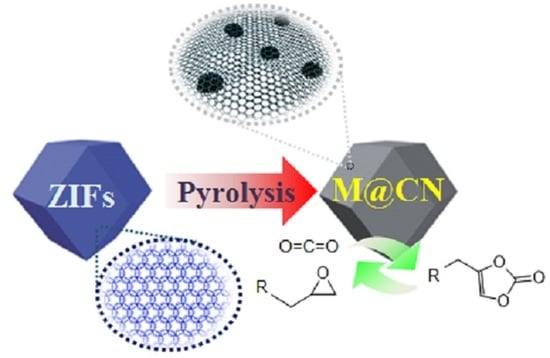
Metal Embedded Porous Carbon for Efficient CO2 Cycloaddition under Mild Conditions
Abstract
:1. Introduction
2. Results
2.1. Material Characterization
2.2. Catalytic Activity
2.2.1. Catalytic Studies
2.2.2. Reaction Mechanism
3. Materials and Methods
3.1. Materials and Synthesis
3.2. Material Characterization
3.3. Cycloaddition Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Georgakilas, V.; Perman, J.A.; Tucek, J.; Zboril, R. Broad family of carbon nanoallotropes: Classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef] [PubMed]
- Su, D.S.; Perathoner, S.; Centi, G. Nanocarbons for the development of advanced catalysts. Chem. Rev. 2013, 113, 5782–5816. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, P.; Deb, A.; Maiti, D.; Jain, S.L. Graphene oxide grafted with iridium complex as a superior heterogeneous catalyst for chemical fixation of carbon dioxide to dimethylformamide. Carbon 2016, 100, 632–640. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, J.; Hyeon, T. Recent progress in the synthesis of porous carbon materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Yang, R.T.; Wang, Y. Catalyzed hydrogen spillover for hydrogen storage. J. Am. Chem. Soc. 2009, 131, 4224–4226. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, K.; Wu, L.; Yu, S.; Antonietti, M.; Titirici, M. Engineering carbon materials from the hydrothermal carbonization process of biomass. Adv. Mater. 2010, 22, 813–828. [Google Scholar] [CrossRef]
- Kyotani, T. Control of pore structure in carbon. Carbon 2000, 38, 269–286. [Google Scholar] [CrossRef]
- Flandrois, S.; Simon, B. Carbon materials for lithium-ion rechargeable batteries. Carbon 1999, 37, 165–180. [Google Scholar] [CrossRef]
- Joo, S.H.; Choi, S.J.; Oh, I.; Kwak, J.; Liu, Z.; Terasaki, O.; Ryoo, R. Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 2001, 412, 169–172. [Google Scholar] [CrossRef]
- Jordá-Beneyto, M.; Suárez-García, F.; Lozano-Castello, D.; Cazorla-Amorós, D.; Linares-Solano, A. Hydrogen storage on chemically activated carbons and carbon nanomaterials at high pressures. Carbon 2007, 45, 293–303. [Google Scholar] [CrossRef]
- Juárez-Pérez, E.J.; Calvo, E.G.; Arenillas, A.; Menéndez, J.A. Precise determination of the point of sol–gel transition in carbon gel synthesis using a microwave heating method. Carbon 2010, 48, 3305–3308. [Google Scholar] [CrossRef]
- Vitillo, J.G.; Crocellà, V.; Bonino, F. ZIF-8 as a Catalyst in ethylene oxide and propylene oxide reaction with CO2 to cyclic organic carbonates. ChemEngineering 2019, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- Kuruppathparambil, R.R.; Babu, R.; Jeong, H.M.; Hwang, G.Y.; Jeong, G.S.; Kim, M.; Kim, D.W.; Park, D.W. A solid solution zeolitic imidazolate framework as a room temperature efficient catalyst for the chemical fixation of CO2. Green Chem. 2016, 18, 6349–6356. [Google Scholar] [CrossRef]
- Xiang, W.L.; Shen, C.Y.; Lu, Z.; Chen, S.; Li, X.; Zou, R.; Zhang, Y.P.; Liu, C.J. CO2 cycloaddition over ionic liquid immobilized hybrid zeolitic imidazolate frameworks: Effect of Lewis acid/base sites. Chem. Eng. Sci. 2021, 233, 116429. [Google Scholar] [CrossRef]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal–organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Zhao, Y.; Song, Z.X.; Li, X.; Sun, Q.; Cheng, N.; Lawes, S.; Sun, X.L. Metal organic frameworks for energy storage and conversion. Energy Storage Mater. 2016, 2, 35–62. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, H.; Bai, C.; Liao, S.; Li, Y. Base-free oxidation of alcohols to esters at room temperature and atmospheric conditions using nanoscale co-based catalysts. ACS Catal. 2015, 5, 1850–1856. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.Q.; Gong, Y.Y.; Wang, J.C.; Yuan, Y.; Cheng, H.; Sang, W.; Chaemchuen, S.; Verpoort, F. Cobalt embedded in nitrogen-doped porous carbon as a robust heterogeneous catalyst for the atom-economic alcohol dehydrogenation to carboxylic acids. Carbon 2021, 174, 284–294. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaén, J.J.D.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.-U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Olivos-Suarez, A.I.; Oar-Arteta, L.; Rozhko, E.; Osadchii, D.; Bavykina, A.; Kapteijn, F.; Gascon, J. Metal-organic-framework mediated cobalt/N-doped carbon hybrids as efficient and chemoselective catalysts for the hydrogenation of nitroarenes. ChemCatChem 2017, 9, 1854. [Google Scholar] [CrossRef]
- Chaemchuen, S.; Xiao, X.; Ghadamyari, M.; Mousavi, B.; Klomkliang, N.; Yuan, Y.; Verpoort, F. Robust and efficient catalyst derived from bimetallic Zn/Co zeolitic imidazolate frameworks for CO2 conversion. J. Catal. 2019, 370, 38–45. [Google Scholar] [CrossRef]
- Khan, I.U.; Othman, M.H.D.; Ismail, A.F.; Ismail, N.; Jaafar, J.; Hashim, H.; Rahman, M.A.; Jilani, A. Structural transition from two-dimensional ZIF-L to three-dimensional ZIF-8 nanoparticles in aqueous room temperature synthesis with improved CO2 adsorption. Mater. Charact. 2018, 136, 407–416. [Google Scholar] [CrossRef]
- Zhou, K.; Mousavi, B.; Luo, Z.; Phatanasri, S.; Chaemchuen, S.; Verpoort, F. Characterization and properties of Zn/Co zeolitic imidazolate frameworks vs. ZIF-8 and ZIF-67. J. Mater. Chem. 2017, 5, 952–957. [Google Scholar] [CrossRef]
- Kaneti, Y.V.; Tang, J.; Salunkhe, R.R.; Jiang, X.; Yu, A.; Wu, K.C.W.; Yamauchi, Y. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks. Adv. Mater. 2017, 29, 1604898. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef]
- Meng, J.S.; Niu, C.J.; Xu, L.H.; Li, J.T.; Liu, X.; Wang, X.P.; Wu, Y.Z.; Xu, X.M.; Chen, W.Y.; Li, Q.; et al. General oriented formation of carbon nanotubes from metal-organic frameworks. J. Am. Chem. Soc. 2017, 139, 8212–8221. [Google Scholar] [CrossRef]
- Xia, B.Y.; Yan, Y.; Li, N.; Wu, H.B.; Lou, X.W.; Wang, X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat. Energy 2016, 1, 15006. [Google Scholar] [CrossRef]
- Hayashi, M. Oxidation using activated carbon and molecular oxygen system. Chem. Rec. 2008, 8, 252–267. [Google Scholar] [CrossRef]
- Konwar, L.J.; Sugano, Y.; Chutia, R.S.; Shchukarev, A.; Mäki-Arvela, P.; Kataki, R.; Mikkola, J.-P. Sustainable synthesis of N and P co-doped porous amorphous carbon using oil seed processing wastes. Mater. Lett. 2016, 173, 145–148. [Google Scholar] [CrossRef]
- Fan, X.Q.; Zhang, L.X.; Zhang, G.B.; Shu, Z.; Shi, J.L. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Sevilla, M.; Valle-Vigón, P.; Fuertes, A.B. N-doped polypyrrole-based porous carbons for CO2 capture. Adv. Funct. Mater. 2011, 21, 2781–2787. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yang, C.-C.; Lin, C.-H.; Jiang, H.-L. Metal–organic-framework-derived hollow n-doped porous carbon with ultrahigh concentrations of single zn atoms for efficient carbon dioxide conversion. Angew. Chem. Int. Ed. 2019, 58, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Kou, Z.K.; Mu, S.C.; Liu, J.P.; He, D.P.; Amiinu, I.S.; Meng, W.; Zhou, K.; Luo, Z.X.; Chaemchuen, S.; et al. 2D Dual-metal zeolitic-imidazolate-framework-(ZIF)-derived bifunctional air electrodes with ultrahigh electrochemical properties for rechargeable zinc–air batteries. Adv. Funct. Mater. 2018, 28, 1705048. [Google Scholar] [CrossRef]
- Wang, N.Y.; Huang, X.H.; Zhang, L.; Hu, J.S.; Chao, Y.M.; Zhao, R.K. Pyrolysis transformation of ZIF-8 wrapped with polytriazine to nitrogen enriched core-shell polyhedrons carbon for supercapacitor. Front. Chem. Sci. Eng. 2021, 15, 944–953. [Google Scholar] [CrossRef]
- Kim, M.; Park, T.; Wang, C.; Tang, J.; Lim, H.; Hossain, M.S.A.; Konarova, M.; Yi, J.W.; Na, J.; Kim, J.; et al. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS Appl. Mater. Interfaces 2020, 12, 34065–34073. [Google Scholar] [CrossRef]
- Banks, C.E.; Crossley, A.; Salter, C.; Wilkins, S.J.; Compton, R.G. Carbon nanotubes contain metal impurities which are responsible for the “electrocatalysis” seen at some nanotube-modified electrodes. Angew. Chem. Int. Ed. 2006, 45, 2533–2537. [Google Scholar] [CrossRef]
- Mousavi, B.; Chaemchuen, S.; Ezugwu, C.I.; Yuan, Y.; Verpoort, F. The effect of synthesis procedure on the catalytic performance of isostructural ZIF-8. Appl. Organomet. Chem. 2017, 32, e4062. [Google Scholar] [CrossRef]
- Ciprian, M.; Ruiz, K.H.; Kassymova, M.; Wang, T.; Zhuiykov, S.; Chaemchuen, S.; Tu, R.; Verpoort, F. 3D derived N-doped carbon matrix from 2D ZIF-L as an enhanced stable catalyst for chemical fixation. Microporous Mesoporous Mater. 2019, 285, 80–88. [Google Scholar] [CrossRef]
- Herskovitz, T. Organic and Bio-organic chemistry of carbon dioxide. Organometallics 1983, 2, 201–202. [Google Scholar] [CrossRef]
- Abla, M.; Choi, J.C.; Sakakura, T. Halogen-free process for the conversion of carbon dioxide to urethanes by homogeneous catalysis. Chem. Commun. 2001, 2238–2239. [Google Scholar] [CrossRef] [PubMed]
- Samikannu, A.; Konwar, L.J.; Mäki-Arvela, P.; Mikkola, J.P. Renewable N-doped active carbons as efficient catalysts for direct synthesis of cyclic carbonates from epoxides and CO2. Appl. Catal. B Environ. 2019, 241, 41–51. [Google Scholar] [CrossRef]
- Guo, Y.C.; Feng, L.; Wu, C.C.; Wang, X.M.; Zhang, X. Confined pyrolysis transformation of ZIF-8 to hierarchically ordered porous Zn-N-C nanoreactor for efficient CO2 photoconversion under mild conditions. J. Catal. 2020, 390, 213–223. [Google Scholar] [CrossRef]
- Chizallet, C.; Lazare, S.; Bazer-Bachi, D.; Bonnier, F.; Lecocq, V.; Soyer, E.; Quoineaud, A.-A.; Bats, N. Catalysis of transesterification by a nonfunctionalized metal–organic framework: Acido-basicity at the external surface of ZIF-8 probed by FTIR and ab initio calculations. J. Am. Chem. Soc. 2010, 132, 12365–12377. [Google Scholar] [CrossRef] [PubMed]
- Leal, O.; Bolívar, C.; Ovalles, C.; García, J.J.; Espidel, Y. Reversible adsorption of carbon dioxide on amine surface-bonded silica gel. Inorg. Chim. Acta 1995, 240, 183–189. [Google Scholar] [CrossRef]
- Cruz-Silva, E.; Cullen, D.A.; Gu, L.; Romo-Herrera, J.M.; Muñoz-Sandoval, E.; López-Urías, F.; Sumpter, B.G.; Meunier, V.; Charlier, J.C.; Smith, D.J.; et al. Heterodoped nanotubes: Theory, synthesis, and characterization of phosphorus−nitrogen doped multiwalled carbon nanotubes. ACS Nano 2008, 2, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zou, B.; Hu, C.; Cao, M. Nitrogen-doped porous carbon nanofiber webs for efficient CO2 capture and conversion. Carbon 2016, 99, 79–89. [Google Scholar] [CrossRef]
- Ma, X.Y.; Zou, B.; Cao, M.H.; Chen, S.L.; Hu, C.W. Nitrogen-doped porous carbon monolith as a highly efficient catalyst for CO2 conversion. J. Mater. Chem. A 2014, 2, 18360–18366. [Google Scholar] [CrossRef]









| Porous Carbon/ZIF Template 1 | Surface Area (m2·g−1) | CO2 Adsorption (cm3·g−1) 3 | Elemental Analysis (% wt.) 4 | |||||
|---|---|---|---|---|---|---|---|---|
| BET | Langmuir | External 2 | Zn | Co | N | C | ||
| ZIF-67 | 664 | 714 | 196 | 21 | 0.00 | 27.84 | 25.15 | 43.08 |
| ZnCo-ZIF | 694 | 747 | 227 | 21 | 16.02 | 16.43 | 24.48 | 41.85 |
| ZIF-8 | 646 | 696 | 238 | 20 | 27.74 | 0.00 | 24.24 | 43.08 |
| Co/CN | 151 | 164 | 158 | 8 | 0.00 | 28 | 6.89 | 27.63 |
| ZnCo/CN | 205 | 221 | 185 | 12 | 0.05 | 5.85 | 1.90 | 53.73 |
| Zn/CN | 1525 | 1650 | 707 | 104 | 1.80 | 0.00 | 3.45 | 61.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, C.; Chaemchuen, S.; Liu, M.; Wang, J.; Zhuiykov, S.; Verpoort, F. Metal Embedded Porous Carbon for Efficient CO2 Cycloaddition under Mild Conditions. Catalysts 2022, 12, 427. https://doi.org/10.3390/catal12040427
Qi C, Chaemchuen S, Liu M, Wang J, Zhuiykov S, Verpoort F. Metal Embedded Porous Carbon for Efficient CO2 Cycloaddition under Mild Conditions. Catalysts. 2022; 12(4):427. https://doi.org/10.3390/catal12040427
Chicago/Turabian StyleQi, Chen, Somboon Chaemchuen, Meng Liu, Jichao Wang, Serge Zhuiykov, and Francis Verpoort. 2022. "Metal Embedded Porous Carbon for Efficient CO2 Cycloaddition under Mild Conditions" Catalysts 12, no. 4: 427. https://doi.org/10.3390/catal12040427
APA StyleQi, C., Chaemchuen, S., Liu, M., Wang, J., Zhuiykov, S., & Verpoort, F. (2022). Metal Embedded Porous Carbon for Efficient CO2 Cycloaddition under Mild Conditions. Catalysts, 12(4), 427. https://doi.org/10.3390/catal12040427