TiO2@UiO-68-CIL: A Metal-Organic-Framework-Based Bifunctional Composite Catalyst for a One-Pot Sequential Asymmetric Morita-Baylis-Hillman Reaction. Inorg. Chem. 2019, 58, 4722–4730. [Google Scholar] [CrossRef] [PubMed]
- Tooriyama, S.; Mimori, Y.; Wu, Y.; Kogure, N.; Kitajima, M. Asymmetric Total Synthesis of Pentacyclic Indole Alkaloid Andranginine and Absolute Configuration of Natural Product Isolated from Kopsia arborea. Org. Lett. 2017, 19, 2722–2725. [Google Scholar] [CrossRef] [PubMed]
- Lacharity, J.J.; Mailyan, A.K.; Chen, K.Y.; Zakarian, A. Concise Synthesis of (+)-[13C4]-Anatoxin-a by Dynamic Kinetic Resolution of a Cyclic Iminium Ion. Angew. Chem. Int. Ed. 2020, 59, 11364–11368. [Google Scholar] [CrossRef]
- Pan, L.; Zheng, C.-W.; Fang, G.-S.; Hong, H.-R.; Liu, J.; Yu, L.-H.; Zhao, G. Asymmetric Total Synthesis of Vincadifformine Enabled by a Thiourea-Phosphonium Salt Catalyzed Mannich -Type Reaction. Chem. Eur. J. 2019, 25, 6306–6310. [Google Scholar] [CrossRef]
- Xiao, Y.-C.; Chen, X.-P.; Deng, J.; Yan, Y.-H.; Zhu, K.-R.; Li, G.; Yu, J.-L.; Brem, J.; Chen, F.; Schofield, C.J.; et al. Design and enantioselective synthesis of 3-(α-acrylic acid) benzoxaboroles to combat carbapenemase resistance. Chem. Commun. 2021, 57, 7709–7712. [Google Scholar] [CrossRef]
- Liu, L.; Sun, Y.; Wang, J.; Ou, W.; Wang, X.; Huang, S. A New Formal Synthetic Route to Entecavir. Synlett 2019, 30, 748–752. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Anwar, S.; Chen, K. Morita-Baylis-Hillman (MBH) Reaction Derived Nitroallylic Alcohols, Acetates and Amines as Synthons in Organocatalysis and Heterocycle Synthesis. Chem. Rec. 2017, 17, 363–381. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Pieczonka, A.M. Optically Pure Aziridinyl Ligands as Useful Catalysts in the Stereocontrolled Synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Jarzyński, S.; Obijalska, E. Lactic acid derived aziridinyl alcohols as highly effective catalysts for asymmetric additions of an organozinc species to aldehydes. Tetrahedron Asymmetry 2013, 24, 1336–1340. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich Reaction Promoted by Chiral Phosphinoyl-Aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef] [Green Version]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Buchcic-Szychowska, A.; Adamczyk, J.; Marciniak, L.; Pieczonka, A.M.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts 2021, 11, 968. [Google Scholar] [CrossRef]
- Nakano, A.; Ushiyama, M.; Iwabuchi, Y.; Hatakeyama, S. Synthesis of Enantiocomplementary Catalyst of β-Isocupreidine (β-ICD) from Quinine. Adv. Synth. Catal. 2005, 347, 1790–1796. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, L.; Song, H.-L.; Hu, Y.; Wu, X.-Y. Chiral phosphinothiourea organocatalyst in the enantioselective Morita-Baylis-Hillman reactions of aromatic aldehydes with methyl vinyl ketone. Tetrahedron Lett. 2008, 49, 6262–6264. [Google Scholar] [CrossRef]





| Entry | Catalyst | Yield [%] | ee [%] a | Abs. Conf. b |
|---|---|---|---|---|
| 1 | 1 | 42 | 54 | (R) |
| 2 | 2 | 48 | 56 | (S) |
| 3 | 3 | 46 | 51 | (S) |
| 4 | 4 | 43 | 44 | (S) |
| 5 | 5 | 95 | 96 | (R) |
| 6 | 6 | 96 | 98 | (S) |
| 7 | 7 | 92 | 96 | (S) |
| 8 | 8 | 94 | 90 | (S) |
| Entry | Catalyst 6 Loading [mol%] | Yield [%] | ee [%] a |
|---|---|---|---|
| 1 | 5 | 72 | 90 |
| 2 | 10 | 85 | 93 |
| 3 | 20 | 96 | 98 |
| Entry | Temperature [°C] | Yield [%] | ee [%] a |
|---|---|---|---|
| 1 | 40 | 90 | 52 |
| 2 | 60 | 91 | 37 |
| Entry | R | Ar | Product | Yield [%] | ee [%] a | Abs. conf. b |
|---|---|---|---|---|---|---|
| 1 | Me | Ph | 12 | 90 | 97 | (S) |
| 2 | Me | 2-Naphthyl | 13 | 93 | 96 | (S) |
| 3 | Me | 4-CF3C6H4 | 14 | 89 | 82 | (S) |
| 4 | Me | 4-BrC6H4 | 15 | 91 | 98 | (S) |
| 5 | Me | 4-MeOC6H4 | 16 | traces | nd c | nd c |
| 6 | OMe | 4-NO2C6H4 | 17 | 85 | 74 | (S) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchcic-Szychowska, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Highly Efficient Asymmetric Morita–Baylis–Hillman Reaction Promoted by Chiral Aziridine-Phosphines. Catalysts 2022, 12, 394. https://doi.org/10.3390/catal12040394
Buchcic-Szychowska A, Zawisza A, Leśniak S, Rachwalski M. Highly Efficient Asymmetric Morita–Baylis–Hillman Reaction Promoted by Chiral Aziridine-Phosphines. Catalysts. 2022; 12(4):394. https://doi.org/10.3390/catal12040394
Chicago/Turabian StyleBuchcic-Szychowska, Aleksandra, Anna Zawisza, Stanisław Leśniak, and Michał Rachwalski. 2022. "Highly Efficient Asymmetric Morita–Baylis–Hillman Reaction Promoted by Chiral Aziridine-Phosphines" Catalysts 12, no. 4: 394. https://doi.org/10.3390/catal12040394
APA StyleBuchcic-Szychowska, A., Zawisza, A., Leśniak, S., & Rachwalski, M. (2022). Highly Efficient Asymmetric Morita–Baylis–Hillman Reaction Promoted by Chiral Aziridine-Phosphines. Catalysts, 12(4), 394. https://doi.org/10.3390/catal12040394