Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure and Chemical Compatibility
2.2. Electrical Conductivity
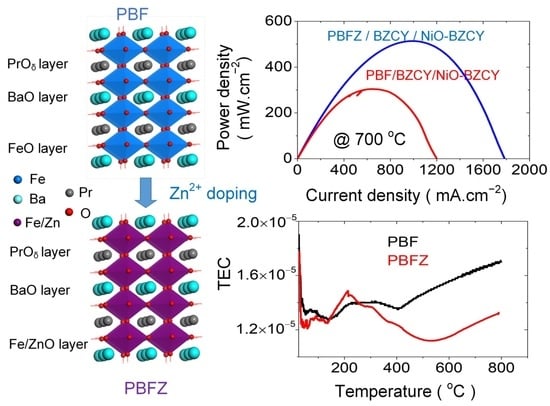
2.3. Thermal Expansion
2.4. ORR Activity
2.5. Single-Cell Performance
2.6. Stability and Microstructure
3. Materials and Methods
3.1. Material Synthesis and Characterization
3.2. Fabrication of Symmetrical and Single Cell
3.3. Electrochemical Performance and Impedance Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Zhong, Y.; Shao, Z. Double Perovskites in Catalysis, Electrocatalysis, and Photo(electro)catalysis. Trends Chem. 2019, 1, 410–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent Progress on Advanced Materials for Solid-Oxide Fuel Cells Operating Below 500 °C. Adv. Mater. 2017, 29, 1700132. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Boldrin, P.; Ruiz-Trejo, E.; Mermelstein, J.; Menéndez, J.M.B.; Reina, T.R.; Brandon, N.P. Strategies for Carbon and Sulfur Tolerant Solid Oxide Fuel Cell Materials, Incorporating Lessons from Heterogeneous Catalysis. Chem. Rev. 2016, 116, 13633–13684. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Manthiram, A. Layered LnBaCo2O5+δ Perovskite Cathodes for Solid Oxide Fuel Cells: An Overview and Perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Wang, H.; Ma, J.; Liu, W.; Wang, X.; Fronzi, M.; Bi, L. Impressive performance of proton-conducting solid oxide fuel cells using a first-generation cathode with tailored cations. J. Mater. Chem. A 2019, 7, 18792–18798. [Google Scholar] [CrossRef]
- Xia, Y.; Jin, Z.; Wang, H.; Gong, Z.; Lv, H.; Peng, R.; Liu, W.; Bi, L. A novel cobalt-free cathode with triple-conduction for proton-conducting solid oxide fuel cells with unprecedented performance. J. Mater. Chem. A 2019, 7, 16136–16148. [Google Scholar] [CrossRef]
- Fabbri, E.; Pergolesi, D.; Traversa, E. Materials challenges toward proton-conducting oxide fuel cells: A critical review. Chem. Soc. Rev. 2010, 39, 4355–4369. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low-temperature proton-conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Sunarso, J.; Song, Y.; Dai, J.; Zhang, J.; Gu, B.; Zhou, W.; Shao, Z. New reduced-temperature ceramic fuel cells with dual-ion conducting electrolyte and triple-conducting double perovskite cathode. J. Mater. Chem. A 2019, 7, 13265–13274. [Google Scholar] [CrossRef]
- Kim, J.; Sengodan, S.; Kwon, G.; Ding, D.; Shin, J.; Liu, M.; Kim, G. Triple-Conducting Layered Perovskites as Cathode Materials for Proton-Conducting Solid Oxide Fuel Cells. ChemSusChem 2014, 7, 2811–2815. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the Electrochemical Performance of Fe-Based Layered Double Perovskite Cathodes by Zn2+ Doping for Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Shin, J.; Kim, G. The electrochemical and thermodynamic characterization of PrBaCo2−xFexO5+δ (x=0, 0.5, 1) infiltrated into yttria-stabilized zirconia scaffold as cathodes for solid oxide fuel cells. J. Power Sources 2012, 201, 10–17. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+δ with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X.; Liu, X.; Meng, G. A novel layered perovskite cathode for proton conducting solid oxide fuel cells. J. Power Sources 2010, 195, 775–778. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Lin, B.; Ding, H.; Wang, S.; Ling, Y.; Peng, R.; Meng, G.; Liu, X. High performance of proton-conducting solid oxide fuel cell with a layered PrBaCo2O5+δ cathode. J. Power Sources 2009, 194, 835–837. [Google Scholar] [CrossRef]
- Grimaud, A.; Bassat, J.-M.; Mauvy, F.; Pollet, M.; Wattiaux, A.; Marrony, M.; Grenier, J.-C. Oxygen reduction reaction of PrBaCo2−xFexO5+δ compounds as H+-SOFC cathodes: Correlation with physical properties. J. Mater. Chem. A 2014, 2, 3594–3604. [Google Scholar] [CrossRef]
- Chen, D.; Wang, F.; Shi, H.; Ran, R.; Shao, Z. Systematic evaluation of Co-free LnBaFe2O5+δ (Ln=Lanthanides or Y) oxides towards the application as cathodes for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2012, 78, 466–474. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, Z.; Xiao, J.; Zhang, H.; Ma, G.; Zhou, Z. A novel cobalt-free layered perovskite-type GdBaFeNiO5+δ cathode material for proton-conducting intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 220, 15–19. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, S.; Brinkman, K.; Chen, F. Layered perovskite PrBa0.5Sr0.5Co2O5+δ as high-performance cathode for solid oxide fuel cells using oxide proton-conducting electrolyte. J. Power Sources 2010, 195, 5468–5473. [Google Scholar] [CrossRef]
- Mao, X.; Wang, W.; Ma, G. A novel cobalt-free double-perovskite NdBaFe1.9Nb0.1O5+δ cathode material for proton-conducting IT-SOFC. Ceram. Int. 2015, 41, 10276–10280. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte-based solid oxide fuel cells with cobalt-free PrBaFe2O5+δ layered perovskite cathode. J. Power Sources 2010, 195, 7038–7041. [Google Scholar] [CrossRef]
- Kim, D.; Son, S.J.; Kim, M.; Park, H.J.; Joo, J.H. PrBaFe2O5+δ Promising Electrode for Redox-Stable Symmetrical Proton-Conducting Solid Oxide Fuel Cells. J. Eur. Ceram. Soc. 2021, 41, 5939–5946. [Google Scholar] [CrossRef]
- Li, F.; Tao, Z.; Dai, H.; Xi, X.; Ding, H. A high-performing proton-conducting solid oxide fuel cell with layered perovskite cathode in intermediate temperatures. Int. J. Hydrogen Energy 2018, 43, 19757–19762. [Google Scholar] [CrossRef]
- Ding, H.; Xue, X. Novel Layered Perovskite GdBaCoFeO5+δ as a Potential Cathode for Proton-Conducting Solid Oxide Fuel Cells. Int. J. Hydrogen Energy 2010, 35, 4311–4315. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Wang, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Tailoring the Oxygen Vacancy to Achieve Fast Intrinsic Proton Transport in a Perovskite Cathode for Protonic Ceramic Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 4914–4922. [Google Scholar] [CrossRef]
- Hashim, S.S.; Liang, F.; Zhou, W.; Sunarso, J. Cobalt-Free Perovskite Cathodes for Solid Oxide Fuel Cells. ChemElectroChem 2019, 6, 3549–3569. [Google Scholar] [CrossRef]
- Ding, H.; Xie, Y.; Xue, X. Electrochemical performance of BaZr0.1Ce0.7Y0.1Yb0.1O3−δ electrolyte-based proton-conducting SOFC solid oxide fuel cell with layered perovskite PrBaCo2O5+δ cathode. J. Power Sources 2011, 196, 2602–2607. [Google Scholar] [CrossRef]
- Xia, Y.; Xu, X.; Teng, Y.; Lv, H.; Jin, Z.; Wang, D.; Peng, R.; Liu, W. A novel BaFe0.8Zn0.1Bi0.1O3−δ cathode for proton conducting solid oxide fuel cells. Ceram. Int. 2020, 46, 25453–25459. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Raimondi, G.; Maier, J. Mixed-Conducting Perovskites as Cathode Materials for Protonic Ceramic Fuel Cells: Understanding the Trends in Proton Uptake. Adv. Funct. Mater. 2018, 28, 1801241. [Google Scholar] [CrossRef]
- Chen, G.; Sunarso, J.; Wang, Y.; Ge, C.; Yang, J.; Liang, F. Evaluation of A-site deficient Sr 1−x Sc 0.175 Nb 0.025 Co 0.8 O 3−δ (x=0, 0.02, 0.05 and 0.1) perovskite cathodes for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2016, 42, 12894–12900. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Huang, X.; Liu, M.; Li, N.; Su, W. Synthesis, electrical and electrochemical properties of Ba0.5Sr0.5Zn0.2Fe0.8O3−δ perovskite oxide for IT-SOFC cathode. J. Power Sources 2008, 176, 1–8. [Google Scholar] [CrossRef]
- Jun, A.; Yoo, S.; Ju, Y.-W.; Hyodo, J.; Choi, S.; Jeong, H.Y.; Shin, J.; Ishihara, T.; Lim, T.-H.; Kim, G. Correlation between fast oxygen kinetics and enhanced performance in Fe doped layered perovskite cathodes for solid oxide fuel cells. J. Mater. Chem. A 2015, 3, 15082–15090. [Google Scholar] [CrossRef]
- Li, P.; Yang, Q.; Zhang, H.; Yao, M.; Yan, F.; Fu, D. Effect of Fe, Ni and Zn dopants in La0·9Sr0·1CoO3−δ on the electrochemical performance of single-component solid oxide fuel cell. Int. J. Hydrogen Energy 2020, 45, 11802–11813. [Google Scholar] [CrossRef]
- Wang, Z.; Lv, P.; Yang, L.; Guan, R.; Jiang, J.; Jin, F.; He, T. Ba0.95La0.05Fe0.8Zn0.2O3-δ cobalt-free perovskite as a triple-conducting cathode for proton-conducting solid oxide fuel cells. Ceram. Int. 2020, 46, 18216–18223. [Google Scholar] [CrossRef]
- Priya, N.S.C.; Sandhya, K.; Rajendran, D.N. Study on Electrical conductivity and Activation Energy of doped Ceria nanostructures. Electrochem. Energy Technol. 2018, 3, 49–53. [Google Scholar] [CrossRef]
- M’Peko, J.-C.; Ruiz-Salvador, A.R.; Rodríguez-Fuentes, G. Conductivity activation energy and analysis of the sintering process of dielectric ceramics. Mater. Lett. 1998, 36, 290–293. [Google Scholar] [CrossRef]
- Chen, T.; Pang, S.; Shen, X.; Jiang, X.; Wang, W. Evaluation of Ba-deficient PrBa1−xFe2O5+δ oxides as cathode materials for intermediate-temperature solid oxide fuel cells. RSC Adv. 2016, 6, 13829–13836. [Google Scholar] [CrossRef]
- Cai, B.; Song, T.-F.; Su, J.-R.; He, H.; Liu, Y. Comparison of (Pr, Ba, Sr) FeO3-δ-SDC composite cathodes in proton-conducting solid oxide fuel cells. Solid State Ionics 2020, 353, 115379. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Novel PrBa0.9Ca0.1Co2-xZnxO5+δ double-perovskite as an active cathode material for high-performance proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 31009–31016. [Google Scholar] [CrossRef]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and Evaluation of Double Perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as Intermediate-Temperature Solid Oxide Fuel Cell Cathodes. J. Power Sources 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y.; Li, J. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode material for IT-SOFCs. Electrochim. Acta 2020, 364, 137274. [Google Scholar] [CrossRef]
- Ling, Y.; Lin, B.; Zhao, L.; Zhang, X.; Yu, J.; Peng, R.; Meng, G.; Liu, X. Layered perovskite LaBaCuMO5+δ (M=Fe, Co) cathodes for intermediate-temperature protonic ceramic membrane fuel cells. J. Alloy. Compd. 2010, 493, 252–255. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Xun, Z.; Wang, H.; Peng, R.; Meng, G.; Liu, X. Characterization and evaluation of NdBaCo2O5+δ cathode for proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2010, 35, 753–756. [Google Scholar] [CrossRef]







| Cathode | Electrolyte | Thickness (μm) | MPD (mW·cm−2) | Reference |
|---|---|---|---|---|
| SmBa0.5Sr0.5Co2O5+δ | BZCY | 15 | 533 | [16] |
| PrBaCo2O5+δ | BZCY | 25 | 545 | [17] |
| NdBaFe1.9Nb0.1O5+δ | BZCY | 30 | 392 | [22] |
| (PrBa)0.95(Fe0.9Mo0.1)2O5+δ | BZCY | 25 | 359 | [25] |
| PrBaCo2O5+δ | BZCYYb | 15 | 490 | [29] |
| LaBaCuFeO5+δ | BZCY | 20 | 327 | [44] |
| NdBaCo2O5+δ | BZCY | 10 | 438 | [45] |
| PrBaF1.9Zn0.1O5+δ | BZCY | 30 | 513 | This work |
| PrBaFe2O5+δ | BZCY | 33 | 304 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teketel, B.S.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Desta, H.G.; Kashif, K.; Chen, Y.; Lin, B. Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping. Catalysts 2022, 12, 488. https://doi.org/10.3390/catal12050488
Teketel BS, Beshiwork BA, Tian D, Zhu S, Desta HG, Kashif K, Chen Y, Lin B. Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping. Catalysts. 2022; 12(5):488. https://doi.org/10.3390/catal12050488
Chicago/Turabian StyleTeketel, Birkneh Sirak, Bayu Admasu Beshiwork, Dong Tian, Shiyue Zhu, Halefom G. Desta, Khan Kashif, Yonghong Chen, and Bin Lin. 2022. "Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping" Catalysts 12, no. 5: 488. https://doi.org/10.3390/catal12050488
APA StyleTeketel, B. S., Beshiwork, B. A., Tian, D., Zhu, S., Desta, H. G., Kashif, K., Chen, Y., & Lin, B. (2022). Promoted Performance of Layered Perovskite PrBaFe2O5+δ Cathode for Protonic Ceramic Fuel Cells by Zn Doping. Catalysts, 12(5), 488. https://doi.org/10.3390/catal12050488