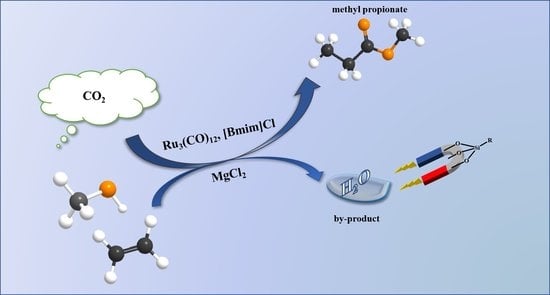
Inorganic Salts and Dehydrating Agents Cooperatively Promoted Ru-Catalyzed Ethylene Methoxycarbonylation Using CO2 as a CO Surrogate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Promoting Effect of Dehydrating Agent and Inorganic Salts
2.2. Exploration of Reaction Mechanism
2.3. Optimization of Reaction Conditions
2.4. Applications of Siloxyl-Functionalized Ionic Liquid
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Synthesis of 1-(3-Trimethoxysilylpropyl)-3-Methylimidazolium Chloride ([TmsPmim]Cl)
3.4. Synthesis of Metal-Based Ionic Liquids: BmimMgCl3
3.5. Synthesis of N-Heterocyclic Carbene Complexes of Ruthenium
3.6. General Procedure for the Hydroesterification Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sang, R.; Hu, Y.; Razzaq, R.; Jackstell, R.; Franke, R.; Beller, M. State-of-the-Art Palladium-Catalyzed Alkoxycarbonylations. Org. Chem. Front. 2021, 8, 799–811. [Google Scholar] [CrossRef]
- Brennführe, A.; Neumann, H.; Beller, M. Palladium-Catalyzed Carbonylation Reactions of Alkenes and Alkynes. ChemCatChem 2009, 1, 28–41. [Google Scholar] [CrossRef]
- Khokarale, S.G.; Mikkola, J.P. Efficient and Catalyst Free Synthesis of Acrylic Plastic Precursors: Methyl Propionate and Methyl Methacrylate Synthesis through Reversible CO2 Capture. Green Chem. 2019, 21, 2138–2147. [Google Scholar] [CrossRef]
- Morimoto, T.; Kakiuchi, K. Evolution of Carbonylation Catalysis: No Need for Carbon Monoxide. Angew. Chem. Int. Ed. 2004, 43, 5580–5588. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, Q.; Jackstell, R.; Beller, M. Carbonylations of Alkenes with CO Surrogates. Angew. Chem. Int. Ed. 2014, 53, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Chhalodia, A.K.; Ahmed, A.; Mukherjee, D. Recent Advances in Metal-Catalyzed Carbonylation Reactions by Using Formic Acid as CO Surrogate. ChemistrySelect 2020, 5, 11272–11290. [Google Scholar] [CrossRef]
- Konishi, H.; Manabe, K. Formic Acid Derivatives as Practical Carbon Monoxide Surrogates for Metal-Catalyzed Carbonylation Reactions. Synlett 2014, 25, 1971–1986. [Google Scholar] [CrossRef]
- Tominaga, K.; Sasaki, Y. Ruthenium Complex-Catalyzed Hydroformylation of Alkenes with Carbon Dioxide. Catal. Commun. 2000, 1, 1–3. [Google Scholar] [CrossRef]
- Yu, D.; Teong, S.P.; Zhang, Y. Transition Metal Complex Catalyzed Carboxylation Reactions with CO2. Coord. Chem. Rev. 2015, 293, 279–291. [Google Scholar] [CrossRef]
- Wu, L.; Liu, Q.; Fleischer, I.; Jackstell, R.; Beller, M. Ruthenium-catalysed alkoxycarbonylation of alkenes with carbon dioxide. Nat. Commun. 2014, 5, 3091. [Google Scholar] [CrossRef] [PubMed]
- Stouten, S.C.; Anastasopoulou, A.; Hessel, V.; Wang, Q. Life Cycle Assessment of Novel Supercritical Methyl Propionate Process with Carbon Dioxide Feedstock. React. Chem. Eng. 2017, 2, 688–695. [Google Scholar] [CrossRef]
- Bi, K.L.; Xu, B.H.; Ding, W.L.; Han, L.J.; Ji, L. Mechanism of CO2 Reduction in Carbonylation Reaction Promoted by Ionic Liquid Additives: A Computational and Experimental Study. Green Energy Environ. 2021. [Google Scholar] [CrossRef]
- Zhang, X.; Shen, C.; Xia, C.; Tian, X.; He, L. Alkoxycarbonylation of Olefins with Carbon Dioxide by Reusable. Green Chem. 2018, 20, 5533–5539. [Google Scholar] [CrossRef]
- Liu, Q.; Wu, L.; Fleischer, I.; Selent, D.; Franke, R.; Jackstell, R.; Beller, M. Development of a Ruthenium/Phosphite Catalyst System for Domino Hydroformylation–Reduction of Olefins with Carbon Dioxide. Chem. Eur. J. 2014, 20, 6888–6894. [Google Scholar] [CrossRef]
- Ali, M.; Gual, A.; Ebeling, G.; Dupont, J. Ruthenium-Catalyzed Hydroformylation of Alkenes by using Carbon Dioxide as the Carbon Monoxide Source in the Presence of Ionic Liquids. ChemCatChem 2014, 6, 2224–2228. [Google Scholar] [CrossRef]
- Jääskeläinen, S.; Haukka, M. The Use of Carbon Dioxide in Ruthenium Carbonyl Catalyzed 1-Hexene Hydroformylation Promoted by Alkali Metal and Alkaline Earth Salts. Appl. Catal. A-Gen. 2003, 247, 95–100. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.M.; Han, H.; Chang, S. Halide Ions as a Highly Efficient Promoter in the Ru-Catalyzed Hydroesterification of Alkenes and Alkynes. Org. Lett. 2006, 8, 4355–4358. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, K.; Sasaki, Y. Ruthenium-Catalyzed One-Pot Hydroformylation of Alkenes Using Carbon Dioxide as a Reactant. J. Mol. Catal. A-Chem. 2004, 220, 159–165. [Google Scholar] [CrossRef]
- Tominaga, K.; Sasaki, Y.; Hagihara, K.; Watanabe, T.; Saito, M. Reverse Water-Gas Shift Reaction Catalyzed by Ruthenium Cluster Anions. Chem. Lett. 1994, 23, 1391–1394. [Google Scholar] [CrossRef]
- Ostapowicz, T.G.; Schmitz, M.; Krystof, M.; Klankermayer, J.; Leitner, W. Carbon Dioxide as a C1 Building Block for the Formation of Carboxylic Acids by Formal Catalytic Hydrocarboxylation. Angew. Chem. 2013, 125, 12341–12345. [Google Scholar] [CrossRef]
- Evans, J.; Gao, J.X.; Leach, H.; Street, A.C. EXAFS Infrared and Kinetic Studies on a Ruthenium Carbonyl Hydrof Ormylation System. J. Organomet. Chem. 1989, 372, 61–66. [Google Scholar] [CrossRef]
- Lavigne, G.; Lugan, N.; Kalck, P.; Soulie, J.M.; Lerouge, O.; Sailladrd, J.Y.; Halet, J.F. New Polymetallic Ruthenium Carbonyl Halide Complexes: Exotic Combinations of Electron Defictent Skeletons with Electron Rich Anions. Their Potential Interest in Ethylene Hydroesterification. J. Am. Chem. Soc. 1992, 114, 10669–10670. [Google Scholar] [CrossRef]
- Dombek, B.D. Hydrogenation of Carbon Monoxide by Ruthenium Complexes with Iodide Promoters: Catalytic and Mechanistic Investigations. J. Organomet. Chem. 1983, 250, 467–483. [Google Scholar] [CrossRef]
- Hidai, M.; Koyasu, Y.; Chikanari, K.; Uchida, Y. Synthesis of Ketones and Esters from Olefins, Carbon Monoxide and Alcohols by Using Ruthenium-Iodide Catalysts. J. Mol. Catal. 1987, 40, 243–254. [Google Scholar] [CrossRef]
- Tamboli, A.H.; Chaugule, A.A.; Kim, H. Catalytic Developments in the Direct Dimethyl Carbonate Synthesis from Carbon Dioxide and Methanol. Chem. Eng. J. 2017, 323, 530–544. [Google Scholar] [CrossRef]
- Tomishige, K.; Kunimori, K. Catalytic and Direct Synthesis of Dimethyl Carbonate Starting from Carbon Dioxide Using CeO2-ZrO2 Solid Solution Heterogeneous Catalyst: Effect of H2O Removal from the Reaction System. Appl. Catal. A-Gen. 2002, 237, 103–109. [Google Scholar] [CrossRef]
- Honda, M.; Kuno, S.; Begum, N.; Fujimoto, K.; Suzuki, K.; Nakagawa, Y.; Tomishige, K. Catalytic synthesis of dialkyl carbonate from low pressure CO2 and alcohols combined with acetonitrile hydration catalyzed by CeO2. Appl. Catal. A-Gen. 2010, 384, 165–170. [Google Scholar] [CrossRef]
- Honda, M.; Tamura, M.; Nakagawa, Y.; Sonehara, S.; Suzuki, K.; Fujimoto, K.; Tomishige, K. Ceria-Catalyzed Conversion of Carbon Dioxide into Dimethyl Carbonate with 2-Cyanopyridine. ChemSusChem 2013, 6, 1341–1344. [Google Scholar] [CrossRef]
- Choi, J.C.; He, L.N.; Yasuda, H.; Sakakura, T. Selective and High Yield Synthesis of Dimethyl Carbonate Directly from Carbon Dioxide and Methanol. Green Chem. 2002, 4, 230–234. [Google Scholar] [CrossRef]
- Zhang, Q.; Yuan, H.Y.; Fukaya, N.; Yasuda, H.; Choi, J.C. A Simple Zinc Catalyst for Carbamate Synthesis Directly from CO2. ChemSusChem 2017, 10, 1501–1508. [Google Scholar] [CrossRef]
- Fukaya, N.; Choi, S.J.; Horikoshi, T.; Kumai, H.; Hasegawa, M.; Yasuda, H.; Sato, K.; Choi, J.C. Synthesis of Tetramethoxysilane from Silica and Methanol Using Carbon Dioxide and an Organic Dehydrating Reagent. Chem. Lett. 2016, 45, 828–830. [Google Scholar] [CrossRef]
- Laine, R.M.; Furgal, J.C.; Doan, P.; Pan, D.; Popova, V.; Zhang, X. Avoiding Carbothermal Reduction: Distillation of Alkoxysilanes from Biogenic, Green, and Sustainable Sources. Angew. Chem. Int. Ed. 2016, 55, 1065–1069. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, J.A.; Rio, I.; Miguel, D.; Sánchez-Vega, M.G. Easy activation of two C-H bonds of an N-heterocyclic carbene N-methyl group. Chem. Commun. 2005, 31, 3956–3958. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.I.; Cole, M.L.; Fung, R.S.C.; Forsyth, C.M.; Hilder, M.; Junk, P.C.; Konstas, K. The reactivity of N-heterocyclic carbenes and their precursors with [Ru3(CO)12]. Dalton Trans. 2008, 31, 4118–4128. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhao, S.; Wang, R. Mechanism of Alkylation of Benzene with Ethylene Catalyzed by [bmim]Cl/FeCl3 Ionic Liquid. Chin. J. Catal. 2004, 25, 247–251. [Google Scholar]
- Ko, N.H.; Lee, J.S.; Huh, E.S.; Lee, H.; Jung, K.D.; Kim, H.S.; Cheong, M. Extractive Desulfurization Using Fe-Containing Ionic Liquids. Energy Fuels 2008, 22, 1687–1690. [Google Scholar] [CrossRef]
- Lim, B.H.; Choe, W.H.; Shim, J.J.; Ra, C.S.; Tuma, D.; Lee, H.; Lee, C.S. High-Pressure Solubility of Carbon Dioxide in Imidazolium-Based Ionic Liquids with Anions [PF6] and [BF4]. Korean J. Chem. Eng. 2009, 26, 1130–1136. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Y.; Ren, R.X.F.; Zhang, Y.; Zhang, J.; Zhang, X. Solubility of CO2 in Sulfonate Ionic Liquids at High Pressure. J. Chem. Eng. Data 2005, 50, 230–233. [Google Scholar] [CrossRef]
- Raut, A.B.; Shende, V.S.; Sasaki, T.; Bhanage, B.M. Reductive Amination of Levulinic Acid to N-Substituted Pyrrolidones over RuCl3 Metal Ion Anchored in Ionic Liquid Immobilized on Graphene Oxide. J. Catal. 2020, 383, 206–214. [Google Scholar] [CrossRef]
- Li, Z.; Cai, Z.; Zeng, Q.; Zhang, T.; France, L.J.; Song, C.; Zhang, Y.; He, H.; Jiang, L.; Long, J.; et al. Selective Catalytic Tailoring of the H Unit in Herbaceous Lignin for Methyl p-Hydroxycinnamate Production over Metal-Based Ionic Liquids. Green Chem. 2018, 20, 3743–3752. [Google Scholar] [CrossRef]

 ) [Bmim]Cl (5.6 mmol); (
) [Bmim]Cl (5.6 mmol); (  ) [TmsPmim]Cl (5.6 mmol); (
) [TmsPmim]Cl (5.6 mmol); (  ) [Bmim]Cl (5.6 mmol) and TMOS (4.2 mmol).
) [Bmim]Cl (5.6 mmol) and TMOS (4.2 mmol).
 ) [Bmim]Cl (5.6 mmol); (
) [Bmim]Cl (5.6 mmol); (  ) [TmsPmim]Cl (5.6 mmol); (
) [TmsPmim]Cl (5.6 mmol); (  ) [Bmim]Cl (5.6 mmol) and TMOS (4.2 mmol).
) [Bmim]Cl (5.6 mmol) and TMOS (4.2 mmol).





| Entry | Ru3(CO)12 [mmol] | Ionic Liquids | Time [h] | Dehydrating Agent | Yield [%] b | TOF [h−1] c |
|---|---|---|---|---|---|---|
| 1 | 0.042 | [Bmim]Cl | 2 | - | 54.8 | 10.6 |
| 2 | 0.042 | [Bmim]Cl | 2 | TMOS | 73.4 | 14.2 |
| 3 | 0.042 | [Bmim]Cl | 2 | TEOS | 69.9 | 13.6 |
| 4 | 0.042 | [Bmim]Cl | 2 | 2-CP | 21.1 | 4.1 |
| 5 | 0.010 | [Bmim]Cl | 4 | - | 40.5 | 16.5 |
| 6 | 0.010 | [TmsPmim]Cl | 4 | - | 61.1 | 23.9 |
| 7 | 0.010 | [Bmim]Cl | 4 | TMOS | 72.1 | 29.1 |
| Entry | Carbene [mmol] | Ru3(CO)12 [mmol] | [Bmim]Cl [mmol] | Salt | Yield [%] b |
|---|---|---|---|---|---|
| 1 | 0.042 | - | - | - | 28.4 |
| 2 | - | 0.042 | - | - | 21.1 |
| 3 | 0.042 | - | - | MgCl2 | 40.2 |
| 4 | - | 0.042 | - | MgCl2 | 34.3 |
| 5 | - | 0.042 | 5.6 | - | 55.6 |
| 6 | - | 0.042 | 5.6 | MgCl2 | 63.4 |
| 7 | - | 0.042 | 5.6 | BmimMgCl3 | 62.3 |
| Entry | Ru3(CO)12 [mmol] | Temperature [°C] | Pressure [Mpa] | Yield [%] b | TOF [h−1] c |
|---|---|---|---|---|---|
| 1 | 0.042 | 160 | 4 | 27.1 | 2.6 |
| 2 | 0.042 | 180 | 4 | 72.5 | 7.0 |
| 3 | 0.042 | 200 | 4 | 97.5 | 9.5 |
| 4 | 0.042 | 200 | 3 | 90.0 | 8.7 |
| 5 | 0.042 | 200 | 2 | 50.5 | 5.0 |
| 6 | 0.032 | 200 | 4 | 90.5 | 11.7 |
| 7 | 0.021 | 200 | 4 | 80.3 | 15.6 |
| 8 | 0.010 | 200 | 4 | 72.1 | 29.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, M.; Dong, T.; Kang, Y.; Zhang, L.; Duan, Z.; Liu, B. Inorganic Salts and Dehydrating Agents Cooperatively Promoted Ru-Catalyzed Ethylene Methoxycarbonylation Using CO2 as a CO Surrogate. Catalysts 2022, 12, 826. https://doi.org/10.3390/catal12080826
Qi M, Dong T, Kang Y, Zhang L, Duan Z, Liu B. Inorganic Salts and Dehydrating Agents Cooperatively Promoted Ru-Catalyzed Ethylene Methoxycarbonylation Using CO2 as a CO Surrogate. Catalysts. 2022; 12(8):826. https://doi.org/10.3390/catal12080826
Chicago/Turabian StyleQi, Meijiao, Tianli Dong, Yu Kang, Li Zhang, Zhongyu Duan, and Binyuan Liu. 2022. "Inorganic Salts and Dehydrating Agents Cooperatively Promoted Ru-Catalyzed Ethylene Methoxycarbonylation Using CO2 as a CO Surrogate" Catalysts 12, no. 8: 826. https://doi.org/10.3390/catal12080826
APA StyleQi, M., Dong, T., Kang, Y., Zhang, L., Duan, Z., & Liu, B. (2022). Inorganic Salts and Dehydrating Agents Cooperatively Promoted Ru-Catalyzed Ethylene Methoxycarbonylation Using CO2 as a CO Surrogate. Catalysts, 12(8), 826. https://doi.org/10.3390/catal12080826